Pan-Cancer Landscape of NEIL3 in Tumor Microenvironment: A Promising Predictor for Chemotherapy and Immunotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples
2.2. NEIL3 mRNA Expression Profiles across Cancers
2.3. Prognostic Analysis
2.4. Cell Culture
2.5. Western Blotting
2.6. Reverse Transcription and Quantitative PCR (RT-qPCR)
2.7. Immunohistochemistry
2.8. NEIL3 Mutational Feature Analysis
2.9. Pan-Cancer Grade and Stage Analysis
2.10. Tumor Immune Infiltration Analysis
2.11. Immune-Related Biomarker and Immune Subtype Analysis
2.12. Tumor Neoantigen, Mutational Burden (TMB), and Microsatellite Instability (MSI) Analysis
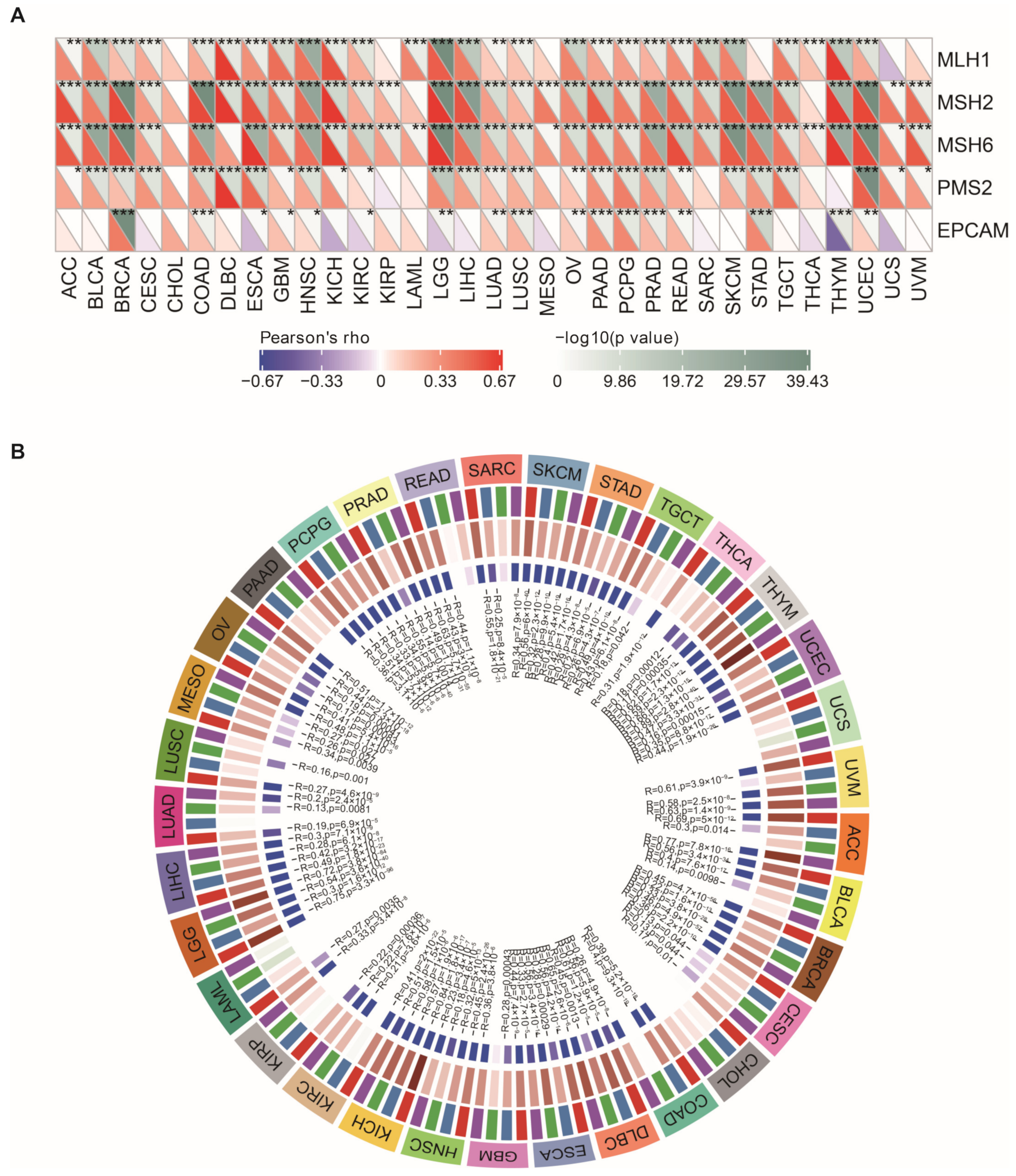
2.13. Mismatch Repair (MMR) Gene Mutation, DNA Methyltransferase, EMT Pathway Activity, and Gene Set Enrichment Analysis (GSEA)
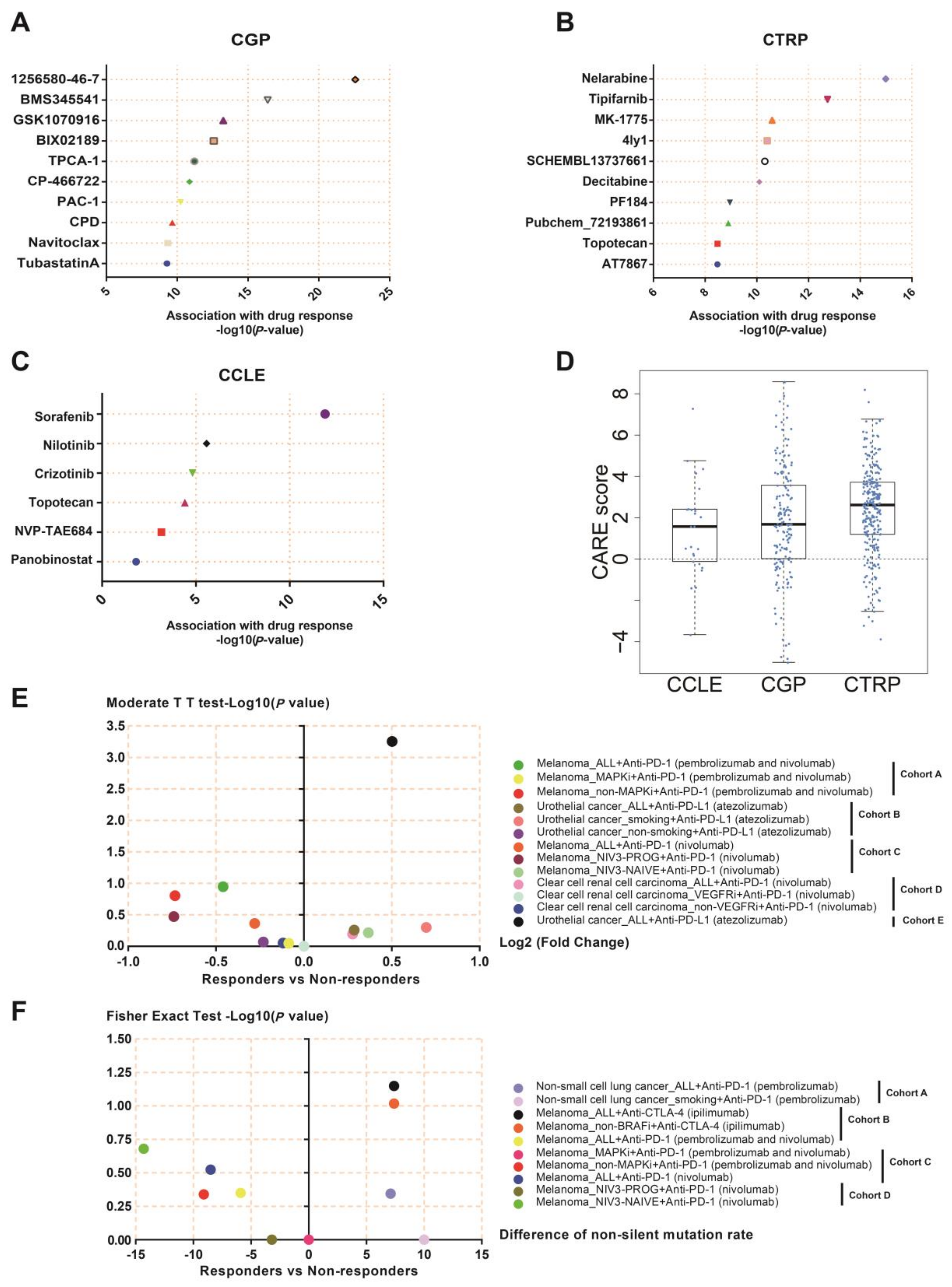
2.14. Chemotherapy and Immunotherapy Response Analysis
2.15. Statistical Analysis
3. Results
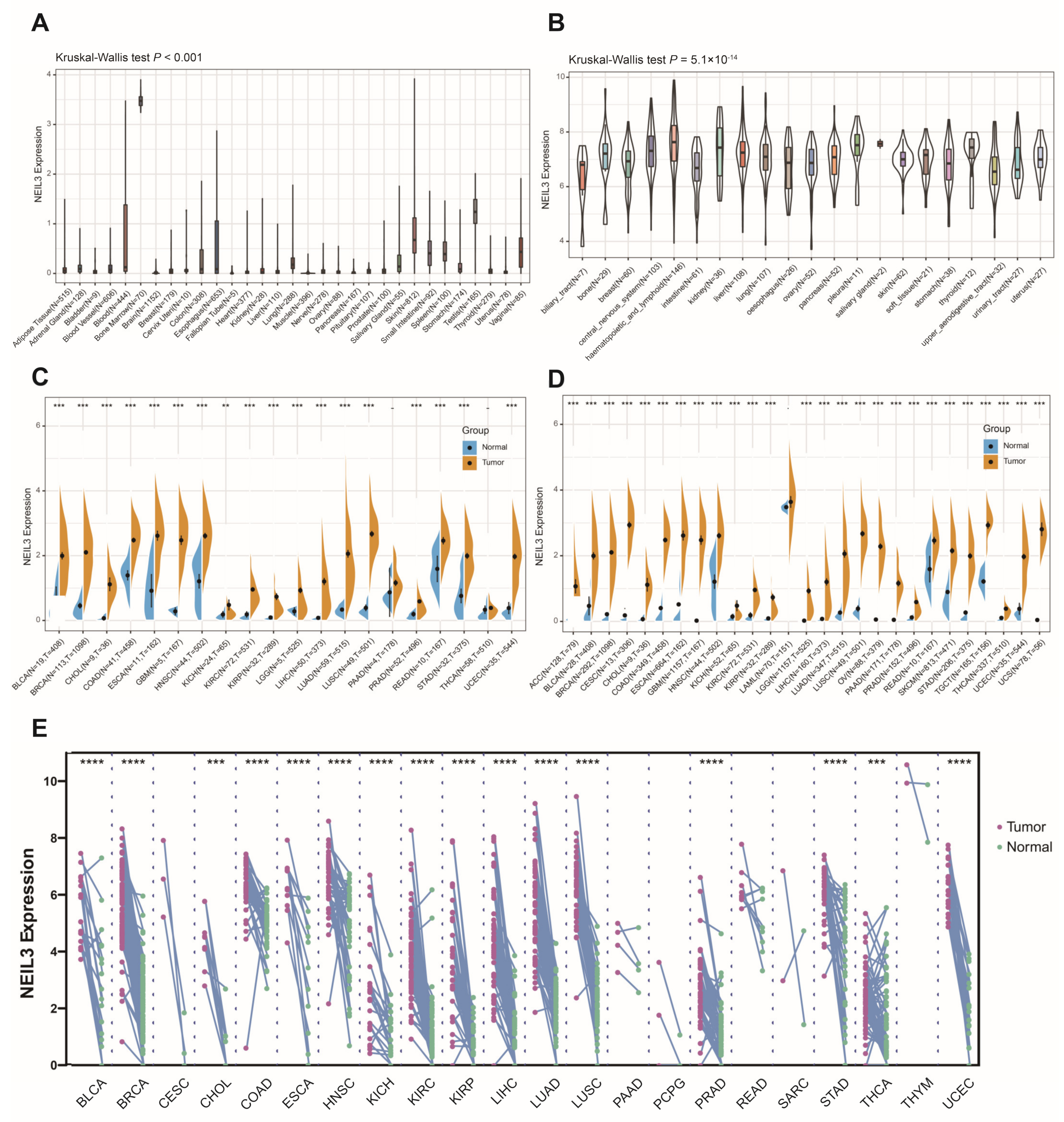
3.1. NEIL3 Expression Is Comparably Increased in Tumor Cells or Tissues across Cancers
3.2. NEIL3 Expression Possesses Prognostic Value in Cancers
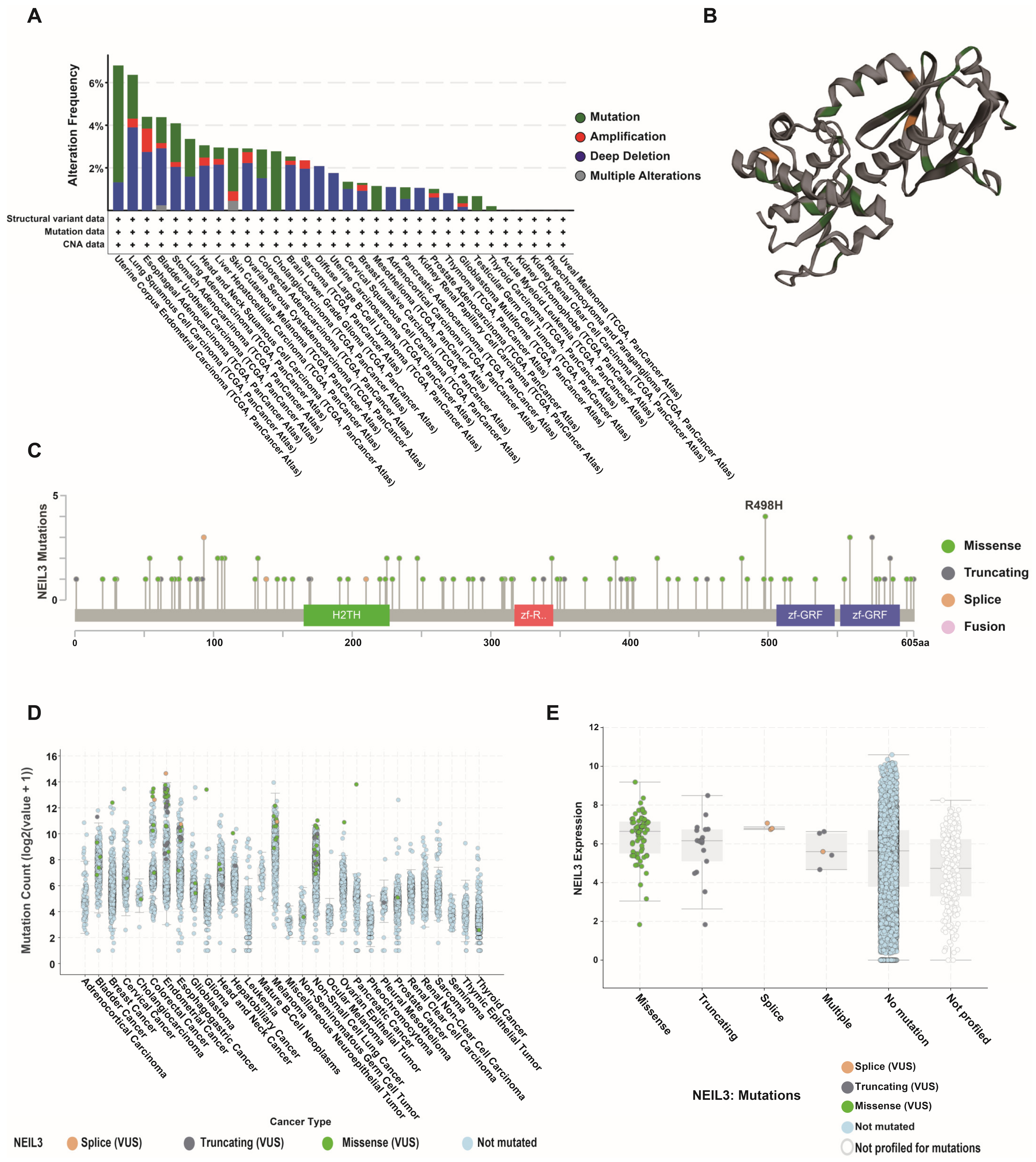
3.3. NEIL3 Presented a High Mutation Frequency among Cancers
3.4. Altered NEIL3 May Have an Impact on Tumors
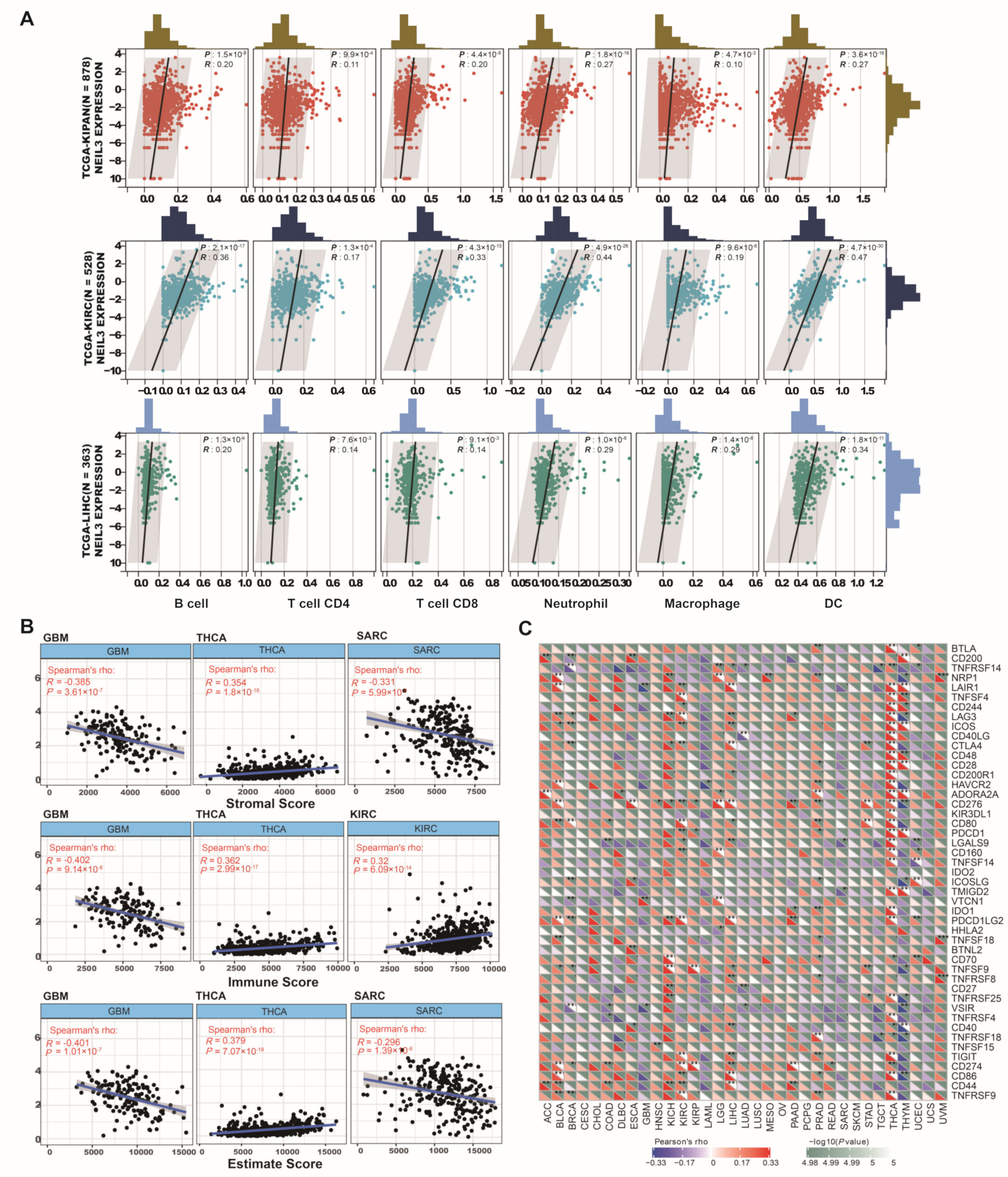
3.5. NEIL3 Contributes to Immune Infiltration among Cancers
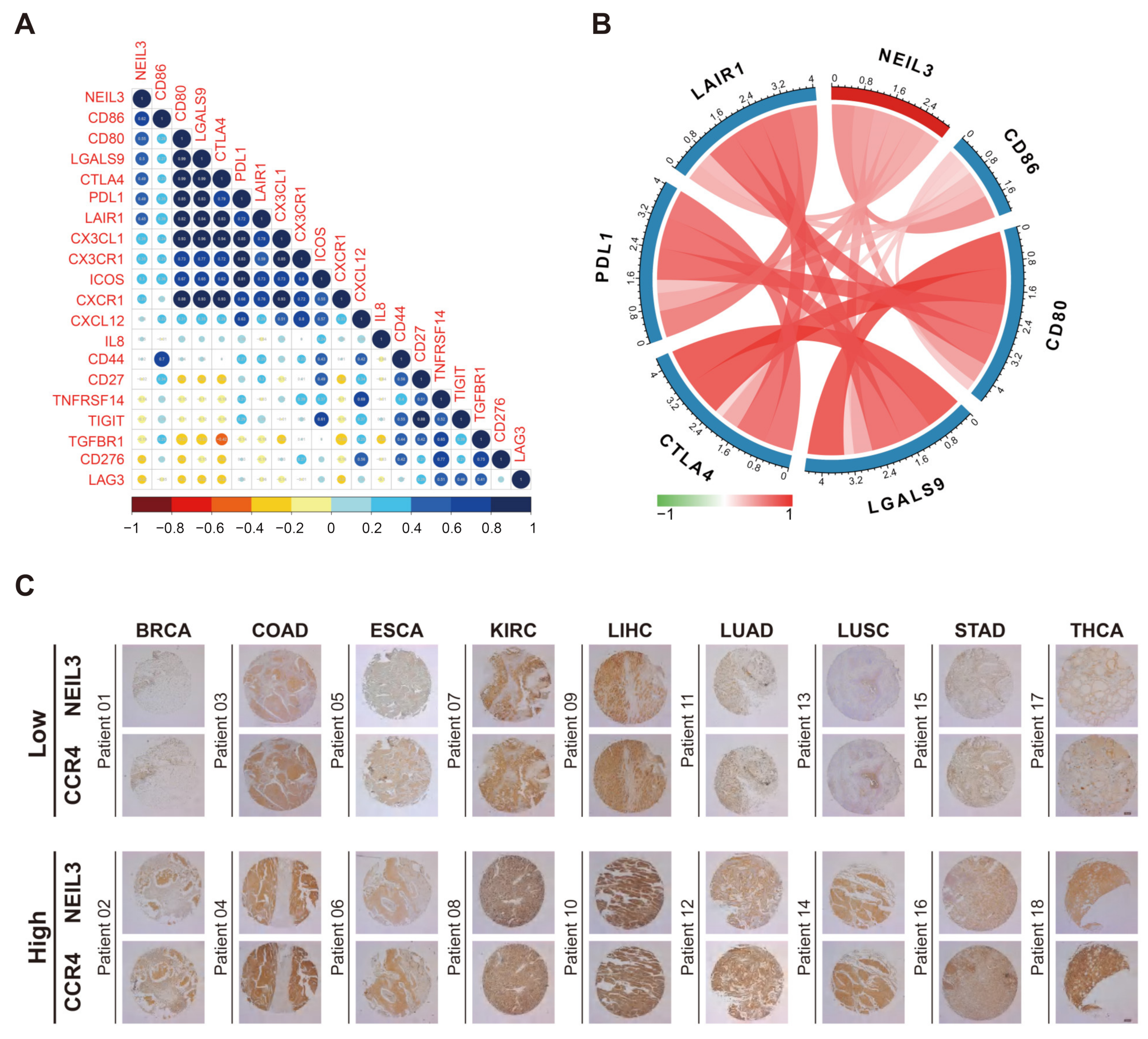
3.6. NEIL3 Has a Close Relationship with Multiple Immune Biomarkers of Cancers
3.7. NEIL3 Exhibited Diverse Expression Patterns among Different Immune Subtypes across Cancers
3.8. NEIL3 Is Involved with Neoantigen, TMB, and MSI in Various Cancers
3.9. NEIL3 Expression Is Correlated with MMR, DNA Methyltransferase Genes, and EMT Pathway Activity across Cancers
3.10. GSEA of Different NEIL3 Expression Levels
3.11. NEIL3 May Be a Novel Indicator for Chemotherapy and Immunotherapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Aldea, M.; Andre, F.; Marabelle, A.; Dogan, S.; Barlesi, F.; Soria, J.-C. Overcoming Resistance to Tumor-Targeted and Immune-Targeted Therapies. Cancer Discov. 2021, 11, 874–899. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer Immunoediting and Resistance to T Cell-Based Immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Nathansen, J.; Meyer, F.; Müller, L.; Schmitz, M.; Borgmann, K.; Dubrovska, A. Beyond the Double-Strand Breaks: The Role of DNA Repair Proteins in Cancer Stem-Cell Regulation. Cancers 2021, 13, 4818. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Jia, K.; Wang, L.; Li, W.; Chen, B.; Liu, Y.; Wang, H.; Zhao, S.; He, Y.; Zhou, C. Alterations of DNA Damage Response Pathway: Biomarker and Therapeutic Strategy for Cancer Immunotherapy. Acta Pharm. Sin. B 2021, 11, 2983–2994. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Onodera, A.; González-Avalos, E.; Lio, C.-W.J.; Georges, R.O.; Bellacosa, A.; Nakayama, T.; Rao, A. Roles of TET and TDG in DNA Demethylation in Proliferating and Non-Proliferating Immune Cells. Genome Biol. 2021, 22, 186. [Google Scholar] [CrossRef]
- Visnes, T.; Cázares-Körner, A.; Hao, W.; Wallner, O.; Masuyer, G.; Loseva, O.; Mortusewicz, O.; Wiita, E.; Sarno, A.; Manoilov, A.; et al. Small Molecule Inhibitor of OGG1 Suppresses Proinflammatory Gene Expression and Inflammation. Science 2018, 362, 834–839. [Google Scholar] [CrossRef]
- RodrÍguez, E.; Schetters, S.T.T.; van Kooyk, Y. The Tumour Glyco-Code as a Novel Immune Checkpoint for Immunotherapy. Nat. Rev. Immunol. 2018, 18, 204–211. [Google Scholar] [CrossRef]
- Liu, M.; Imamura, K.; Averill, A.M.; Wallace, S.S.; Doublié, S. Structural Characterization of a Mouse Ortholog of Human NEIL3 with a Marked Preference for Single-Stranded DNA. Structure 2013, 21, 247–256. [Google Scholar] [CrossRef]
- Zhao, Z.; Gad, H.; Benitez-Buelga, C.; Sanjiv, K.; Xiangwei, H.; Kang, H.; Feng, M.; Zhao, Z.; Berglund, U.W.; Xia, Q.; et al. NEIL3 Prevents Senescence in Hepatocellular Carcinoma by Repairing Oxidative Lesions at Telomeres during Mitosis. Cancer Res. 2021, 81, 4079–4093. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, L.; Shi, S.; Wu, S.; Meng, R.; Chen, H.; Jiang, Z. Deficiency of NEIL3 Enhances the Chemotherapy Resistance of Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 4098. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.M.; Ngoi, N.Y.L.; Peng, G.; Tan, D.S.P.; Yap, T.A. Development of Poly(ADP-Ribose) Polymerase Inhibitor and Immunotherapy Combinations: Progress, Pitfalls, and Promises. Trends Cancer 2021, 7, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A View on Drug Resistance in Cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Kraehenbuehl, L.; Weng, C.