Pathologic Response of Associated Ductal Carcinoma In Situ to Neoadjuvant Systemic Therapy: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Literature Search
3.2. Effect of NACT
3.3. Effect of NET
3.4. Subgroup Analyses
3.4.1. NACT vs. NET
3.4.2. HER2 Positive vs. TNBC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldberg, H.; Zandbank, J.; Kent, V.; Leonov-Polak, M.; Livoff, A.; Chernihovsky, A.; Guindy, M.; Evron, E. Chemotherapy may eradicate ductal carcinoma in situ (DCIS) but not the associated microcalcifications. Eur. J. Surg. Oncol. 2017, 43, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.; Lau, S.; Yau, T.; Cheung, P.; Epstein, R.J. Presence of an in situ component is associated with reduced biological aggressiveness of size-matched invasive breast cancer. Br. J. Cancer 2010, 102, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Pilewskie, M.; Morrow, M. Margins in breast cancer: How much is enough? Cancer 2018, 124, 1335–1341. [Google Scholar] [CrossRef]
- Ploumen, R.A.W.; Keymeulen, K.; Kooreman, L.F.S.; van Kuijk, S.M.J.; Siesling, S.; Smidt, M.L.; van Nijnatten, T.J.A. The percentage of residual DCIS in patients diagnosed with primary invasive breast cancer treated with neoadjuvant systemic therapy: A nationwide retrospective study. Eur. J. Surg. Oncol. 2022, 48, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Mazouni, C.; Peintinger, F.; Wan-Kau, S.; Andre, F.; Gonzalez-Angulo, A.M.; Symmans, W.F.; Meric-Bernstam, F.; Valero, V.; Hortobagyi, G.N.; Pusztai, L. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J. Clin. Oncol. 2007, 25, 2650–2655. [Google Scholar] [CrossRef]
