Splanchnic Vein Thrombosis in Myeloproliferative Neoplasms: Treatment Considerations and Unmet Needs
Abstract
Simple Summary
Abstract
1. Introduction
1.1. SVT as a Manifestation of MPNs
1.2. Pathophysiology of MPN-SVT
1.3. Prognosis of MPN-SVT
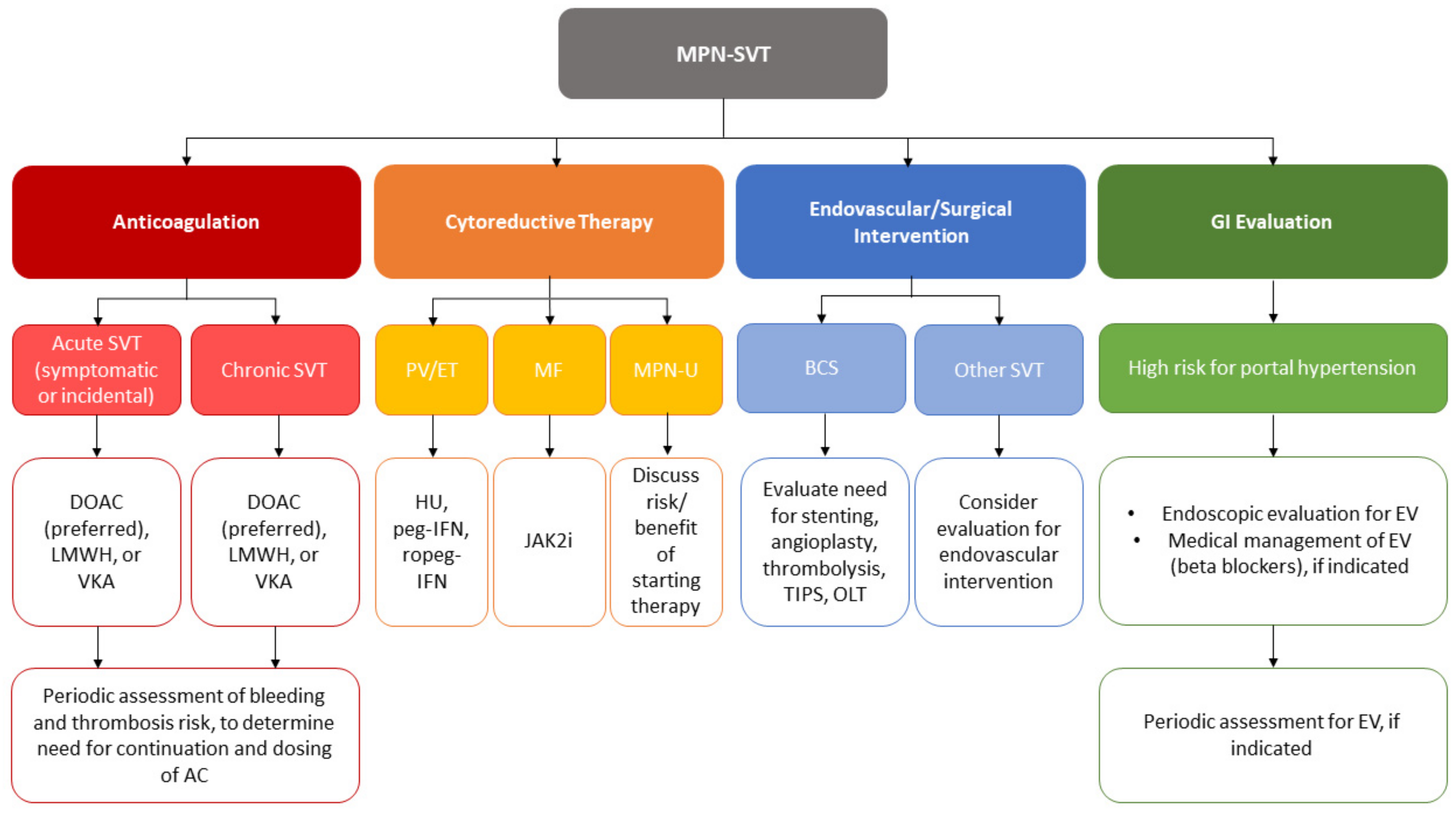
2. Anticoagulation
2.1. Considerations Prior to Anticoagulation
2.2. Choice of Anticoagulant
2.3. Duration of Anticoagulation
2.4. Efficacy of Anticoagulation and the Role of Endovascular and Surgical Intervention
3. Cytoreductive Therapy
4. Unmet Needs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Finazzi, G.; De Stefano, V.; Barbui, T. Splanchnic vein thrombosis in myeloproliferative neoplasms: Treatment algorithm 2018. Blood Cancer J. 2018, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Pardanani, A. Myeloproliferative Neoplasms: A Contemporary Review. JAMA Oncol. 2015, 1, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, D.; Yacoub, A.; Hoffman, R. Overview of Myeloproliferative Neoplasms: History, Pathogenesis, Diagnostic Criteria, and Complications. Hematol. Oncol. Clin. N. Am. 2021, 35, 159–176. [Google Scholar] [CrossRef]
- Sekhar, M.; McVinnie, K.; Burroughs, A.K. Splanchnic vein thrombosis in myeloproliferative neoplasms. Br. J. Haematol. 2013, 162, 730–747. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, V.; Martinelli, I. Splanchnic vein thrombosis: Clinical presentation, risk factors and treatment. Intern. Emerg. Med. 2010, 5, 487–494. [Google Scholar] [CrossRef]
- Valeriani, E.; Riva, N.; Di Nisio, M.; Ageno, W. Splanchnic Vein Thrombosis: Current Perspectives. Vasc. Health Risk. Manag. 2019, 15, 449–461. [Google Scholar] [CrossRef]
- Smalberg, J.H.; Arends, L.R.; Valla, D.C.; Kiladjian, J.J.; Janssen, H.L.; Leebeek, F.W. Myeloproliferative neoplasms in Budd-Chiari syndrome and portal vein thrombosis: A meta-analysis. Blood 2012, 120, 4921–4928. [Google Scholar] [CrossRef]
- Hoekstra, J.; Bresser, E.L.; Smalberg, J.H.; Spaander, M.C.; Leebeek, F.W.; Janssen, H.L. Long-term follow-up of patients with portal vein thrombosis and myeloproliferative neoplasms. J. Thromb. Haemost. 2011, 9, 2208–2214. [Google Scholar] [CrossRef]
- Alvarez-Larran, A.; Pereira, A.; Magaz, M.; Hernandez-Boluda, J.C.; Garrote, M.; Cuevas, B.; Ferrer-Marin, F.; Gomez-Casares, M.T.; Garcia-Gutierrez, V.; Mata-Vazquez, M.I.; et al. Natural history of polycythemia vera and essential thrombocythemia presenting with splanchnic vein thrombosis. Ann. Hematol. 2020, 99, 791–798. [Google Scholar] [CrossRef]
- Anger, B.R.; Seifried, E.; Scheppach, J.; Heimpel, H. Budd-Chiari syndrome and thrombosis of other abdominal vessels in the chronic myeloproliferative diseases. Klin. Wochenschr. 1989, 67, 818–825. [Google Scholar] [CrossRef]