-H.; Eghbali, S.; Wolchok, J.D.; Merghoub, T. Enhancing Immunotherapy in Cancer by Targeting Emerging Immunomodulatory Pathways. Nat. Rev. Clin. Oncol. 2022, 19, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Alteber, Z.; Kotturi, M.F.; Whelan, S.; Ganguly, S.; Weyl, E.; Pardoll, D.M.; Hunter, J.; Ophir, E. Therapeutic Targeting of Checkpoint Receptors within the DNAM1 Axis. Cancer Discov. 2021, 11, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Hildrestrand, G.A.; Neurauter, C.G.; Diep, D.B.; Castellanos, C.G.; Krauss, S.; Bjørås, M.; Luna, L. Expression Patterns of Neil3 during Embryonic Brain Development and Neoplasia. BMC Neurosci. 2009, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.-F.; Huang, Y.-L.; Wang, J.-L.; Deng, M.-H.; Xia, T.-L.; Zeng, M.-S.; Chen, M.-S.; Wang, H.-B.; Huang, Y.-H. Anillin Is Required for Tumor Growth and Regulated by MiR-15a/MiR-16-1 in HBV-Related Hepatocellular Carcinoma. Aging 2018, 10, 1884–1901. [Google Scholar] [CrossRef] [PubMed]
- ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. Pan-Cancer Analysis of Whole Genomes. Nature 2020, 578, 82–93. [CrossRef]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416.e11. [Google Scholar] [CrossRef]
- Lian, Y.-F.; Huang, Y.-L.; Zhang, Y.-J.; Chen, D.-M.; Wang, J.-L.; Wei, H.; Bi, Y.-H.; Jiang, Z.-W.; Li, P.; Chen, M.-S.; et al. CACYBP Enhances Cytoplasmic Retention of P27Kip1 to Promote Hepatocellular Carcinoma Progression in the Absence of RNF41 Mediated Degradation. Theranostics 2019, 9, 8392–8408. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.-Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis. Oncol. 2017, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.-H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [PubMed]
- Azimi, F.; Scolyer, R.A.; Rumcheva, P.; Moncrieff, M.; Murali, R.; McCarthy, S.W.; Saw, R.P.; Thompson, J.F. Tumor-Infiltrating Lymphocyte Grade Is an Independent Predictor of Sentinel Lymph Node Status and Survival in Patients with Cutaneous Melanoma. J. Clin. Oncol. 2012, 30, 2678–2683. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Humeau, J.; Buqué, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with Chemotherapy in the Era of Immune Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Algarra, I.; Garrido, F.; Garcia-Lora, A.M. MHC Heterogeneity and Response of Metastases to Immunotherapy. Cancer Metastasis Rev. 2021, 40, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Neefjes, J.; Jongsma, M.L.M.; Paul, P.; Bakke, O. Towards a Systems Understanding of MHC Class I and MHC Class II Antigen Presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the Cancer Microenvironment and Their Relevance in Cancer Immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef]