- Pusztai, L.; Siddik, Z.H.; Mills, G.B.; Bast, R.C., Jr. Physiologic and pathologic drug resistance in ovarian carcinoma--a hypothesis based on a clonal progression model. Acta Oncol. 1998, 37, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Groen, E.J.; van der Noordaa, M.E.M.; Schaapveld, M.; Sonke, G.S.; Mann, R.M.; van Ramshorst, M.S.; Lips, E.H.; Vrancken Peeters, M.; van Duijnhoven, F.H.; Wesseling, J. Pathologic response of ductal carcinoma in situ to neoadjuvant systemic treatment in HER2-positive breast cancer. Breast Cancer Res. Treat. 2021, 189, 213–224. [Google Scholar] [CrossRef]
- Labrosse, J.; Morel, C.; Lam, T.; Laas, E.; Feron, J.G.; Coussy, F.; Lae, M.; Reyal, F.; Hamy, A.S. The Presence of an In Situ Component on Pre-Treatment Biopsy Is Not Associated with Response to Neoadjuvant Chemotherapy for Breast Cancer. Cancers 2021, 13, 235. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Darb-Esfahani, S.; Loibl, S.; Huober, J.; Tesch, H.; Solbach, C.; Holms, F.; Eidtmann, H.; Dietrich, K.; Just, M.; et al. Responsiveness of adjacent ductal carcinoma in situ and changes in HER2 status after neoadjuvant chemotherapy/trastuzumab treatment in early breast cancer--results from the GeparQuattro study (GBG 40). Breast Cancer Res. Treat. 2012, 132, 863–870. [Google Scholar] [CrossRef]
- Sun, S.; van la Parra, R.F.D.; Rauch, G.M.; Checka, C.; Tadros, A.B.; Lucci, A., Jr.; Teshome, M.; Black, D.; Hwang, R.F.; Smith, B.D.; et al. Patient Selection for Clinical Trials Eliminating Surgery for HER2-Positive Breast Cancer Treated with Neoadjuvant Systemic Therapy. Ann. Surg. Oncol. 2019, 26, 3071–3079. [Google Scholar] [CrossRef]
