Interpatient Heterogeneity in Drug Response and Protein Biomarker Expression of Recurrent Ovarian Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tumor Samples
2.2. Ovarian Cancer Spheroid Model and Treatment
2.3. Immunohistochemistry
2.4. Statistics
3. Results
3.1. Patient Characteristics and Clinical Treatment
3.2. Tumor Sample Characteristics
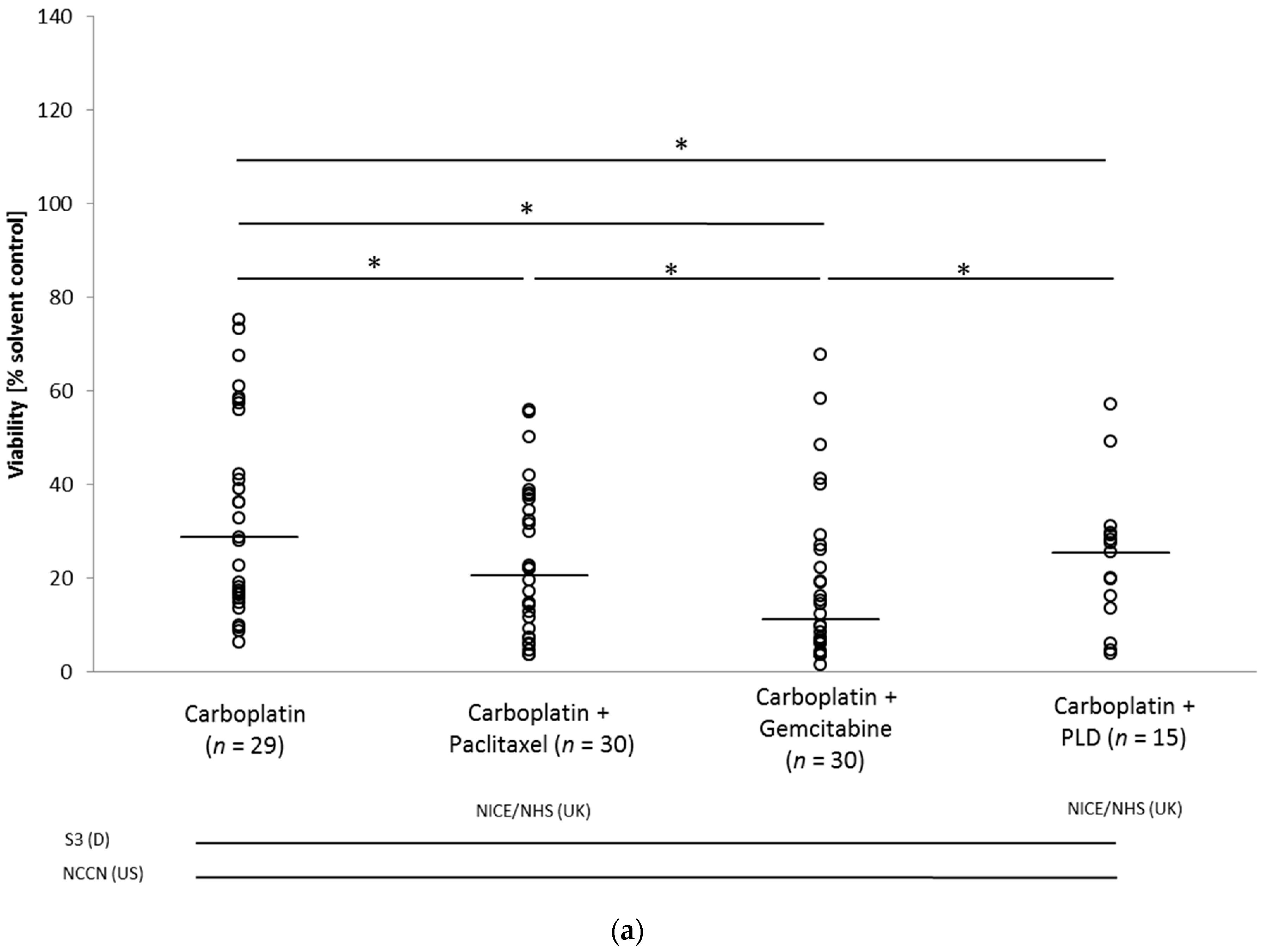
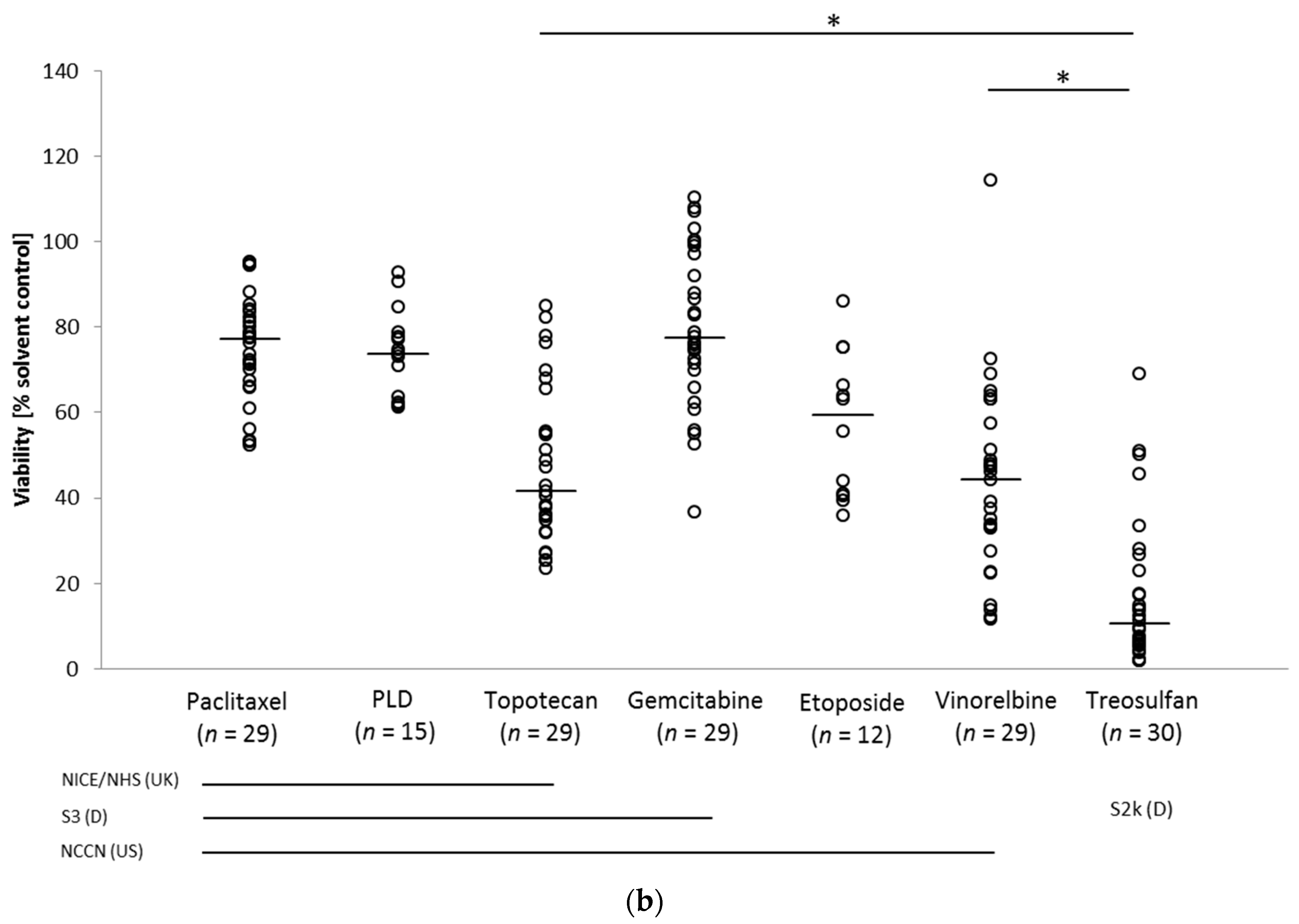
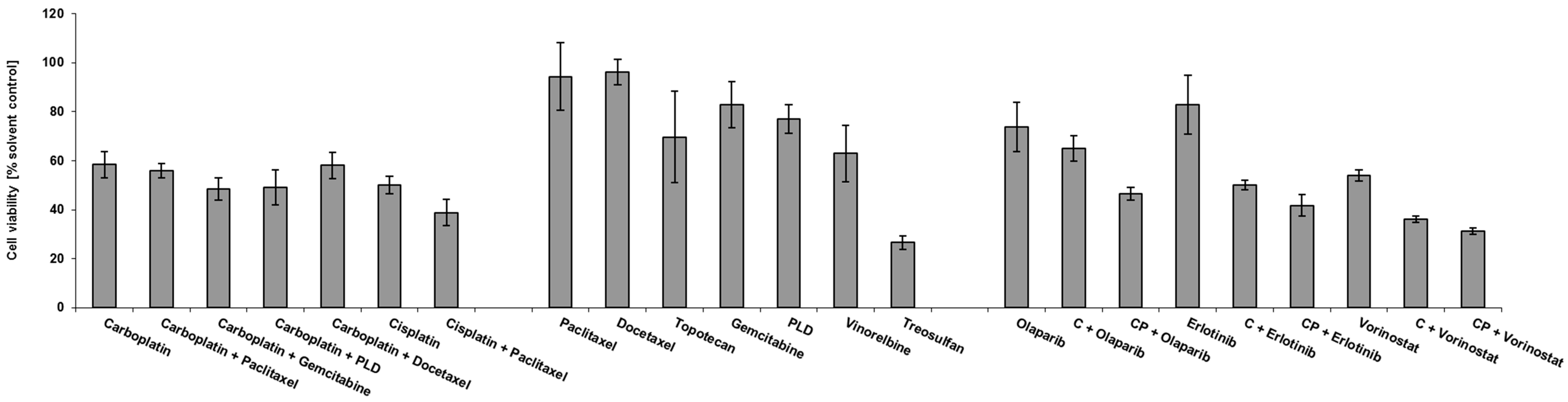
3.3. Drug Response in the Ovarian Cancer Spheroid Model
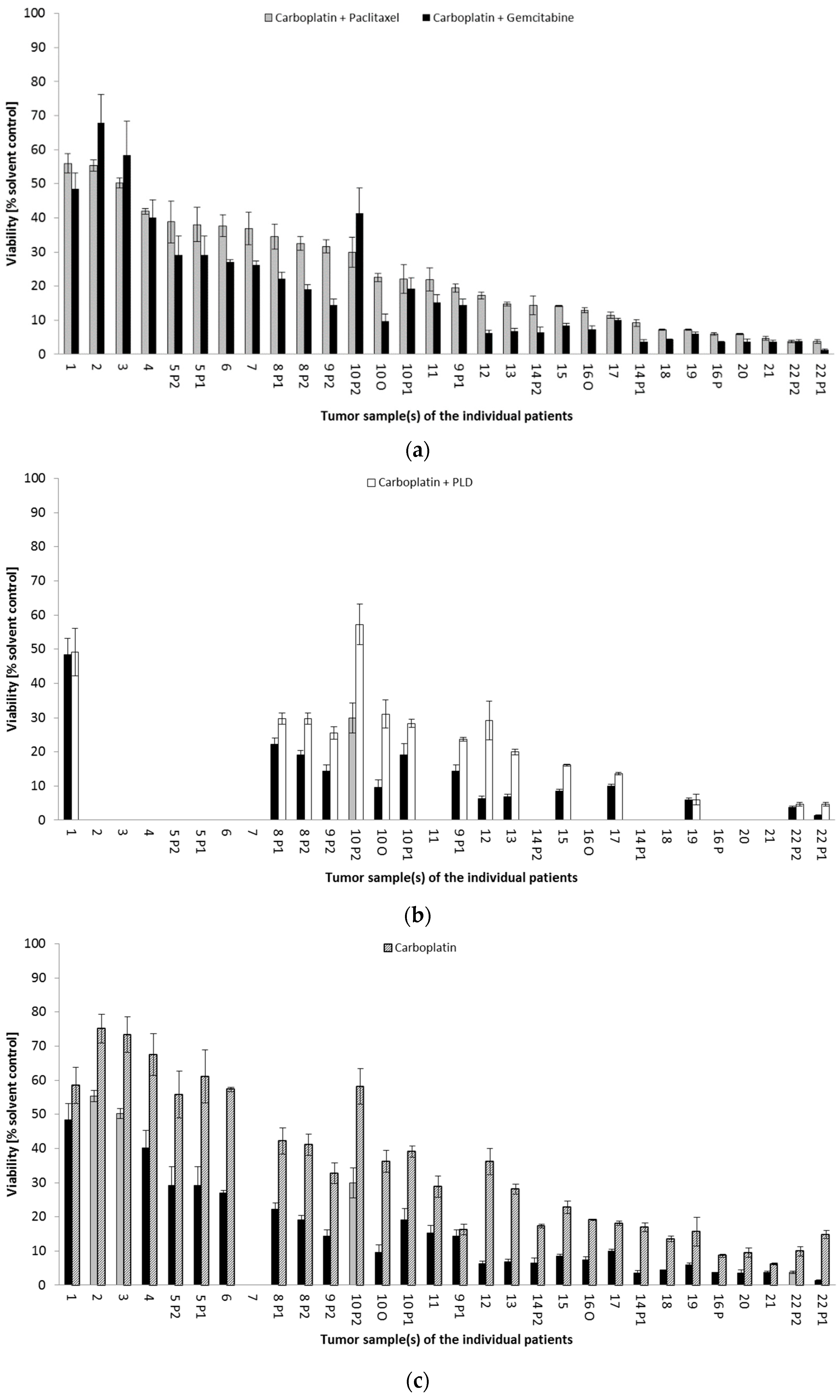
Drug Response Pattern in Autologous Metastatic Lesions
3.4. Biomarker Profile of Recurrent Ovarian Cancer
Biomarker Expression in Autologous Metastatic Lesions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanker, L.C.; Loibl, S.; Burchardi, N.; Pfisterer, J.; Meier, W.; Pujade-Lauraine, E.; Ray-Coquard, I.; Sehouli, J.; Harter, P.; du Bois, A.; et al. The Impact of Second to Sixth Line Therapy on Survival of Relapsed Ovarian Cancer after Primary Taxane/Platinum-Based Therapy. Ann. Oncol. 2012, 23, 2605–2612. [Google Scholar] [CrossRef]