- De Stefano, V.; Za, T.; Rossi, E.; Vannucchi, A.M.; Ruggeri, M.; Elli, E.; Mico, C.; Tieghi, A.; Cacciola, R.R.; Santoro, C.; et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: Incidence, risk factors, and effect of treatments. Haematologica 2008, 93, 372–380. [Google Scholar] [CrossRef]
- Gangat, N.; Wolanskyj, A.P.; Tefferi, A. Abdominal vein thrombosis in essential thrombocythemia: Prevalence, clinical correlates, and prognostic implications. Eur. J. Haematol. 2006, 77, 327–333. [Google Scholar] [CrossRef]
- Barbui, T.; Carobbio, A.; Cervantes, F.; Vannucchi, A.M.; Guglielmelli, P.; Antonioli, E.; Alvarez-Larran, A.; Rambaldi, A.; Finazzi, G.; Barosi, G. Thrombosis in primary myelofibrosis: Incidence and risk factors. Blood 2010, 115, 778–782. [Google Scholar] [CrossRef]
- Dentali, F.; Squizzato, A.; Brivio, L.; Appio, L.; Campiotti, L.; Crowther, M.; Grandi, A.M.; Ageno, W. JAK2V617F mutation for the early diagnosis of Ph- myeloproliferative neoplasms in patients with venous thromboembolism: A meta-analysis. Blood 2009, 113, 5617–5623. [Google Scholar] [CrossRef]
- Sant’Antonio, E.; Guglielmelli, P.; Pieri, L.; Primignani, M.; Randi, M.L.; Santarossa, C.; Rumi, E.; Cervantes, F.; Delaini, F.; Carobbio, A.; et al. Splanchnic vein thromboses associated with myeloproliferative neoplasms: An international, retrospective study on 518 cases. Am. J. Hematol. 2020, 95, 156–166. [Google Scholar] [CrossRef]
- Tremblay, D.; Vogel, A.S.; Moshier, E.; Hoffman, R.; Kremyanskaya, M.; Zhou, S.; Schiano, T.; Mascarenhas, J. Outcomes of splanchnic vein thrombosis in patients with myeloproliferative neoplasms in a single center experience. Eur. J. Haematol. 2020, 104, 72–73. [Google Scholar] [CrossRef]
- Valeriani, E.; Di Nisio, M.; Riva, N.; Cohen, O.; Garcia-Pagan, J.C.; Magaz, M.; Porreca, E.; Ageno, W. Anticoagulant therapy for splanchnic vein thrombosis: A systematic review and meta-analysis. Blood 2021, 137, 1233–1240. [Google Scholar] [CrossRef]
- Saha, P.; Black, S.; Breen, K.; Patel, A.; Modarai, B.; Smith, A. Contemporary management of acute and chronic deep venous thrombosis. Br. Med. Bull. 2016, 117, 107–120. [Google Scholar] [CrossRef]
- Lussana, F.; Carobbio, A.; Randi, M.L.; Elena, C.; Rumi, E.; Finazzi, G.; Bertozzi, I.; Pieri, L.; Ruggeri, M.; Palandri, F.; et al. A lower intensity of treatment may underlie the increased risk of thrombosis in young patients with masked polycythaemia vera. Br. J. Haematol. 2014, 167, 541–546. [Google Scholar] [CrossRef]
- Stein, B.L.; Williams, D.M.; Wang, N.Y.; Rogers, O.; Isaacs, M.A.; Pemmaraju, N.; Spivak, J.L.; Moliterno, A.R. Sex differences in the JAK2 V617F allele burden in chronic myeloproliferative disorders. Haematologica 2010, 95, 1090–1097. [Google Scholar] [CrossRef]