- van la Parra, R.F.D.; Tadros, A.B.; Checka, C.M.; Rauch, G.M.; Lucci, A., Jr.; Smith, B.D.; Krishnamurthy, S.; Valero, V.; Yang, W.T.; Kuerer, H.M. Baseline factors predicting a response to neoadjuvant chemotherapy with implications for non-surgical management of triple-negative breast cancer. Br. J. Surg. 2018, 105, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Hyslop, T.; Hendrix, L.H.; Duong, S.; Bedrosian, I.; Price, E.; Caudle, A.; Hieken, T.; Guenther, J.; Hudis, C.A.; et al. Phase II Single-Arm Study of Preoperative Letrozole for Estrogen Receptor-Positive Postmenopausal Ductal Carcinoma In Situ: CALGB 40903 (Alliance). J. Clin. Oncol. 2020, 38, 1284–1292. [Google Scholar] [CrossRef]
- Doebar, S.C.; van den Broek, E.C.; Koppert, L.B.; Jager, A.; Baaijens, M.H.A.; Obdeijn, I.A.M.; van Deurzen, C.H.M. Extent of ductal carcinoma in situ according to breast cancer subtypes: A population-based cohort study. Breast Cancer Res. Treat. 2016, 158, 179–187. [Google Scholar] [CrossRef] [PubMed]
- de Boniface, J.; Szulkin, R.; Johansson, A.L.V. Survival After Breast Conservation vs Mastectomy Adjusted for Comorbidity and Socioeconomic Status: A Swedish National 6-Year Follow-up of 48986 Women. JAMA Surg. 2021, 156, 628–637. [Google Scholar] [CrossRef]
