Predictors of Early Thrombotic Events in Adult Patients with Acute Myeloid Leukemia: A Real-World Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients’ Characteristics
2.2. Thrombotic Events Diagnosis and Management
2.3. Statistical Analyses
3. Results
3.1. Development of Venous Thromboembolism Prediction Score
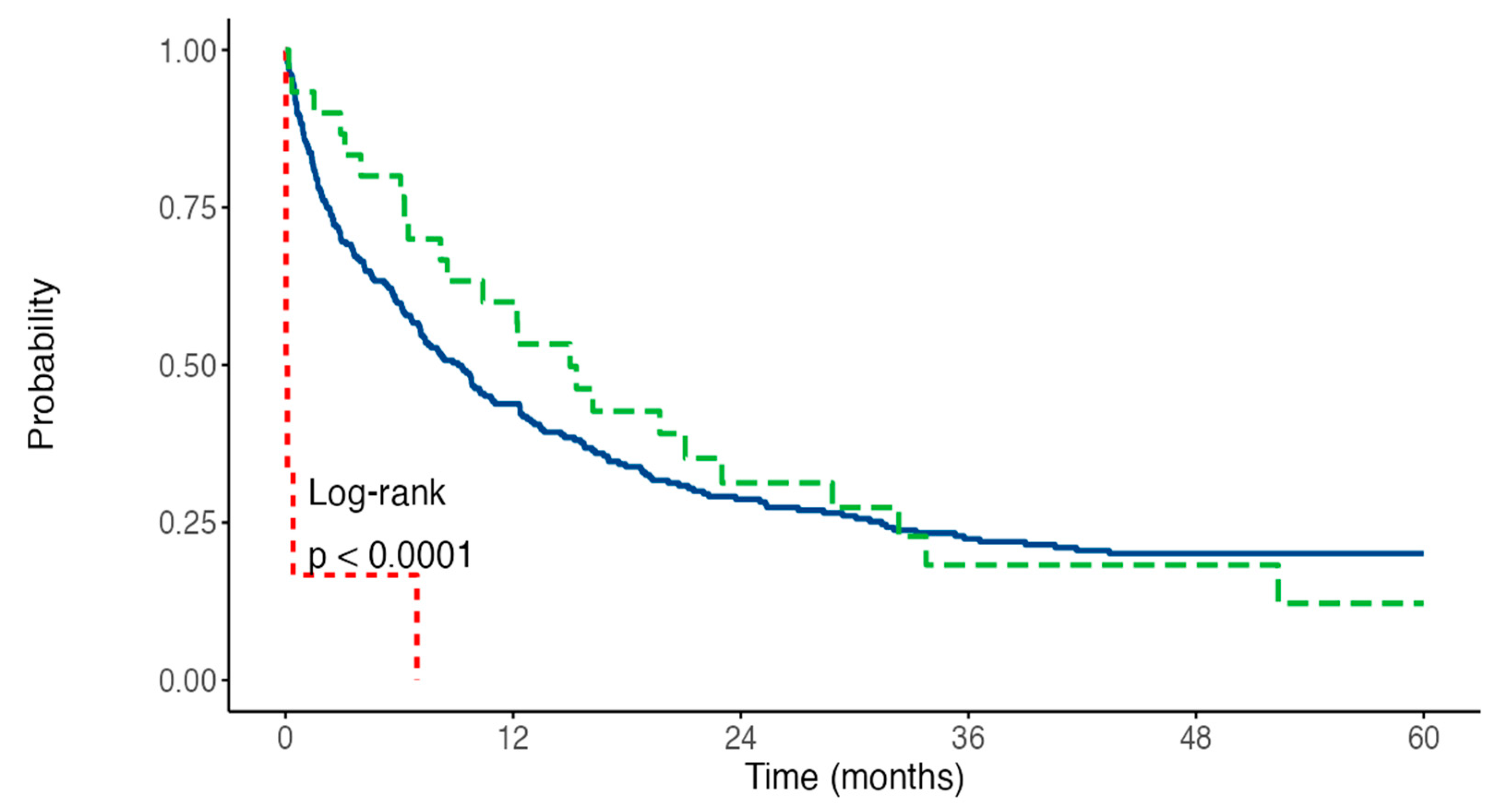
3.2. Impact of Thrombosis on Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bouillaud, J.; Bouillaud, S. De l’obliteration des veines et de son influence sur la formation des hydropisies partielles: Consideration sur la hydropisies passive et general. Arch. Gen. Intern. Med. 1823, 1, 188–204. [Google Scholar]
- Timp, J.F.; Braekkan, S.K.; Versteeg, H.H.; Cannegieter, S.C. Epidemiology of cancer-associated venous thrombosis. Blood 2013, 122, 1712–1723. [Google Scholar] [CrossRef]
- Blom, J.W.; Doggen, C.J.M.; Osanto, S.; Rosendaal, F.R. Malignancies, Prothrombotic Mutations, and the Risk of Venous Thrombosis. JAMA 2005, 293, 715–722. [Google Scholar] [CrossRef]
- Khorana, A.A. Venous thromboembolism and prognosis in cancer. Thromb. Res. 2010, 125, 490–493. [Google Scholar] [CrossRef]
- Donnellan, E.; Khorana, A.A. Cancer and Venous Thromboembolic Disease: A Review. Oncologist 2017, 22, 199–207. [Google Scholar] [CrossRef]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 2007, 5, 632–634. [Google Scholar] [CrossRef]
- Kekre, N.; Connors, J.M. Venous thromboembolism incidence in hematologic malignancies. Blood Rev. 2019, 33, 24–32. [Google Scholar] [CrossRef]
- Colombo, R.; Gallipoli, P.; Castelli, R. Thrombosis and hemostatic abnormalities in hematological malignancies. Clin. Lymphoma Myeloma Leuk. 2014, 14, 441–450. [Google Scholar] [CrossRef]
- Palumbo, A.; Rajkumar, S.V.; Dimopoulos, M.A.; Richardson, P.G.; San Miguel, J.; Barlogie, B.; Harousseau, J.; Zonder, J.A.; Cavo, M.; Zangari, M.; et al. Prevention of thalidomide-and lenalidomide-associated thrombosis in myeloma. Leukemia 2008, 22, 414–423. [Google Scholar] [CrossRef]
- Hohaus, S.; Bartolomei, F.; Cuccaro, A.; Maiolo, E.; Alma, E.; D’alò, F.; Bellesi, S.; Rossi, E.; De Stefano, V. Venous thromboembolism in lymphoma: Risk stratification and antithrombotic prophylaxis. Cancers 2020, 12, 1291. [Google Scholar] [CrossRef]
- Falanga, A.; Marchetti, M. Thrombosis in myeloproliferative neoplasms. Semin. Thromb. Hemost. 2014, 40, 348–358. [Google Scholar] [CrossRef]
- Montesinos, P.; de la Serna, J.; Vellenga, E.; Rayon, C.; Bergua, J.; Parody, R.; Esteve, J.; Gonzalez, M.; Brunet, S.; Sanz, M. Incidence and Risk Factors for Thrombosis in Patients with Acute Promyelocytic Leukemia. Experience of the PETHEMA LPA96 and LPA99 Protocols. Blood 2006, 108, 1503. [Google Scholar] [CrossRef]