- Corrado, G.; Salutari, V.; Palluzzi, E.; Distefano, M.G.; Scambia, G.; Ferrandina, G. Optimizing Treatment in Recurrent Epithelial Ovarian Cancer. Expert Rev. Anticancer Ther. 2017, 17, 1147–1158. [Google Scholar] [CrossRef]
- Hoogstad-van Evert, J.S.; Bekkers, R.; Ottevanger, N.; Jansen, J.H.; Massuger, L.; Dolstra, H. Harnessing Natural Killer Cells for the Treatment of Ovarian Cancer. Gynecol. Oncol. 2020, 157, 810–816. [Google Scholar] [CrossRef]
- Francis, J.-A.; Coakley, N.; Elit, L.; Mackay, H. Systemic Therapy for Recurrent Epithelial Ovarian Cancer: A Clinical Practice Guideline. Curr. Oncol. 2017, 24, 540–546. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network® (NCCN). NCCN Clinical Practice Guidelines in OncologyTM. Ovarian Cancer; Version 2.2020; NCCN: Plymouth Meeting, PA, USA; Available online: www.nccn.org (accessed on 15 March 2021).
- Krebsgesellschaft, D.; Krebshilfe, D. AWMF S3-Leitlinie Diagnostik, Therapie Und Nachsorge Maligner Ovarialtumoren; Leitlinienprogramm Onkologie: Berlin, Germany, 2020. [Google Scholar]