- Xiang, W.; Wu, C.; Wu, H.; Fang, S.; Liu, N.; Yu, H. Survival Comparisons between Breast Conservation Surgery and Mastectomy Followed by Postoperative Radiotherapy in Stage I-III Breast Cancer Patients: Analysis of the Surveillance, Epidemiology, and End Results (Seer) Program Database. Curr. Oncol. 2022, 29, 5731–5747. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Casas, S.E.; Castilla-Tarra, J.A.; Pena-Torres, E.; Orozco-Ospino, M.; Mendoza-Diaz, S.; Nunez-Lemus, M.; Garcia-Angulo, O.; Garcia-Mora, M.; Guzman-AbiSaab, L.; Lehmann-Mosquera, C.; et al. Pathological Response to Neoadjuvant Chemotherapy and the Molecular Classification of Locally Advanced Breast Cancer in a Latin American Cohort. Oncologist 2019, 24, e1360–e1370. [Google Scholar] [CrossRef]
- Osdoit, M.; Yau, C.; Symmans, W.F.; Boughey, J.C.; Ewing, C.A.; Balassanian, R.; Chen, Y.Y.; Krings, G.; Wallace, A.M.; Zare, S.; et al. Association of Residual Ductal Carcinoma In Situ With Breast Cancer Recurrence in the Neoadjuvant I-SPY2 Trial. JAMA Surg. 2022, 157, 1034–1041. [Google Scholar] [CrossRef]
- Heil, J.; Kuerer, H.M.; Pfob, A.; Rauch, G.; Sinn, H.P.; Golatta, M.; Liefers, G.J.; Vrancken Peeters, M.J. Eliminating the breast cancer surgery paradigm after neoadjuvant systemic therapy: Current evidence and future challenges. Ann. Oncol. 2020, 31, 61–71. [Google Scholar] [CrossRef]
- Kuerer, H.M.; Rauch, G.M.; Krishnamurthy, S.; Adrada, B.E.; Caudle, A.S.; DeSnyder, S.M.; Black, D.M.; Santiago, L.; Hobbs, B.P.; Lucci, A., Jr.; et al. A Clinical Feasibility Trial for Identification of Exceptional Responders in Whom Breast Cancer Surgery Can Be Eliminated Following Neoadjuvant Systemic Therapy. Ann. Surg. 2018, 267, 946–951. [Google Scholar] [CrossRef]