- Gurnari, C.; Breccia, M.; Di Giuliano, F.; Scalzulli, E.; Divona, M.; Piciocchi, A.; Cicconi, L.; De Bellis, E.; Venditti, A.; Del Principe, M.I.; et al. Early intracranial haemorrhages in acute promyelocytic leukaemia: Analysis of neuroradiological and clinico-biological parameters. Br. J. Haematol. 2021, 193, 129–132. [Google Scholar] [CrossRef]
- Ku, G.H.; White, R.H.; Chew, H.K.; Harvey, D.J.; Zhou, H.; Wun, T. Venous thromboembolism in patients with acute leukemia: Incidence, risk factors, and effect on survival. Blood 2009, 113, 3911–3917. [Google Scholar] [CrossRef]
- De Stefano, V.; Sorà, F.; Rossi, E.; Chiusolo, P.; Laurenti, L.; Fianchi, L.; Zini, G.; Pagano, L.; Sica, S.; Leone, G. The risk of thrombosis in patients with acute leukemia: Occurrence of thrombosis at diagnosis and during treatment. J. Thromb. Haemost. 2005, 3, 1985–1992. [Google Scholar] [CrossRef]
- Libourel, E.J.; Klerk, C.P.W.; van Norden, Y.; de Maat, M.P.M.; Kruip, M.J.; Sonneveld, P.; Löwenberg, B.; Leebeek, F.W.G. Disseminated intravascular coagulation at diagnosis is a strong predictor for thrombosis in acute myeloid leukemia. Blood 2016, 128, 1854–1861. [Google Scholar] [CrossRef]
- Martella, F.; Cerrano, M.; Di Cuonzo, D.; Secreto, C.; Olivi, M.; Apolito, V.; D’Ardia, S.; Frairia, C.; Giai, V.; Lanzarone, G.; et al. Frequency and risk factors for thrombosis in acute myeloid leukemia and high-risk myelodysplastic syndromes treated with intensive chemotherapy: A two centers observational study. Ann. Hematol. 2022, 101, 855–867. [Google Scholar] [CrossRef]
- Falanga, A.; Barbui, T.; Rickles, F.R. Hypercoagulability and tissue factor gene upregulation in hematologic malignancies. Semin. Thromb. Hemost. 2008, 34, 204–210. [Google Scholar] [CrossRef]
- Falanga, A.; Schieppati, F.; Russo, D. Cancer Tissue Procoagulant Mechanisms and the Hypercoagulable State of Patients with Cancer. Semin. Thromb. Hemost. 2015, 41, 756–764. [Google Scholar] [CrossRef]
- Kwaan, H.C. Double hazard of thrombophilia and bleeding in leukemia. Hematol. Am. Soc. Hematol. Educ. Program 2007, 2007, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Eckert, L.; Wang, Y.; Wang, H.; Cohen, A. Venous Thromboembolism Risk in Patients with Cancer Receiving Chemotherapy: A Real-World Analysis. Oncologist 2013, 18, 1321–1329. [Google Scholar] [CrossRef]
- Franchini, M.; Di Minno, M.N.D.; Coppola, A. Disseminated intravascular coagulation in hematologic malignancies. Semin. Thromb. Hemost. 2010, 36, 388–403. [Google Scholar] [CrossRef]
- Levi, M.; Sivapalaratnam, S. Disseminated intravascular coagulation: An update on pathogenesis and diagnosis. Expert Rev. Hematol. 2018, 11, 663–672. [Google Scholar] [CrossRef]