- Pignata, S.; Cecere, S.C.; Du Bois, A.; Harter, P.; Heitz, F. Treatment of Recurrent Ovarian Cancer. Ann. Oncol. 2017, 28, viii51–viii56. [Google Scholar] [CrossRef]
- Testa, U.; Petrucci, E.; Pasquini, L.; Castelli, G.; Pelosi, E. Ovarian Cancers: Genetic Abnormalities, Tumor Heterogeneity and Progression, Clonal Evolution and Cancer Stem Cells. Medicines 2018, 5, 16. [Google Scholar] [CrossRef]
- Li, C.; Bonazzoli, E.; Bellone, S.; Choi, J.; Dong, W.; Menderes, G.; Altwerger, G.; Han, C.; Manzano, A.; Bianchi, A.; et al. Mutational Landscape of Primary, Metastatic, and Recurrent Ovarian Cancer Reveals c-MYC Gains as Potential Target for BET Inhibitors. Proc. Natl. Acad. Sci. USA 2019, 116, 619–624. [Google Scholar] [CrossRef]
- Alexandrova, E.; Pecoraro, G.; Sellitto, A.; Melone, V.; Ferravante, C.; Rocco, T.; Guacci, A.; Giurato, G.; Nassa, G.; Rizzo, F.; et al. An Overview of Candidate Therapeutic Target Genes in Ovarian Cancer. Cancers 2020, 12, 1470. [Google Scholar] [CrossRef]