- How, J.; Zhou, A.; Oh, S.T. Splanchnic vein thrombosis in myeloproliferative neoplasms: Pathophysiology and molecular mechanisms of disease. Ther. Adv. Hematol. 2017, 8, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, D.; Winters, A.; Beckman, J.D.; Naymagon, L.; Patel, R.; Mascarenhas, J.; Schiano, T.D. Splanchnic vein thrombosis associated with myeloproliferative neoplasms. Thromb. Res. 2022, 218, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Kushner, A.; West, W.P.; Pillarisetty, L.S. Virchow Triad; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Rusak, T.; Misztal, T.; Piszcz, J.; Tomasiak, M. Nitric oxide scavenging by cell-free hemoglobin may be a primary factor determining hypertension in polycythemic patients. Free Radic. Res. 2014, 48, 230–238. [Google Scholar] [CrossRef]
- Gadomska, G.; Rosc, D.; Stankowska, K.; Boinska, J.; Ruszkowska-Ciastek, B.; Wieczor, R. Selected parameters of hemostasis in patients with myeloproliferative neoplasms. Blood Coagul. Fibrinolysis 2014, 25, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.K.; de Nully Brown, P.; Lund, B.V.; Nielsen, O.J.; Hasselbalch, H.C. Increased platelet activation and abnormal membrane glycoprotein content and redistribution in myeloproliferative disorders. Br. J. Haematol. 2000, 110, 116–124. [Google Scholar] [CrossRef]
- Falanga, A.; Marchetti, M.; Barbui, T.; Smith, C.W. Pathogenesis of thrombosis in essential thrombocythemia and polycythemia vera: The role of neutrophils. Semin. Hematol. 2005, 42, 239–247. [Google Scholar] [CrossRef]
- Wautier, M.P.; El Nemer, W.; Gane, P.; Rain, J.D.; Cartron, J.P.; Colin, Y.; Le Van Kim, C.; Wautier, J.L. Increased adhesion to endothelial cells of erythrocytes from patients with polycythemia vera is mediated by laminin alpha5 chain and Lu/BCAM. Blood 2007, 110, 894–901. [Google Scholar] [CrossRef]
- Sozer, S.; Fiel, M.I.; Schiano, T.; Xu, M.; Mascarenhas, J.; Hoffman, R. The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood 2009, 113, 5246–5249. [Google Scholar] [CrossRef]
- Rosti, V.; Villani, L.; Riboni, R.; Poletto, V.; Bonetti, E.; Tozzi, L.; Bergamaschi, G.; Catarsi, P.; Dallera, E.; Novara, F.; et al. Spleen endothelial cells from patients with myelofibrosis harbor the JAK2V617F mutation. Blood 2013, 121, 360–368. [Google Scholar] [CrossRef]
- Guy, A.; Danaee, A.; Paschalaki, K.; Boureau, L.; Riviere, E.; Etienne, G.; Mansier, O.; Laffan, M.; Sekhar, M.; James, C. Absence of JAK2V617F Mutated Endothelial Colony-Forming Cells in Patients With JAK2V617F Myeloproliferative Neoplasms and Splanchnic Vein Thrombosis. Hemasphere 2020, 4, e364. [Google Scholar] [CrossRef]
- Debureaux, P.E.; Cassinat, B.; Soret-Dulphy, J.; Mora, B.; Verger, E.; Maslah, N.; Plessier, A.; Rautou, P.E.; Ollivier-Hourman, I.; De Ledinghen, V.; et al. Molecular profiling and risk classification of patients with myeloproliferative neoplasms and splanchnic vein thromboses. Blood Adv. 2020, 4, 3708–3715. [Google Scholar] [CrossRef]