- Suzuki, K.; Wada, H.; Imai, H.; Iba, T.; Thachil, J.; Toh, C.H. A re-evaluation of the D-dimer cut-off value for making a diagnosis according to the ISTH overt-DIC diagnostic criteria: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1442–1444. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Tallman, M.S.; Wang, E.S.; Altman, J.K.; Appelbaum, F.R.; Bhatt, V.R.; Bixby, D.; Coutre, S.E.; De Lima, M.; Fathi, A.T.; Fiorella, M.; et al. Acute Myeloid Leukemia, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 721–749. [Google Scholar] [CrossRef]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 78, e187–e285. [Google Scholar] [CrossRef]
- Napolitano, M.; Saccullo, G.; Marietta, M.; Carpenedo, M.; Castaman, G.; Cerchiara, E.; Chistolini, A.; Contino, L.; De Stefano, V.; Falanga, A.; et al. Platelet cut-off for anticoagulant therapy in thrombocytopenic patients with blood cancer and venous thromboembolism: An expert consensus. Blood Transfus. 2019, 17, 171–180. [Google Scholar] [CrossRef]
- Napolitano, M.; Valore, L.; Malato, A.; Saccullo, G.; Vetro, C.; Mitra, M.E.; Fabbiano, F.; Mannina, D.; Casuccio, A.; Lucchesi, A.; et al. Management of venous thromboembolism in patients with acute leukemia at high bleeding risk: A multi-center study. Leuk. Lymphoma 2016, 57, 116–119. [Google Scholar] [CrossRef]
- Falanga, A.; Leader, A.; Ambaglio, C.; Bagoly, Z.; Castaman, G.; Elalamy, I.; Lecumberri, R.; Niessner, A.; Pabinger, I.; Szmit, S.; et al. EHA Guidelines on Management of Antithrombotic Treatments in Thrombocytopenic Patients with Cancer. HemaSphere 2022, 6, e750. [Google Scholar] [CrossRef]
- Del Principe, M.I.; Del Principe, D.; Venditti, A. Thrombosis in adult patients with acute leukemia. Curr. Opin. Oncol. 2017, 29, 448–454. [Google Scholar] [CrossRef]
- Vu, K.; Luong, N.V.; Hubbard, J.; Zalpour, A.; Faderl, S.; Thomas, D.A.; Yang, D.; Kantarjian, H.; Kroll, M.H. A retrospective study of venous thromboembolism in acute leukemia patients treated at the university of texas MD anderson cancer center. Cancer Med. 2015, 4, 27–35. [Google Scholar] [CrossRef]
- Mirza, A.S.; Yun, S.; Ali, N.A.; Shin, H.; O’Neil, J.L.; Elharake, M.; Schwartz, D.; Robinson, K.; Nowell, E.; Engle, G.; et al. Validation of the Khorana score in acute myeloid leukemia patients: A single-institution experience. Thromb. J. 2019, 17, 13. [Google Scholar] [CrossRef]
- Al-Ani, F.; Wang, Y.P.; Lazo-Langner, A. Development of a Clinical Prediction Rule for Venous Thromboembolism in Patients with Acute Leukemia. Thromb. Haemost. 2020, 120, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Razak, N.B.A.; Jones, G.; Bhandari, M.; Berndt, M.C.; Metharom, P. Cancer-associated thrombosis: An overview of mechanisms, risk factors, and treatment. Cancers 2018, 10, 380. [Google Scholar] [CrossRef]