- Singh, T.; Neal, A.S.; Moatamed, N.A.; Memarzadeh, S. Exploring the Potential of Drug Response Assays for Precision Medicine in Ovarian Cancer. Int. J. Mol. Sci. 2020, 22, 305. [Google Scholar] [CrossRef]
- Hoffmann, O.I.; Ilmberger, C.; Magosch, S.; Joka, M.; Jauch, K.-W.; Mayer, B. Impact of the Spheroid Model Complexity on Drug Response. J. Biotechnol. 2015, 205, 14–23. [Google Scholar] [CrossRef]
- Mayer, B.; Karakhanova, S.; Bauer, N.; Liu, L.; Zhu, Y.; Philippov, P.P.; Werner, J.; Bazhin, A.V. A Marginal Anticancer Effect of Regorafenib on Pancreatic Carcinoma Cells In Vitro, Ex Vivo, and In Vivo. Naunyn-Schmiedeberg’s Arch. Pharm. 2017, 390, 1125–1134. [Google Scholar] [CrossRef]
- Halfter, K.; Hoffmann, O.; Ditsch, N.; Ahne, M.; Arnold, F.; Paepke, S.; Grab, D.; Bauerfeind, I.; Mayer, B. Testing Chemotherapy Efficacy in HER2 Negative Breast Cancer Using Patient-Derived Spheroids. J. Transl. Med. 2016, 14, 112. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network® (NCCN). NCCN Clinical Practice Guidelines in OncologyTM. Ovarian Cancer; Version 2.2012; NCCN: Plymouth Meeting, PA, USA; Available online: www.nccn.org (accessed on 18 March 2013).