- De Stefano, V.; Ruggeri, M.; Cervantes, F.; Alvarez-Larran, A.; Iurlo, A.; Randi, M.L.; Elli, E.; Finazzi, M.C.; Finazzi, G.; Zetterberg, E.; et al. High rate of recurrent venous thromboembolism in patients with myeloproliferative neoplasms and effect of prophylaxis with vitamin K antagonists. Leukemia 2016, 30, 2032–2038. [Google Scholar] [CrossRef]
- De Stefano, V.; Vannucchi, A.M.; Ruggeri, M.; Cervantes, F.; Alvarez-Larran, A.; Iurlo, A.; Randi, M.L.; Pieri, L.; Rossi, E.; Guglielmelli, P.; et al. Splanchnic vein thrombosis in myeloproliferative neoplasms: Risk factors for recurrences in a cohort of 181 patients. Blood Cancer J. 2016, 6, e493. [Google Scholar] [CrossRef]
- Naymagon, L. Venous thrombosis of the liver: Current and emerging concepts in management. Transl. Res. 2020, 225, 54–69. [Google Scholar] [CrossRef]
- Turnes, J.; Garcia-Pagan, J.C.; Gonzalez, M.; Aracil, C.; Calleja, J.L.; Ripoll, C.; Abraldes, J.G.; Banares, R.; Villanueva, C.; Albillos, A.; et al. Portal hypertension-related complications after acute portal vein thrombosis: Impact of early anticoagulation. Clin. Gastroenterol. Hepatol. 2008, 6, 1412–1417. [Google Scholar] [CrossRef]
- Northup, P.G.; Garcia-Pagan, J.C.; Garcia-Tsao, G.; Intagliata, N.M.; Superina, R.A.; Roberts, L.N.; Lisman, T.; Valla, D.C. Vascular Liver Disorders, Portal Vein Thrombosis, and Procedural Bleeding in Patients With Liver Disease: 2020 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 73, 366–413. [Google Scholar] [CrossRef]
- Seijo, S.; Plessier, A.; Hoekstra, J.; Dell’era, A.; Mandair, D.; Rifai, K.; Trebicka, J.; Morard, I.; Lasser, L.; Abraldes, J.G.; et al. Good long-term outcome of Budd-Chiari syndrome with a step-wise management. Hepatology 2013, 57, 1962–1968. [Google Scholar] [CrossRef]
- Naymagon, L.; Tremblay, D.; Zubizarreta, N.; Moshier, E.; Schiano, T.; Mascarenhas, J. Portal vein thrombosis patients harboring JAK2V617F have poor long-term outcomes despite anticoagulation. J. Thromb. Thrombolysis 2020, 50, 652–660. [Google Scholar] [CrossRef]
- Ageno, W.; Beyer-Westendorf, J.; Garcia, D.A.; Lazo-Langner, A.; McBane, R.D.; Paciaroni, M. Guidance for the management of venous thrombosis in unusual sites. J. Thromb. Thrombolysis 2016, 41, 129–143. [Google Scholar] [CrossRef]
- Naymagon, L.; Tremblay, D.; Zubizarreta, N.; Moshier, E.; Troy, K.; Schiano, T.; Mascarenhas, J. The efficacy and safety of direct oral anticoagulants in noncirrhotic portal vein thrombosis. Blood Adv. 2020, 4, 655–666. [Google Scholar] [CrossRef]
- Janczak, D.T.; Mimier, M.K.; McBane, R.D.; Kamath, P.S.; Simmons, B.S.; Bott-Kitslaar, D.M.; Lenz, C.J.; Vargas, E.R.; Hodge, D.O.; Wysokinski, W.E. Rivaroxaban and Apixaban for Initial Treatment of Acute Venous Thromboembolism of Atypical Location. Mayo Clin. Proc. 2018, 93, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Ageno, W.; Beyer Westendorf, J.; Contino, L.; Bucherini, E.; Sartori, M.T.; Senzolo, M.; Grandone, E.; Santoro, R.; Carrier, M.; Delluc, A.; et al. Rivaroxaban for the treatment of noncirrhotic splanchnic vein thrombosis: An interventional prospective cohort study. Blood Adv. 2022, 6, 3569–3578. [Google Scholar] [CrossRef] [PubMed]