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in Cancer: From Biology to Therapy. Nat. Rev. Cancer 2021, 21, 481–499. [Google Scholar] [CrossRef]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.-J. CXCL9, CXCL10, CXCL11/CXCR3 Axis for Immune Activation—A Target for Novel Cancer Therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef]
- Guldner, I.H.; Wang, Q.; Yang, L.; Golomb, S.M.; Zhao, Z.; Lopez, J.A.; Brunory, A.; Howe, E.N.; Zhang, Y.; Palakurthi, B.; et al. CNS-Native Myeloid Cells Drive Immune Suppression in the Brain Metastatic Niche through Cxcl10. Cell 2020, 183, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Tardáguila, M.; Mira, E.; García-Cabezas, M.A.; Feijoo, A.M.; Quintela-Fandino, M.; Azcoitia, I.; Lira, S.A.; Mañes, S. CX3CL1 Promotes Breast Cancer via Transactivation of the EGF Pathway. Cancer Res. 2013, 73, 4461–4473. [Google Scholar] [CrossRef] [PubMed]
- Sciumè, G.; Soriani, A.; Piccoli, M.; Frati, L.; Santoni, A.; Bernardini, G. CX3CR1/CX3CL1 Axis Negatively Controls Glioma Cell Invasion and Is Modulated by Transforming Growth Factor-Β1. Neuro Oncol. 2010, 12, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Tosolini, M.; Kirilovsky, A.; Mlecnik, B.; Fredriksen, T.; Mauger, S.; Bindea, G.; Berger, A.; Bruneval, P.; Fridman, W.-H.; Pagès, F.; et al. Clinical Impact of Different Classes of Infiltrating T Cytotoxic and Helper Cells (Th1, Th2, Treg, Th17) in Patients with Colorectal Cancer. Cancer Res. 2011, 71, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Dulos, J.; Carven, G.J.; van Boxtel, S.J.; Evers, S.; Driessen-Engels, L.J.A.; Hobo, W.; Gorecka, M.A.; de Haan, A.F.J.; Mulders, P.; Punt, C.J.A.; et al. PD-1 Blockade Augments Th1 and Th17 and Suppresses Th2 Responses in Peripheral Blood from Patients with Prostate and Advanced Melanoma Cancer. J. Immunother. 2012, 35, 169–178. [Google Scholar] [CrossRef]