- Kuerer, H.M.; Newman, L.A.; Buzdar, A.U.; Dhingra, K.; Hunt, K.K.; Buchholz, T.A.; Binkley, S.M.; Strom, E.A.; Ames, F.C.; Ross, M.I.; et al. Pathologic tumor response in the breast following neoadjuvant chemotherapy predicts axillary lymph node status. Cancer J. Sci. Am. 1998, 4, 230–236. [Google Scholar]
- Park, S.; Yoon, J.H.; Sohn, J.; Park, H.S.; Moon, H.J.; Kim, M.J.; Kim, E.K.; Kim, S.I.; Park, B.W. Magnetic Resonance Imaging after Completion of Neoadjuvant Chemotherapy Can Accurately Discriminate between No Residual Carcinoma and Residual Ductal Carcinoma In Situ in Patients with Triple-Negative Breast Cancer. PLoS ONE 2016, 11, e0149347. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.; Taube, J.M.; Elwood, H.; Sharma, R.; Meeker, A.; Warzecha, H.N.; Argani, P.; Cimino-Mathews, A.; Emens, L.A. The immune microenvironment of breast ductal carcinoma in situ. Mod. Pathol. 2016, 29, 249–258. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmana, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Kim, S.B.; Chung, W.P.; Im, S.A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.M.; Petry, V.; Chung, C.F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Crook, T.; Leonard, R.; Mokbel, K.; Thompson, A.; Michell, M.; Page, R.; Vaid, A.; Mehrotra, R.; Ranade, A.; Limaye, S.; et al. Accurate Screening for Early-Stage Breast Cancer by Detection and Profiling of Circulating Tumor Cells. Cancers 2022, 14, 3341. [Google Scholar] [CrossRef]
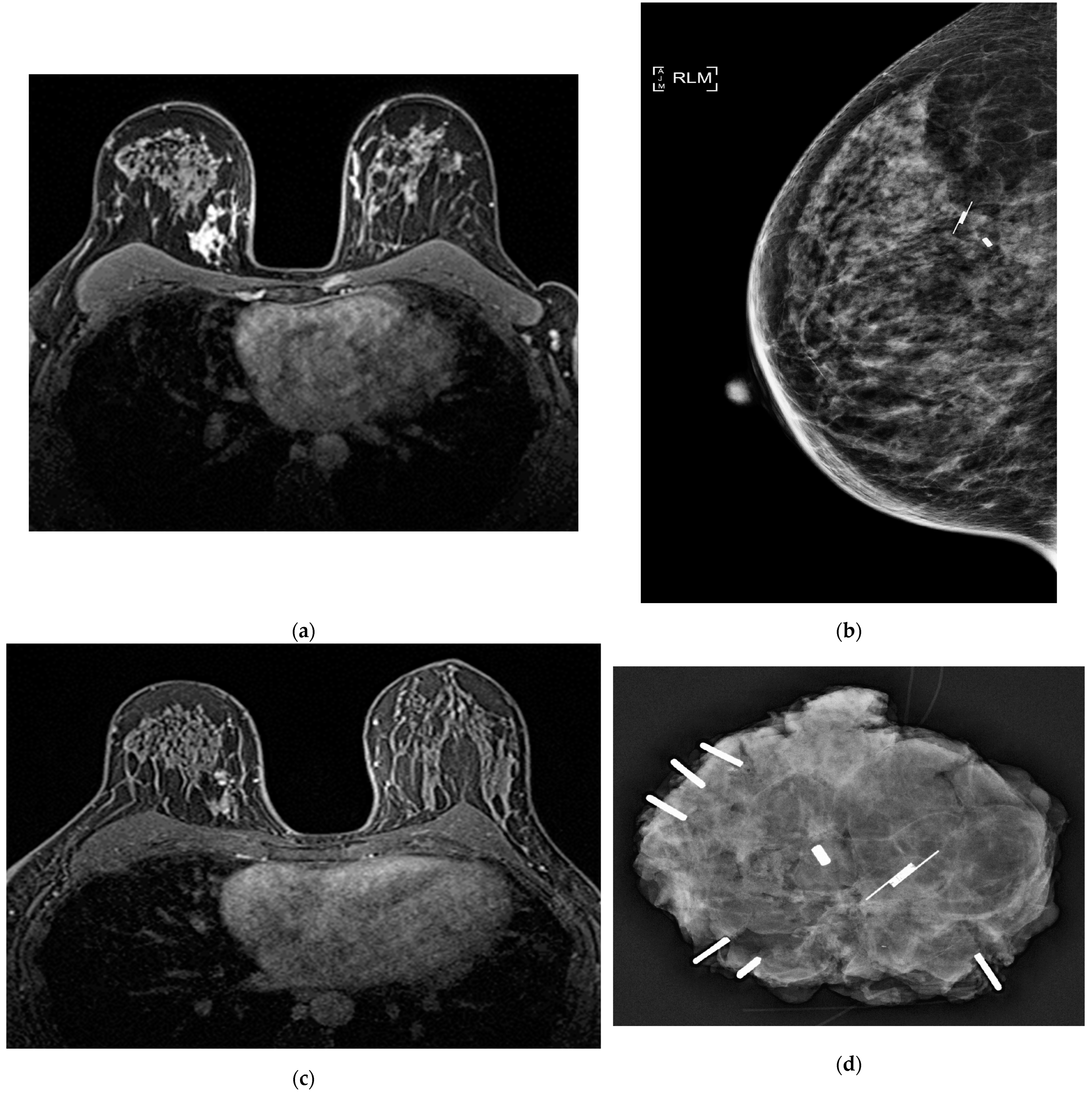
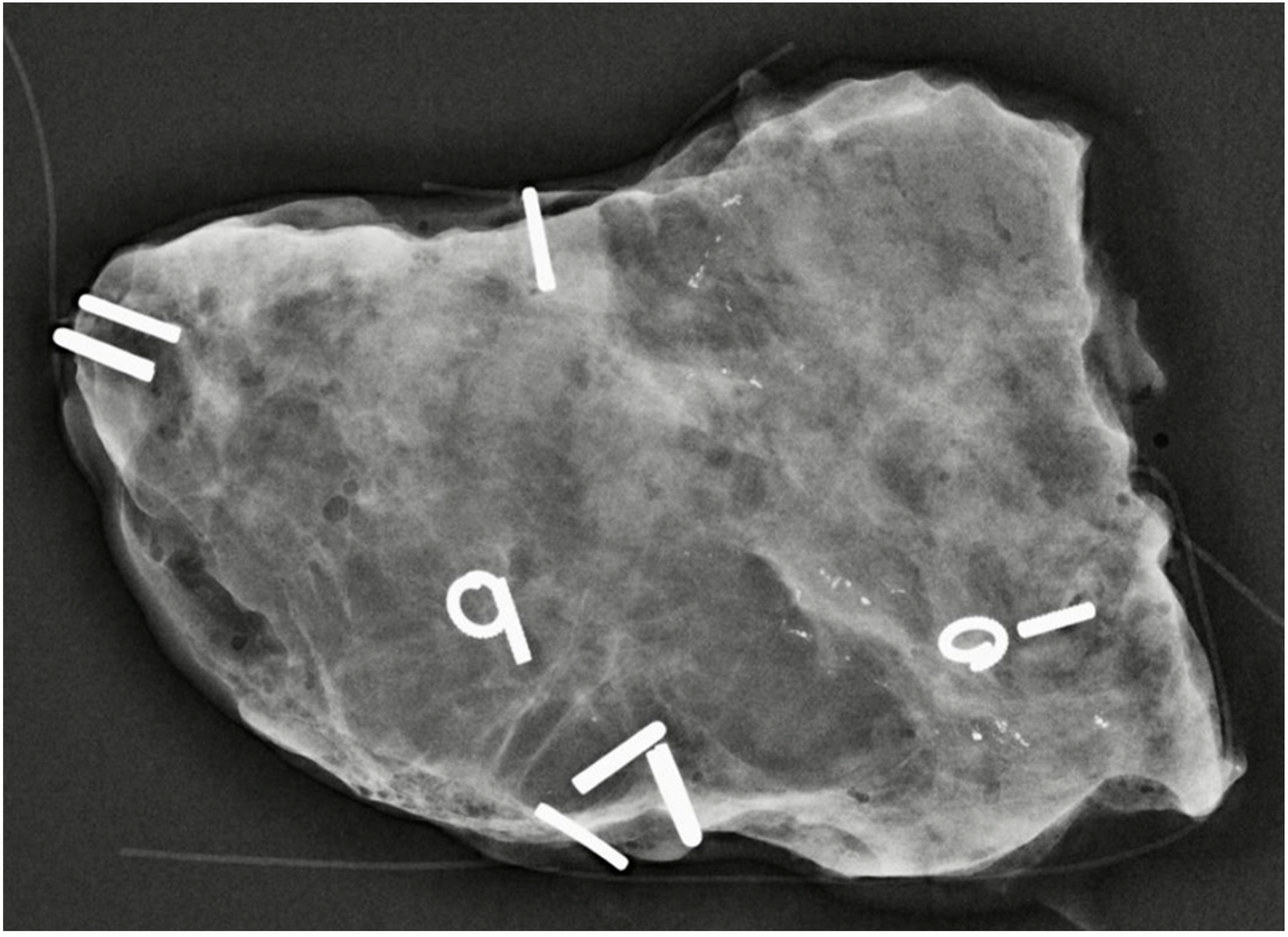
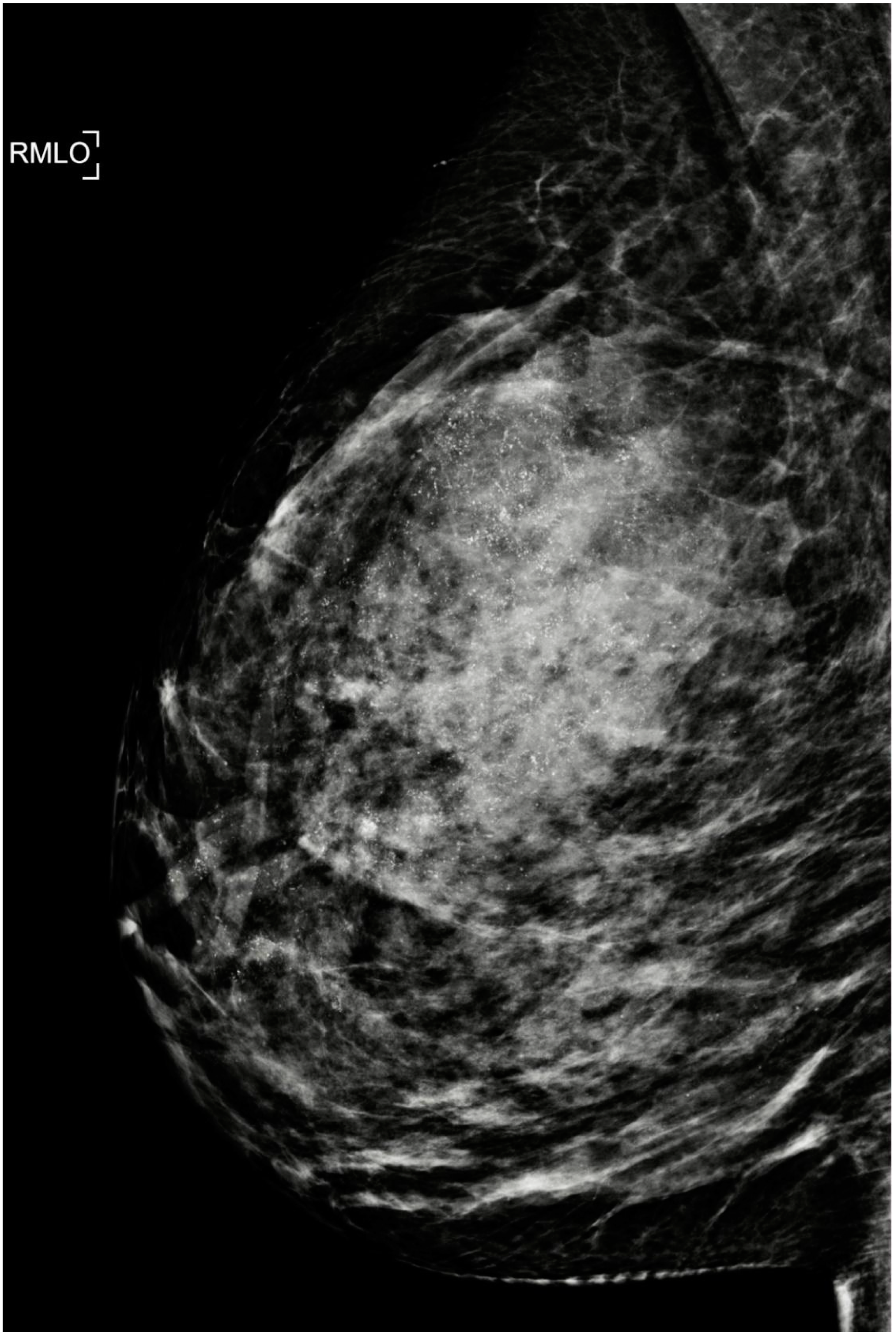