- Barbui, T.; De Stefano, V.; Carobbio, A.; Iurlo, A.; Alvarez-Larran, A.; Cuevas, B.; Ferrer Marin, F.; Vannucchi, A.M.; Palandri, F.; Harrison, C.; et al. Direct oral anticoagulants for myeloproliferative neoplasms: Results from an international study on 442 patients. Leukemia 2021, 35, 2989–2993. [Google Scholar] [CrossRef] [PubMed]
- Di Nisio, M.; Valeriani, E.; Riva, N.; Schulman, S.; Beyer-Westendorf, J.; Ageno, W. Anticoagulant therapy for splanchnic vein thrombosis: ISTH SSC Subcommittee Control of Anticoagulation. J. Thromb. Haemost. 2020, 18, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Barbui, T.; Finazzi, G.; Falanga, A. Myeloproliferative neoplasms and thrombosis. Blood 2013, 122, 2176–2184. [Google Scholar] [CrossRef]
- Potthoff, A.; Attia, D.; Pischke, S.; Mederacke, I.; Beutel, G.; Rifai, K.; Deterding, K.; Heiringhoff, K.; Klempnauer, J.; Strassburg, C.P.; et al. Long-term outcome of liver transplant patients with Budd-Chiari syndrome secondary to myeloproliferative neoplasms. Liver Int. 2015, 35, 2042–2049. [Google Scholar] [CrossRef]
- Mesa, R.A.; Jamieson, C.; Bhatia, R.; Deininger, M.W.; Fletcher, C.D.; Gerds, A.T.; Gojo, I.; Gotlib, J.; Gundabolu, K.; Hobbs, G.; et al. NCCN Guidelines Insights: Myeloproliferative Neoplasms, Version 2.2018. J. Natl. Compr. Cancer Netw. 2017, 15, 1193–1207. [Google Scholar] [CrossRef]
- Barbui, T.; Tefferi, A.; Vannucchi, A.M.; Passamonti, F.; Silver, R.T.; Hoffman, R.; Verstovsek, S.; Mesa, R.; Kiladjian, J.J.; Hehlmann, R.; et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: Revised management recommendations from European LeukemiaNet. Leukemia 2018, 32, 1057–1069. [Google Scholar] [CrossRef]
- Marchetti, M.; Vannucchi, A.M.; Griesshammer, M.; Harrison, C.; Koschmieder, S.; Gisslinger, H.; Alvarez-Larran, A.; De Stefano, V.; Guglielmelli, P.; Palandri, F.; et al. Appropriate management of polycythaemia vera with cytoreductive drug therapy: European LeukemiaNet 2021 recommendations. Lancet Haematol. 2022, 9, e301–e311. [Google Scholar] [CrossRef]
- Tremblay, D.; Kosiorek, H.E.; Dueck, A.C.; Hoffman, R. Evaluation of Therapeutic Strategies to Reduce the Number of Thrombotic Events in Patients With Polycythemia Vera and Essential Thrombocythemia. Front. Oncol. 2020, 10, 636675. [Google Scholar] [CrossRef]
- De Stefano, V.; Rossi, E.; Carobbio, A.; Ghirardi, A.; Betti, S.; Finazzi, G.; Vannucchi, A.M.; Barbui, T. Hydroxyurea prevents arterial and late venous thrombotic recurrences in patients with myeloproliferative neoplasms but fails in the splanchnic venous district. Pooled analysis of 1500 cases. Blood Cancer J. 2018, 8, 112. [Google Scholar] [CrossRef]