- Kießler, M.; Plesca, I.; Sommer, U.; Wehner, R.; Wilczkowski, F.; Müller, L.; Tunger, A.; Lai, X.; Rentsch, A.; Peuker, K.; et al. Tumor-Infiltrating Plasmacytoid Dendritic Cells Are Associated with Survival in Human Colon Cancer. J. Immunother. Cancer 2021, 9, e001813. [Google Scholar] [CrossRef]
- Rui, X.; Shao, S.; Wang, L.; Leng, J. Identification of Recurrence Marker Associated with Immune Infiltration in Prostate Cancer with Radical Resection and Build Prognostic Nomogram. BMC Cancer 2019, 19, 1179. [Google Scholar] [CrossRef]
- Yoshie, O.; Matsushima, K. CCR4 and Its Ligands: From Bench to Bedside. Int. Immunol. 2015, 27, 11–20. [Google Scholar] [CrossRef]
- De Mattos-Arruda, L.; Vazquez, M.; Finotello, F.; Lepore, R.; Porta, E.; Hundal, J.; Amengual-Rigo, P.; Ng, C.K.Y.; Valencia, A.; Carrillo, J.; et al. Neoantigen Prediction and Computational Perspectives towards Clinical Benefit: Recommendations from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 978–990. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An Immunogenic Personal Neoantigen Vaccine for Patients with Melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen Vaccine Generates Intratumoral T Cell Responses in Phase Ib Glioblastoma Trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Carreno, B.M.; Magrini, V.; Becker-Hapak, M.; Kaabinejadian, S.; Hundal, J.; Petti, A.A.; Ly, A.; Lie, W.-R.; Hildebrand, W.H.; Mardis, E.R.; et al. Cancer Immunotherapy. A Dendritic Cell Vaccine Increases the Breadth and Diversity of Melanoma Neoantigen-Specific T Cells. Science 2015, 348, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Baretti, M.; Le, D.T. DNA Mismatch Repair in Cancer. Pharmacol. Ther. 2018, 189, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Dudley, J.C.; Lin, M.-T.; Le, D.T.; Eshleman, J.R. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin. Cancer Res. 2016, 22, 813–820. [Google Scholar] [CrossRef]
- Koch, A.; Joosten, S.C.; Feng, Z.; de Ruijter, T.C.; Draht, M.X.; Melotte, V.; Smits, K.M.; Veeck, J.; Herman, J.G.; Van Neste, L.; et al. Analysis of DNA Methylation in Cancer: Location Revisited. Nat. Rev. Clin. Oncol. 2018, 15, 459–466. [Google Scholar] [CrossRef]
- Qiao, M.; Jiang, T.; Liu, X.; Mao, S.; Zhou, F.; Li, X.; Zhao, C.; Chen, X.; Su, C.; Ren, S.; et al. Immune Checkpoint Inhibitors in EGFR-Mutated NSCLC: Dusk or Dawn? J. Thorac. Oncol. 2021, 16, 1267–1288. [Google Scholar] [CrossRef]