- Connolly, G.C.; Khorana, A.A. Emerging risk stratification approaches to cancer-associated thrombosis: Risk factors, biomarkers and a risk score. Thromb. Res. 2010, 125, S1–S7. [Google Scholar] [CrossRef]
- Wun, T.; White, R.H. Venous thromboembolism (VTE) in patients with cancer: Epidemiology and risk factors. Cancer Investig. 2009, 27, 63–74. [Google Scholar] [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef]
- Levi, M.; Toh, C.H.; Thachil, J.; Watson, H.G. Guidelines for the diagnosis and management of disseminated intravascular coagulation. Br. J. Haematol. 2009, 145, 24–33. [Google Scholar] [CrossRef]
- Weltermann, A.; Pabinger, I.; Geissler, K.; Jäger, U.; Gisslinger, H.; Knöbl, P.; Eichinger, S.; Kyrie, P.A.; Valent, P.; Speiser, W.; et al. Hypofibrinogenemia in non-M3 acute myeloid leukemia. Incidence, clinical and laboratory characteristics and prognosis. Leukemia 1998, 12, 1182–1186. [Google Scholar] [CrossRef]
- Murray, J.; Precious, E.; Alikhan, R. Catheter-related thrombosis in cancer patients. Br. J. Haematol. 2013, 162, 746–757. [Google Scholar] [CrossRef]
- Geerts, W. Central venous catheter-related thrombosis. Hematol. Am. Soc. Hematol. Educ. Program 2014, 1, 306–311. [Google Scholar] [CrossRef]
- Grilz, E.; Königsbrügge, O.; Posch, F.; Schmidinger, M.; Pirker, R.; Lang, I.M.; Pabinger, I.; Ay, C. Frequency, risk factors, and impact on mortality of arterial thromboembolism in patients with cancer. Haematologica 2018, 103, 1549–1556. [Google Scholar] [CrossRef]
- Poh, C.; Brunson, A.; Keegan, T.; Wun, T.; Mahajan, A. Incidence of Upper Extremity Deep Vein Thrombosis in Acute Leukemia and Effect on Mortality. TH Open 2020, 04, e309–e317. [Google Scholar] [CrossRef]
- Mohamed, M.O.; Lopez-Mattei, J.C.; Parwani, P.; Iliescu, C.A.; Bharadwaj, A.; Kim, P.Y.; Palaskas, N.L.; Rashid, M.; Potts, J.; Kwok, C.S.; et al. Management strategies and clinical outcomes of acute myocardial infarction in leukaemia patients: Nationwide insights from United States hospitalizations. Int. J. Clin. Pract. 2020, 74, e13476. [Google Scholar] [CrossRef]


| No TE n = 264 | TE n = 36 | p | VTE, n = 30 | p | ATE, n = 6 | p | |
|---|---|---|---|---|---|---|---|
| Sex M/F | 159/105 | 24/12 | 0.585 | 18/12 | 1 | 6/0 | 0.085 |
| Median Age (interval) | 64 (21–90) | 64 (30–82) | 0.998 | 64 (30–82) | 0.708 | 66 (53–77) | 0.584 |
| AML Classification | |||||||
| de Novo AML | 183 | 24 | 0.203 | 20 | 0.137 | 4 | 0.59 |
| Treatment-related AML | 35 | 2 | 0.219 | 1 | 0.195 | 1 | 0.318 |
| Secondary-AML | 46 | 10 | 0.152 | 9 | 0.179 | 1 | 0.876 |