- Pieri, L.; Paoli, C.; Arena, U.; Marra, F.; Mori, F.; Zucchini, M.; Colagrande, S.; Castellani, A.; Masciulli, A.; Rosti, V.; et al. Safety and efficacy of ruxolitinib in splanchnic vein thrombosis associated with myeloproliferative neoplasms. Am. J. Hematol. 2017, 92, 187–195. [Google Scholar] [CrossRef]
- Mascarenhas, J.; Kosiorek, H.; Prchal, J.; Yacoub, A.; Berenzon, D.; Baer, M.R.; Ritchie, E.; Silver, R.T.; Kessler, C.; Winton, E.; et al. A prospective evaluation of pegylated interferon alfa-2a therapy in patients with polycythemia vera and essential thrombocythemia with a prior splanchnic vein thrombosis. Leukemia 2019, 33, 2974–2978. [Google Scholar] [CrossRef]
- Beauverd, Y.; Radia, D.; Cargo, C.; Knapper, S.; Drummond, M.; Pillai, A.; Harrison, C.; Robinson, S. Pegylated interferon alpha-2a for essential thrombocythemia during pregnancy: Outcome and safety. A case series. Haematologica 2016, 101, e182–e184. [Google Scholar] [CrossRef]
- Agnelli, G.; Buller, H.R.; Cohen, A.; Curto, M.; Gallus, A.S.; Johnson, M.; Porcari, A.; Raskob, G.E.; Weitz, J.I.; Investigators, A.-E. Apixaban for extended treatment of venous thromboembolism. N. Engl. J. Med. 2013, 368, 699–708. [Google Scholar] [CrossRef]
- Weitz, J.I.; Lensing, A.W.A.; Prins, M.H.; Bauersachs, R.; Beyer-Westendorf, J.; Bounameaux, H.; Brighton, T.A.; Cohen, A.T.; Davidson, B.L.; Decousus, H.; et al. Rivaroxaban or Aspirin for Extended Treatment of Venous Thromboembolism. N. Engl. J. Med. 2017, 376, 1211–1222. [Google Scholar] [CrossRef]
- Guy, A.; Gourdou-Latyszenok, V.; Le Lay, N.; Peghaire, C.; Kilani, B.; Dias, J.V.; Duplaa, C.; Renault, M.A.; Denis, C.; Villeval, J.L.; et al. Vascular endothelial cell expression of JAK2(V617F) is sufficient to promote a pro-thrombotic state due to increased P-selectin expression. Haematologica 2019, 104, 70–81. [Google Scholar] [CrossRef]
- Poisson, J.; Plessier, A.; Kiladjian, J.J.; Turon, F.; Cassinat, B.; Andreoli, A.; De Raucourt, E.; Goria, O.; Zekrini, K.; Bureau, C.; et al. Selective testing for calreticulin gene mutations in patients with splanchnic vein thrombosis: A prospective cohort study. J. Hepatol. 2017, 67, 501–507. [Google Scholar] [CrossRef]
- Magaz, M.; Alvarez-Larran, A.; Colomer, D.; Lopez-Guerra, M.; Garcia-Criado, M.A.; Mezzano, G.; Belmonte, E.; Olivas, P.; Soy, G.; Cervantes, F.; et al. Next-generation sequencing in the diagnosis of non-cirrhotic splanchnic vein thrombosis. J. Hepatol. 2021, 74, 89–95. [Google Scholar] [CrossRef]
- Kiladjian, J.J.; Debureaux, P.E.; Plessier, A.; Soret-Dulphy, J.; Valla, D.; Cassinat, B.; Rautou, P.E. Benefits of molecular profiling with next-generation sequencing for the diagnosis and prognosis of myeloproliferative neoplasms in splanchnic vein thrombosis. J. Hepatol. 2021, 74, 251–252. [Google Scholar] [CrossRef]
- Wolach, O.; Sellar, R.S.; Martinod, K.; Cherpokova, D.; McConkey, M.; Chappell, R.J.; Silver, A.J.; Adams, D.; Castellano, C.A.; Schneider, R.K.; et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci. Transl. Med. 2018, 10, eaan8292. [Google Scholar] [CrossRef] [PubMed]