- Hilmi, M.; Vienot, A.; Rousseau, B.; Neuzillet, C. Immune Therapy for Liver Cancers. Cancers 2019, 12, 77. [Google Scholar] [CrossRef]
- Tran, O.T.; Tadesse, S.; Chu, C.; Kidane, D. Overexpression of NEIL3 Associated with Altered Genome and Poor Survival in Selected Types of Human Cancer. Tumor Biol. 2020, 42, 1010428320918404. [Google Scholar] [CrossRef]
- Li, N.; Wang, J.; Wallace, S.S.; Chen, J.; Zhou, J.; D’Andrea, A.D. Cooperation of the NEIL3 and Fanconi Anemia/BRCA Pathways in Interstrand Crosslink Repair. Nucleic Acids Res. 2020, 48, 3014–3028. [Google Scholar] [CrossRef]
- Fleming, A.M.; Zhu, J.; Howpay Manage, S.A.; Burrows, C.J. Human NEIL3 Gene Expression Regulated by Epigenetic-Like Oxidative DNA Modification. J. Am. Chem. Soc. 2019, 141, 11036–11049. [Google Scholar] [CrossRef]
- Zhou, J.; Chan, J.; Lambelé, M.; Yusufzai, T.; Stumpff, J.; Opresko, P.L.; Thali, M.; Wallace, S.S. NEIL3 Repairs Telomere Damage during S Phase to Secure Chromosome Segregation at Mitosis. Cell Rep. 2017, 20, 2044–2056. [Google Scholar] [CrossRef] [PubMed]
- King, K.E.; Losier, T.T.; Russell, R.C. Regulation of Autophagy Enzymes by Nutrient Signaling. Trends Biochem. Sci. 2021, 46, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Jellusova, J. The Role of Metabolic Checkpoint Regulators in B Cell Survival and Transformation. Immunol. Rev. 2020, 295, 39–53. [Google Scholar] [CrossRef]
- Olson, M.E.; Harris, R.S.; Harki, D.A. APOBEC Enzymes as Targets for Virus and Cancer Therapy. Cell Chem. Biol. 2018, 25, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tao, Y.; Xu, X.; Cai, F.; Yu, Y.; Ma, L. Bufalin Inhibits Cell Proliferation and Migration of Hepatocellular Carcinoma Cells via APOBEC3F Induced Intestinal Immune Network for IgA Production Signaling Pathway. Biochem. Biophys. Res. Commun. 2018, 503, 2124–2131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lu, M.; Qin, Y.; Gao, W.; Tao, L.; Su, W.; Zhong, J. Neoantigen: A New Breakthrough in Tumor Immunotherapy. Front. Immunol. 2021, 12, 672356. [Google Scholar] [CrossRef]
- Joshi, S.S.; Badgwell, B.D. Current Treatment and Recent Progress in Gastric Cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef]
- Lai, H.; Hung, L.; Yen, C.; Hung, H.; Chen, R.; Ku, Y.; Lo, H.; Tsai, H.; Lee, Y.; Yang, T.; et al. NEIL3 promotes hepatoma epithelial-mesenchymal transition by activating the BRAF/MEK/ERK/TWIST signaling pathway. J. Pathol. 2022, 258, 339–352. [Google Scholar] [CrossRef]