- Kreienberg, R.; du Bois, A.; Pfisterer, J.; Schindelmann, S.; Schmalfeldt, B. Interdisziplinäre S2k-Leitlinie Für Die Diagnostik Und Therapie Maligner Ovarialtumoren. In Management des Ovarialkarzinoms: Interdisziplinäres Vorgehen; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- NICE. Ovarian Cancer: Recognition and Initial Management; NICE: London, UK, 2011; ISBN 978-1-4731-2766-1. [Google Scholar]
- Harris, J.R.; Burton, P.; Knoppers, B.M.; Lindpaintner, K.; Bledsoe, M.; Brookes, A.J.; Budin-Ljøsne, I.; Chisholm, R.; Cox, D.; Deschênes, M.; et al. Toward a Roadmap in Global Biobanking for Health. Eur. J. Hum. Genet. 2012, 20, 1105–1111. [Google Scholar] [CrossRef]
- Hirte, H. Profile of Erlotinib and Its Potential in the Treatment of Advanced Ovarian Carcinoma. OTT 2013, 6, 427–435. [Google Scholar] [CrossRef]
- Grassadonia, A.; Cioffi, P.; Simiele, F.; Iezzi, L.; Zilli, M.; Natoli, C. Role of Hydroxamate-Based Histone Deacetylase Inhibitors (Hb-HDACIs) in the Treatment of Solid Malignancies. Cancers 2013, 5, 919–942. [Google Scholar] [CrossRef]
- Mayer, B.; Funke, I.; Johnson, J. High Expression of a Lewis(x)-Related Epitope in Gastric Carcinomas Indicates Metastatic Potential and Poor Prognosis. Gastroenterology 1996, 111, 1433–1446. [Google Scholar] [CrossRef]
- Dötzer, K.; Schlüter, F.; Koch, F.E.V.; Brambs, C.E.; Anthuber, S.; Frangini, S.; Czogalla, B.; Burges, A.; Werner, J.; Mahner, S.; et al. Integrin α2β1 Represents a Prognostic and Predictive Biomarker in Primary Ovarian Cancer. Biomedicines 2021, 9, 289. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.S.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. JCO 2013, 31, 3997–4013. [Google Scholar] [CrossRef]
- Rüschoff, J.; Dietel, M.; Baretton, G.; Arbogast, S.; Walch, A.; Monges, G.; Chenard, M.-P.; Penault-Llorca, F.; Nagelmeier, I.; Schlake, W.; et al. HER2 Diagnostics in Gastric Cancer—Guideline Validation and Development of Standardized Immunohistochemical Testing. Virchows Arch. 2010, 457, 299–307. [Google Scholar] [CrossRef]
- Hammond, M.E.H.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. JCO 2010, 28, 2784–2795. [Google Scholar] [CrossRef]
- Weiss, L.; Huemer, F.; Mlineritsch, B.; Greil, R. Immune Checkpoint Blockade in Ovarian Cancer. Memo 2016, 9, 82–84. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Gonzalez Bosquet, J.; Newtson, A.M.; Chung, R.K.; Thiel, K.W.; Ginader, T.; Goodheart, M.J.; Leslie, K.K.; Smith, B.J. Prediction of Chemo-Response in Serous Ovarian Cancer. Mol. Cancer 2016, 15, 66. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Wagner, U.; Aavall-Lundqvist, E.; Gebski, V.; Heywood, M.; Vasey, P.A.; Volgger, B.; Vergote, I.; Pignata, S.; Ferrero, A.; et al. Pegylated Liposomal Doxorubicin and Carboplatin Compared With Paclitaxel and Carboplatin for Patients With Platinum-Sensitive Ovarian Cancer in Late Relapse. JCO 2010, 28, 3323–3329. [Google Scholar] [CrossRef]