- Cordua, S.; Kjaer, L.; Skov, V.; Pallisgaard, N.; Hasselbalch, H.C.; Ellervik, C. Prevalence and phenotypes of JAK2 V617F and calreticulin mutations in a Danish general population. Blood 2019, 134, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Larran, A.; Ancochea, A.; Angona, A.; Pedro, C.; Garcia-Pallarols, F.; Martinez-Aviles, L.; Bellosillo, B.; Besses, C. Red cell mass measurement in patients with clinically suspected diagnosis of polycythemia vera or essential thrombocythemia. Haematologica 2012, 97, 1704–1707. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A. The rise and fall of red cell mass measurement in polycythemia vera. Curr. Hematol. Rep. 2005, 4, 213–217. [Google Scholar]
- Alvarez-Larran, A.; Pereira, A.; Cervantes, F.; Arellano-Rodrigo, E.; Hernandez-Boluda, J.C.; Ferrer-Marin, F.; Angona, A.; Gomez, M.; Muina, B.; Guillen, H.; et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood 2012, 119, 1363–1369. [Google Scholar] [CrossRef]
- Hernandez-Boluda, J.C.; Alvarez-Larran, A.; Gomez, M.; Angona, A.; Amat, P.; Bellosillo, B.; Martinez-Aviles, L.; Navarro, B.; Teruel, A.; Martinez-Ruiz, F.; et al. Clinical evaluation of the European LeukaemiaNet criteria for clinicohaematological response and resistance/intolerance to hydroxycarbamide in essential thrombocythaemia. Br. J. Haematol. 2011, 152, 81–88. [Google Scholar] [CrossRef]
- Tremblay, D.; Srisuwananukorn, A.; Ronner, L.; Podoltsev, N.; Gotlib, J.; Heaney, M.L.; Kuykendall, A.; O’Connell, C.L.; Shammo, J.M.; Fleischman, A.; et al. European LeukemiaNet Response Predicts Disease Progression but Not Thrombosis in Polycythemia Vera. Hemasphere 2022, 6, e721. [Google Scholar] [CrossRef]
| MPN | Prevalence of SVT | References |
|---|---|---|
| PV | 5.5–10.0% | 14/140 with SVT (10%) [10] |
| 13/235 (5.5%) with BCS and PMVT [11] | ||
| ET | 4.0–13.0% | 3/23 (13%) with SVT [10] |
| 19/460 (4%) with SVT [12] | ||
| 24/259 (9.2%) with BCS and PMVT [11] | ||
| PMF | 0.6–1.0% | 1/106 (1%) with SVT [10] |
| 4/707 (0.6%) with SVT [13] | ||
| SVT | Prevalence of MPN | References |
| PVT | 31.5% | 188/615 (31.5%) with MPN [7] |
| 27.5% PV | ||
| 26.2% ET | ||
| 12.8% MF | ||
| 17.7% MPN-U | ||
| 24.0% solitary JAK2V617F+ | ||
| BCS | 40.9% | 180/440 (40.9%) with MPN [7] |
| 52.9% PV | ||
| 24.6% ET | ||
| 6.7% MF | ||
| 17.0% MPN-U | ||
| 6.5% solitary JAK2V617F+ |
| Complication | Findings from MPN-SVT Studies | References |
|---|---|---|
| Bleeding | Occurrence 20–39% | 13/64 (20%) [16], 17/44 (39%) [8] |
| Time to first bleeding event: 2.3 yr (0.5–5.1) | [8] | |
| Incidence 1.2–2.4 per 100 pt-yr | 1.2 per 100 pt-yr [15], 2.0 per 100 pt-yr [34], 2.43 per 100 pt-yr [9] | |
| IRR 3.6, 95% CI 2.3–5.5, p < 0.001 Higher risk of bleeding compared to MPN pts w/o SVT | [9] | |