- Gajewski, T.F. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Semin. Oncol. 2015, 42, 663–671. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The Future of Immune Checkpoint Therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Hegde, P.S.; Karanikas, V.; Evers, S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin. Cancer Res. 2016, 22, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Chen, Y.; Ding, D.; Dai, H.; Liu, G.; Li, C. Immunosuppressive Effect of Renal Cell Carcinoma on Phenotype and Function of Dendritic Cells. Int. Urol. Nephrol. 2014, 46, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Suthen, S.; Lim, C.J.; Nguyen, P.H.D.; Dutertre, C.-A.; Lai, H.L.H.; Wasser, M.; Chua, C.; Lim, T.K.H.; Leow, W.Q.; Loh, T.J.; et al. Hypoxia-Driven Immunosuppression by Treg and Type-2 Conventional Dendritic Cells in HCC. Hepatology 2022, 76, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.B.; Hildrestrand, G.A.; Scheffler, K.; Vinge, L.E.; Alfsnes, K.; Palibrk, V.; Wang, J.; Neurauter, C.G.; Luna, L.; Johansen, J.; et al. NEIL3-Dependent Regulation of Cardiac Fibroblast Proliferation Prevents Myocardial Rupture. Cell Rep. 2017, 18, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Kumai, T.; Ohkuri, T.; Kosaka, A.; Nagato, T.; Hirata, Y.; Ohara, K.; Oikawa, K.; Aoki, N.; Akiyama, N.; et al. Epigenetic Modification Augments the Immunogenicity of Human Leukocyte Antigen G Serving as a Tumor Antigen for T Cell-Based Immunotherapy. OncoImmunology 2016, 5, e1169356. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.-Y.; Zhang, S. Safety and Efficacy of Personalized Cancer Vaccines in Combination with Immune Checkpoint Inhibitors in Cancer Treatment. Front. Oncol. 2021, 11, 663264. [Google Scholar] [CrossRef] [PubMed]
- Economopoulou, P.; Kotsantis, I.; Bamias, A. A Drug Safety Evaluation of Atezolizumab in Locally Advanced or Metastatic Urothelial Carcinoma. Expert Opin. Drug Saf. 2020, 19, 955–960. [Google Scholar] [CrossRef]
- Pham, L.M.; Poudel, K.; Ou, W.; Phung, C.D.; Nguyen, H.T.; Nguyen, B.L.; Karmacharya, P.; Pandit, M.; Chang, J.-H.; Jeong, J.-H.; et al. Combination Chemotherapeutic and Immune-Therapeutic Anticancer Approach via Anti-PD-L1 Antibody Conjugated Albumin Nanoparticles. Int. J. Pharm. 2021, 605, 120816. [Google Scholar] [CrossRef]