- Eng, K.H.; Hanlon, B.M.; Bradley, W.H.; Szender, J.B. Prognostic Factors Modifying the Treatment-Free Interval in Recurrent Ovarian Cancer. Gynecol. Oncol. 2015, 139, 228–235. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Integrated Genomic Analyses of Ovarian Carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Piccirillo, M.C.; Scambia, G.; Bologna, A.; Signoriello, S.; Vergote, I.; Baumann, K.; Lorusso, D.; Murgia, V.; Sorio, R.; Ferrandina, G.; et al. Quality-of-Life Analysis of the MITO-8, MaNGO, BGOG-Ov1, AGO-Ovar2.16, ENGOT-Ov1, GCIG Study Comparing Platinum-Based versus Non-Platinum-Based Chemotherapy in Patients with Partially Platinum-Sensitive Recurrent Ovarian Cancer. Ann. Oncol. 2018, 29, 1189–1194. [Google Scholar] [CrossRef]
- Sehouli, J.; Tomè, O.; Dimitrova, D.; Camara, O.; Runnebaum, I.B.; Tessen, H.W.; Rautenberg, B.; Chekerov, R.; Muallem, M.Z.; Lux, M.P.; et al. A Phase III, Open Label, Randomized Multicenter Controlled Trial of Oral versus Intravenous Treosulfan in Heavily Pretreated Recurrent Ovarian Cancer: A Study of the North-Eastern German Society of Gynecological Oncology (NOGGO). J. Cancer Res. Clin. Oncol. 2017, 143, 541–550. [Google Scholar] [CrossRef]
- Mahner, S.; Oskay-Özcelik, G.; Heidrich-Lorsbach, E.; Fuxius, S.; Sommer, H.; Klare, P.; Belau, A.; Ruhmland, B.; Heuser, T.; Kölbl, H.; et al. A Prospective Multicenter Study of Treosulfan in Elderly Patients with Recurrent Ovarian Cancer: Results of a Planned Safety Analysis. J. Cancer Res. Clin. Oncol. 2012, 138, 1413–1419. [Google Scholar] [CrossRef]
- On behalf of the Ovarian Cancer Study Group of the North-Eastern German Society of Gynaecological Oncology (NOGGO); Chekerov, R.; Harter, P.; Fuxius, S.; Hanker, L.C.; Woelber, L.; Müller, L.; Klare, P.; Abenhardt, W.; Nedkova, Y.; et al. Preference of Elderly Patients’ to Oral or Intravenous Chemotherapy in Heavily Pre-Treated Recurrent Ovarian Cancer: Final Results of a Prospective Multicenter Trial. Gynecol. Oncol. Res. Pract. 2017, 4, 6. [Google Scholar] [CrossRef]
- Garziera, M.; Roncato, R.; Montico, M.; De Mattia, E.; Gagno, S.; Poletto, E.; Scalone, S.; Canzonieri, V.; Giorda, G.; Sorio, R.; et al. New Challenges in Tumor Mutation Heterogeneity in Advanced Ovarian Cancer by a Targeted Next-Generation Sequencing (NGS) Approach. Cells 2019, 8, 584. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.-M.; Coquan, E.; Weiswald, L.-B.; Lambert, B.; Vaur, D.; Poulain, L. Identifying Patients Eligible for PARP Inhibitor Treatment: From NGS-Based Tests to 3D Functional Assays. Br. J. Cancer 2021, 125, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Dizon, D.; Vathipadiekal, V.; Birrer, M.J. Ovarian Cancer: Genomic Analysis. Ann. Oncol. 2013, 24, x7–x15. [Google Scholar] [CrossRef] [PubMed]
- Zolotovskaia, M.A.; Sorokin, M.I.; Petrov, I.V.; Poddubskaya, E.V.; Moiseev, A.A.; Sekacheva, M.I.; Borisov, N.M.; Tkachev, V.S.; Garazha, A.V.; Kaprin, A.D.; et al. Disparity between Inter-Patient Molecular Heterogeneity and Repertoires of Target Drugs Used for Different Types of Cancer in Clinical Oncology. Int. J. Mol. Sci. 2020, 21, 1580. [Google Scholar] [CrossRef] [PubMed]