| Recurrent thrombosis | Occurrence 27% (12/ 44) [25% intrasplanchnic VTE, 33% extrasplanchnic VTE, 42% arterial] | [8] |
| Time to recurrent thrombosis: 7.5 yr (0–18) | [8] | |
| Incidence recurrent thrombosis 4.2 per 100 pt-yr | [34] | |
| Incidence VTE 2.55 per 100 pt-yr [75% cases intrasplanchnic, 25% cases extrasplanchnic] | [9] | |
| Incidence SVT recurrence 1.6 per 100 pt-yr | [15] | |
| Incidence extrasplanchnic VTE 1.6 per 100 pt-yr | [15] | |
| Incidence arterial thrombosis 0.9–1.6 per 100 pt-yr | 0.90 per 100 pt-yr [9], 1.6 per 100 pt-yr [15] | |
| Risk factors for recurrent thrombosis: BCS (HR 3.03), history of prior thrombosis (HR 3.62), splenomegaly (HR 2.66), leukocytosis (HR 2.8) | [34] | |
| Gastrointestinal complications | EV occurrence 67% (307/458) | [15] |
| Ascites occurrence 47% (30/64) | [16] | |
| Hepatic encephalopathy occurrence 9% (6/64) | [16] | |
| Splenomegaly occurrence: 56.3% PV-SVT, 46.9% ET-SVT | [15] | |
| MPN progression | Occurrence 27% (12/44) | [8] |
| Time to disease progression 9.7 yr (1–17) | [8] | |
| 11% developed MF (7/80) or MPN-BP (2/80) | [32] | |
| 6% progressed to MF (7/118), no cases of MPN-BP | [9] | |
| No difference in progression to MPN-BP compared to MPN pts w/o SVT Evolution to secondary MF was lower in PV-SVT (1.5 vs. 1.7, p = 0.034) compared to PV pts w/o SVT | [15] | |
| Risk factors for MPN progression or death: JAK2V617F allele burden ≥ 50% (OR 14.7), presence of chromatin/spliceosome/TP53 mutations (OR 9.0) | [32] | |
| Death | Occurrence 39% (17/44), most died from end-stage MPN Median age at death 64 yr (30–88) 1-yr OS, 98%; 5-yr OS 88% | Deaths: 8/17 (47%) from end-stage MPN, 3/17 (18%) from thrombosis [8] |
| HR 2.47, 95% CI 1.5–4.01, p < 0.001, most died from hepatic disease, major bleeding, or second cancer | [9] | |
| Median OS similar in PV-SVT (24.9 vs. 23.8 yr, p = 0.76), shorter in ET-SVT (p < 0.001), longer in PMF-SVT (22.1 vs. 6.1 yr, p < 0.001) compared to controls (MPN pts w/o SVT) | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, A.; Naymagon, L.; Tremblay, D. Splanchnic Vein Thrombosis in Myeloproliferative Neoplasms: Treatment Considerations and Unmet Needs. Cancers 2023, 15, 11. https://doi.org/10.3390/cancers15010011
Liu A, Naymagon L, Tremblay D. Splanchnic Vein Thrombosis in Myeloproliferative Neoplasms: Treatment Considerations and Unmet Needs. Cancers. 2023; 15(1):11. https://doi.org/10.3390/cancers15010011
Chicago/Turabian StyleLiu, Angela, Leonard Naymagon, and Douglas Tremblay. 2023. "Splanchnic Vein Thrombosis in Myeloproliferative Neoplasms: Treatment Considerations and Unmet Needs" Cancers 15, no. 1: 11. https://doi.org/10.3390/cancers15010011
APA StyleLiu, A., Naymagon, L., & Tremblay, D. (2023). Splanchnic Vein Thrombosis in Myeloproliferative Neoplasms: Treatment Considerations and Unmet Needs. Cancers, 15(1), 11. https://doi.org/10.3390/cancers15010011