| Citation | Author (Year) | Study Design | Patients (N) | Overall pCR | IDC-DCIS | DCIS-specific pCR |
|---|---|---|---|---|---|---|
| [7] | Groen (2021) | Prospective | 316 | 46% | 138 | 64 |
| [8] | Labrosse (2021) | Retrospective | 1148 | 19.4% (283) | 225 | 82 |
| [9] | von Minckowitz (2021) | RCT (subset) | 158 | 50.8% (30) | 59 | 30 |
| [10] | Sun (2019) | Retrospective | 280 | 36.4% (102) | 129 | 46 |
| [1] | Goldberg (2017) | Prospective | 92 | 42% (39) | 30 | 10 |
| [11] | Van la Parra (2018) | Prospective | 328 | 36.9% | 78 | 35 |
| Total | 659 | 267 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wazir, U.; Patani, N.; Balalaa, N.; Mokbel, K. Pathologic Response of Associated Ductal Carcinoma In Situ to Neoadjuvant Systemic Therapy: A Systematic Review. Cancers 2023, 15, 13. https://doi.org/10.3390/cancers15010013
Wazir U, Patani N, Balalaa N, Mokbel K. Pathologic Response of Associated Ductal Carcinoma In Situ to Neoadjuvant Systemic Therapy: A Systematic Review. Cancers. 2023; 15(1):13. https://doi.org/10.3390/cancers15010013
Chicago/Turabian StyleWazir, Umar, Neill Patani, Nahed Balalaa, and Kefah Mokbel. 2023. "Pathologic Response of Associated Ductal Carcinoma In Situ to Neoadjuvant Systemic Therapy: A Systematic Review" Cancers 15, no. 1: 13. https://doi.org/10.3390/cancers15010013
APA StyleWazir, U., Patani, N., Balalaa, N., & Mokbel, K. (2023). Pathologic Response of Associated Ductal Carcinoma In Situ to Neoadjuvant Systemic Therapy: A Systematic Review. Cancers, 15(1), 13. https://doi.org/10.3390/cancers15010013