- Symeonides, S.; Gourley, C. Ovarian Cancer Molecular Stratification and Tumor Heterogeneity: A Necessity and a Challenge. Front. Oncol. 2015, 5, 229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arienti, C.; Tesei, A.; Verdecchia, G.; Framarini, M.; Virzì, S.; Grassi, A.; Scarpi, E.; Turci, L.; Silvestrini, R.; Amadori, D.; et al. Peritoneal Carcinomatosis from Ovarian Cancer: Chemosensitivity Test and Tissue Markers as Predictors of Response to Chemotherapy. J. Transl. Med. 2011, 9, 94. [Google Scholar] [CrossRef]
- Langdon, S.P.; Herrington, C.S.; Hollis, R.L.; Gourley, C. Estrogen Signaling and Its Potential as a Target for Therapy in Ovarian Cancer. Cancers 2020, 12, 1647. [Google Scholar] [CrossRef]
- Tuefferd, M.; Couturier, J.; Penault-Llorca, F.; Vincent-Salomon, A.; Broët, P.; Guastalla, J.-P.; Allouache, D.; Combe, M.; Weber, B.; Pujade-Lauraine, E.; et al. HER2 Status in Ovarian Carcinomas: A Multicenter GINECO Study of 320 Patients. PLoS ONE 2007, 2, e1138. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Wang, T.; George, S.L. On Enrichment Strategies for Biomarker Stratified Clinical Trials. J. Biopharm. Stat. 2018, 28, 292–308. [Google Scholar] [CrossRef]
- Khalique, L.; Ayhan, A.; Weale, M.; Jacobs, I.; Ramus, S.; Gayther, S. Genetic Intra-Tumour Heterogeneity in Epithelial Ovarian Cancer and Its Implications for Molecular Diagnosis of Tumours. J. Pathol. 2007, 211, 286–295. [Google Scholar] [CrossRef]
- Hoogstraat, M.; de Pagter, M.S.; Cirkel, G.A.; van Roosmalen, M.J.; Harkins, T.T.; Duran, K.; Kreeftmeijer, J.; Renkens, I.; Witteveen, P.O.; Lee, C.C.; et al. Genomic and Transcriptomic Plasticity in Treatment-Naive Ovarian Cancer. Genome Res. 2014, 24, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Kossaï, M.; Leary, A.; Scoazec, J.-Y.; Genestie, C. Ovarian Cancer: A Heterogeneous Disease. Pathobiology 2018, 85, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Morden, C.R.; Farrell, A.C.; Sliwowski, M.; Lichtensztejn, Z.; Altman, A.D.; Nachtigal, M.W.; McManus, K.J. Chromosome instability is prevalent and dynamic in high-grade serous ovarian cancer patient samples. Gynecol. Oncol. 2021, 161, 769–778. [Google Scholar] [CrossRef]
- Ballabio, S.; Craparotta, I.; Paracchini, L.; Mannarino, L.; Corso, S.; Pezzotta, M.G.; Vescio, M.; Fruscio, R.; Romualdi, C.; Dainese, E.; et al. Multisite analysis of high-grade serous epithelial ovarian cancers identifies genomic regions of focal and recurrent copy number alteration in 3q26.2 and 8q24.3. Int. J. Cancer 2019, 145, 2670–2681. [Google Scholar] [CrossRef] [PubMed]
- de Witte, C.J.; Kutzera, J.; van Hoeck, A.; Nguyen, L.; Boere, I.A.; Jalving, M.; Ottevanger, P.B.; de Mheen, C.va.; Stevense, M.; Kloosterman, W.P.; et al. Distinct Genomic Profiles Are Associated with Treatment Response and Survival in Ovarian Cancer. Cancer 2022, 14, 1511. [Google Scholar] [CrossRef]