| ELN 2017-risk stratification | |||||||
| Favorable | 46 | 6 | 0.908 | 5 | 0.919 | 1 | 0.93 |
| Intermediate | 108 | 16 | 0.803 | 13 | 0.862 | 2 | 0.885 |
| Adverse | 73 | 12 | 0.665 | 10 | 0.696 | 2 | 0.868 |
| Non-Classifiable | 37 | 2 | 2 | 1 | |||
| FLT3 | 49 | 8 | 0.652 | 7 | 0.621 | 1 | 1 |
| NPM1 | 54 | 5 | 0.379 | 4 | 0.467 | 1 | 1 |
| Comorbidities ≥ 1 | 171 | 32 | 0.004 | 27 | 0.006 | 6 | 0.182 |
| Cardiovascular comorbidities ≥ 2 | 58 | 16 | 0.006 | 10 | 0.266 | 6 | <0.001 |
| BMI ≥ 1 | 24 | 5 | 0.367 | 4 | 0.511 | 1 | 0.46 |
| ISTH-DIC score ≥ 4 | 56 | 5 | 0.382 | 5 | 0.811 | 0 | 0.605 |
| Smokers | 107 | 18 | 0.286 | 15 | 0.337 | 3 | 0.696 |
| History of VTE | 7 | 5 | 0.008 | 5 | 0.003 | 0 | |
| Central Venous Catheter | 221 | 31 | 0.813 | 25 | 1 | 6 | |
| Hemoglobin Count g/dL | 8.55 (3.5–13.8) | 8.95 (5.2–13.9) | 0.371 | 8.7 (5.2–11.7) | 0.939 | 9.7 (8.4–13.9) | 0.059 |
| Leukocyte Count × 109/L | 9.11 (10–472.3) | 8.77 (0.47–200) | 0.614 | 7.83 (0.47–200) | 0.708 | 6.93 (0.82–26) | 0.286 |
| Platelet Count × 109/L | 44.5 (3–1130) | 71 (5–597) | 0.032 | 76 (5–597) | 0.05 | 44.5 (29–95) | 0.998 |
| Serum LDH level, U/L | 350 (70–4000) | 300 (120–1687) | 0.425 | 292 (120–1687) | 0.262 | 364 (234–811) | 0.707 |
| Serum Fibrinogen level mg/dL | 364 (63–2878) | 373 (82–1025) | 0.842 | 364 (82–1025) | 0.677 | 506 (332–872) | 0.027 |
| ATIII, % | 92 (25–142) | 97.3 (70–125) | 0.147 | 97.3 (71–125) | 0.2 | 88.3 (70–115) | 0.727 |
| PT, seconds | 14.6 (11.1–29.6) | 14.5 (11.4–20.4) | 0.439 | 14.85 (11.4–20.4) | 0.66 | 14.7 (12.4–16.5) | 0.981 |
| aPTT, ratio | 0.980 (0.59–1.86) | 0.925 (0.68–2.01) | 0.136 | 0.935 (0.68–2.01) | 0.379 | 0.950 (0.8–0.94) | 0.076 |
| Serum D-Dimer level ng/mL | 834 (52–121,450) | 886 (77–48,065) | 0.936 | 999 (77–48,065) | 0.948 | 1279 (532–20,650) | 0.324 |
| Age | Sex | Type of TE | Onset from AML Diagnosis (Days) | History of VTE | n° of Comorbidities | TE Pre/Post AML-Therapy | |
|---|---|---|---|---|---|---|---|
| 1 | 66 | M | hepatic veins thrombosis | 1 | no | 2 | pre |
| 2 | 47 | F | CRT | 8 | no | 2 | pre |
| 3 | 63 | M | CRT | 10 | no | 1 | post |
| 4 | 68 | M | DVT+PE | 1 | no | 3 | pre |
| 5 | 53 | M | MI | 1 | no | 2 | pre |
| 6 | 44 | F | CRT | 11 | no | 1 | post |
| 7 | 71 | F | PE | 12 | no | 2 | post |
| 8 | 63 | M | MI | 35 | no | 2 | post |
| 9 | 81 | M | CRT | 18 | no | 2 | pre |
| 10 | 70 | M | DVT | 1 | no | 2 | pre |
| 11 | 68 | M | CRT | 4 | no | 2 | pre |
| 12 | 49 | F | CRT | 11 | no | 2 | post |
| 13 | 64 | F | CRT | 10 | no | 1 | pre |
| 14 | 30 | M | CRT | 8 | no | 0 | pre |
| 15 | 58 | M | CRT | 5 | no | 2 | pre |
| 16 | 63 | F | DVT | 1 | yes | 1 | pre |
| 17 | 64 | M | DVT | 3 | no | 3 | pre |