- Pusztai, L.; Yau, C.; Wolf, D.M.; Han, H.S.; Du, L.; Wallace, A.M.; String-Reasor, E.; Boughey, J.C.; Chien, A.J.; Elias, A.D.; et al. Durvalumab with Olaparib and Paclitaxel for High-Risk HER2-Negative Stage II/III Breast Cancer: Results from the Adaptively Randomized I-SPY2 Trial. Cancer Cell 2021, 39, 989–998.e5. [Google Scholar] [CrossRef]




| Position | AA1 | AA2 | Result | Score | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| 76 | V | L | Possibly Damaging | 0.604 | 0.87 | 0.91 |
| 96 | R | W | Possibly Damaging | 0.918 | 0.81 | 0.94 |
| 103 | M | I | Benign | 0.000 | 1.00 | 0.00 |
| 106 | P | L | Benign | 0.102 | 0.93 | 0.85 |
| 132 | D | H | Probably Damaging | 0.998 | 0.27 | 0.99 |
| 225 | I | M | Possibly Damaging | 0.950 | 0.79 | 0.95 |
| 234 | R | M | Probably Damaging | 0.986 | 0.74 | 0.96 |
| 247 | K | T | Probably Damaging | 1.000 | 0.00 | 1.00 |
| 344 | R | K | Benign | 0.145 | 0.92 | 0.86 |
| 390 | L | V | Probably Damaging | 0.999 | 0.14 | 0.99 |
| 481 | N | D | Benign | 0.097 | 0.93 | 0.85 |
| 498 | R | H | Benign | 0.000 | 1.00 | 0.00 |
| 559 | R | C | Probably Damaging | 1.000 | 0.00 | 1.00 |
| 559 | R | H | Probably Damaging | 1.000 | 0.00 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, W.; Huang, S.; Li, L.; Wang, J.; Li, J.; Chen, Y.; Chen, L.; Lian, Y.; Huang, Y. Pan-Cancer Landscape of NEIL3 in Tumor Microenvironment: A Promising Predictor for Chemotherapy and Immunotherapy. Cancers 2023, 15, 109. https://doi.org/10.3390/cancers15010109
Liao W, Huang S, Li L, Wang J, Li J, Chen Y, Chen L, Lian Y, Huang Y. Pan-Cancer Landscape of NEIL3 in Tumor Microenvironment: A Promising Predictor for Chemotherapy and Immunotherapy. Cancers. 2023; 15(1):109. https://doi.org/10.3390/cancers15010109
Chicago/Turabian StyleLiao, Weixin, Shaozhuo Huang, Lin Li, Jialiang Wang, Jing Li, Yongjian Chen, Lubiao Chen, Yifan Lian, and Yuehua Huang. 2023. "Pan-Cancer Landscape of NEIL3 in Tumor Microenvironment: A Promising Predictor for Chemotherapy and Immunotherapy" Cancers 15, no. 1: 109. https://doi.org/10.3390/cancers15010109
APA StyleLiao, W., Huang, S., Li, L., Wang, J., Li, J., Chen, Y., Chen, L., Lian, Y., & Huang, Y. (2023). Pan-Cancer Landscape of NEIL3 in Tumor Microenvironment: A Promising Predictor for Chemotherapy and Immunotherapy. Cancers, 15(1), 109. https://doi.org/10.3390/cancers15010109