| 18 | 76 | M | CRT | 8 | no | 3 | post |
| 19 | 61 | M | CRT | 6 | no | 1 | post |
| 20 | 73 | F | PE | 40 | no | 1 | post |
| 21 | 59 | M | CRT | 36 | yes | 1 | post |
| 22 | 40 | F | DVT+PE | 10 | no | 0 | post |
| 23 | 82 | F | CRT | 28 | no | 2 | pre |
| 24 | 41 | M | CRT | 34 | no | 0 | post |
| 25 | 55 | M | CRT | 12 | no | 1 | pre |
| 26 | 64 | M | MI | 24 | no | 2 | post |
| 27 | 75 | M | CRT | 31 | yes | 1 | post |
| 28 | 72 | M | MI | 13 | no | 1 | post |
| 29 | 69 | F | CRT | 2 | no | 1 | pre |
| 30 | 73 | M | CRT | 6 | yes | 1 | pre |
| 31 | 48 | F | hepatic veins thrombosis | 1 | no | 0 | pre |
| 32 | 72 | M | CRT | 3 | no | 1 | pre |
| 33 | 55 | M | CRT | 10 | no | 3 | pre |
| 34 | 68 | M | MI | 24 | no | 2 | pre |
| 35 | 67 | F | CRT | 10 | yes | 3 | pre |
| 36 | 77 | M | MI | 25 | no | 4 | post |
| Risk Factor | β-Coefficient | 95% Coefficient Interval for β-Coefficient | p-Value | p-Value (Bootstrapped) |
|---|---|---|---|---|
| Platelet count > 50 × 109/L at baseline | 3.238 | 1.361–7.704 | 0.008 | 0.004 |
| History of venous thromboembolism | 5.447 | 1.521–19.514 | 0.009 | 0.004 |
| Presence of one or more comorbidities | 3.974 | 1.150–13.734 | 0.029 | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paterno, G.; Palmieri, R.; Forte, V.; Del Prete, V.; Gurnari, C.; Guarnera, L.; Mallegni, F.; Pascale, M.R.; Buzzatti, E.; Mezzanotte, V.; et al. Predictors of Early Thrombotic Events in Adult Patients with Acute Myeloid Leukemia: A Real-World Experience. Cancers 2022, 14, 5640. https://doi.org/10.3390/cancers14225640
Paterno G, Palmieri R, Forte V, Del Prete V, Gurnari C, Guarnera L, Mallegni F, Pascale MR, Buzzatti E, Mezzanotte V, et al. Predictors of Early Thrombotic Events in Adult Patients with Acute Myeloid Leukemia: A Real-World Experience. Cancers. 2022; 14(22):5640. https://doi.org/10.3390/cancers14225640
Chicago/Turabian StylePaterno, Giovangiacinto, Raffaele Palmieri, Vittorio Forte, Valentina Del Prete, Carmelo Gurnari, Luca Guarnera, Flavia Mallegni, Maria Rosaria Pascale, Elisa Buzzatti, Valeria Mezzanotte, and et al. 2022. "Predictors of Early Thrombotic Events in Adult Patients with Acute Myeloid Leukemia: A Real-World Experience" Cancers 14, no. 22: 5640. https://doi.org/10.3390/cancers14225640
APA StylePaterno, G., Palmieri, R., Forte, V., Del Prete, V., Gurnari, C., Guarnera, L., Mallegni, F., Pascale, M. R., Buzzatti, E., Mezzanotte, V., Cerroni, I., Savi, A., Buccisano, F., Maurillo, L., Venditti, A., & Del Principe, M. I. (2022). Predictors of Early Thrombotic Events in Adult Patients with Acute Myeloid Leukemia: A Real-World Experience. Cancers, 14(22), 5640. https://doi.org/10.3390/cancers14225640