- Arend, R.C.; Scalise, C.B.; Gordon, E.R.; Davis, A.M.; Foxall, M.E.; Johnston, B.E.; Crossman, D.K.; Cooper, S.J. Metabolic Alterations and WNT Signaling Impact Immune Response in HGSOC. Clin. Cancer Res. 2022, 28, 1433–1445. [Google Scholar] [CrossRef]
- Duffy, M.J.; Harbeck, N.; Nap, M.; Molina, R.; Nicolini, A.; Senkus, E.; Cardoso, F. Clinical Use of Biomarkers in Breast Cancer: Updated Guidelines from the European Group on Tumor Markers (EGTM). Eur. J. Cancer 2017, 75, 284–298. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Braicu, I.; Berger, R.; Mahner, S.; Sehouli, J.; Pujade-Lauraine, E.; Cassier, P.A.; Moll, U.M.; Ulmer, H.; Leunen, K.; et al. Part I of GANNET53: A European Multicenter Phase I/II Trial of the Hsp90 Inhibitor Ganetespib Combined With Weekly Paclitaxel in Women With High-Grade, Platinum-Resistant Epithelial Ovarian Cancer—A Study of the GANNET53 Consortium. Front. Oncol. 2019, 9, 832. [Google Scholar] [CrossRef]
- Liu, J.F.; Gordon, M.; Veneris, J.; Braiteh, F.; Balmanoukian, A.; Eder, J.P.; Oaknin, A.; Hamilton, E.; Wang, Y.; Sarkar, I.; et al. Safety, Clinical Activity and Biomarker Assessments of Atezolizumab from a Phase I Study in Advanced/Recurrent Ovarian and Uterine Cancers. Gynecol. Oncol. 2019, 154, 314–322. [Google Scholar] [CrossRef]


| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Mean/median | 60/61 | |
| Range | 37–76 | |
| Relapse number | ||
| 1 | 15 | 68.18 |
| 2 | 3 | 13.64 |
| >2 | 4 | 18.18 |
| Histology | ||
| Serous | 19 | 86.36 |
| Other | 3 | 13.64 |
| Grading | ||
| Grade 3 | 22 | 100 |
| Macroscopic residual tumor after surgery of the current relapse | ||
| Yes | 9 | 40.91 |
| No | 13 | 59.09 |
| Number of analyzed samples per patient | ||
| 1 | 15 | 68.18 |
| 2 | 6 | 27.27 |
| 3 | 1 | 4.55 |
| Relapse Number | Patient No. | PFI (mo) | Second-Line Chemotherapy | PFS (mo) | OS (mo) | Tumor-Related Death |
| 1 | 3 | 13 | CP + B | 17 | 47 | - |
| 4 | 20 | C(AUC4)G(800 mg/m2) -> B | 30 | 33 | Yes | |
| 6 | 22 | 2 × C(AUC2)G, 4 × C(AUC1)D(35 mg/m2) | 25 | 36 | Yes | |
| 9 | 11 | CPLD (80%Cy3–6) | 7 | 57 | - | |
| 10 | 1 | 4 × CP, 2 × CP(75%) | 4 | 11 | Yes | |
| 11 | 8 | C(AUC4)G + B(75%Cy2,3 Ød8Cy4,5) | 22 | 32 | Yes | |
| 12 | 15 | C(AUC4)G + B | 17 | 51 | - | |
| 13 | 23 | 1 × CPLD, 5 × CG | 43 | 75 | - | |
| 17 | 67 | 2 × CPLD(85%), 2 × C(AUC5), 2 × C(AUC4) | 12 | 43 | - | |
| 18 | 11 | CPLD + B | 27 | 31 | Yes | |
| 21 | 13 | C(AUC4)G (Ød8Cy2,3,6) | 8 | 41 | Yes | |
| 22 | 59 | C(AUC4)G + B(red. Cy1,2,6 d8Cy5,6) | 17 | 71 | - | |
| 20 | 4 | 1 × G, 5 × T(3 mg/m2), 14 × T(4 mg/m2) | 16 | 18 | Yes | |
| 5 | 13 | None | 5 | 11 | Yes | |
| 19 | 262 | None | 61 | 61 | - | |
| Relapse Number | Patient No. | TFI (mo) | Subsequent Chemotherapy | PFS (mo) | OS (mo) | Tumor-Related Death |
| 2 | 16 | 13 | 3 × CG, 3 × C | 17 | 77 | - |
| 2 | 15 | 15 | CP + B | 9 | 29 | Yes |
| 3 | 14 | 8 | 4 × C(AUC4)G(800 mg/m2), 2 × Cis(50 mg/m2)G(800 mg/m2) | 6 | 13 | Yes |
| 3 | 8 | 59 | 2 × C, 6 × PLD | 65 | 65 | - |
| 5 | 7 | 5 | Cis(20 mg/m2)D(30 mg/m2) + B(Ød15Cy3, aborted Cy6) | 8 | 9 | Yes |
| 2 | 1 | 1 | 9 × T(1.25 mg/m2) | 54 | 54 | - |
| 4 | 2 | 1 | None | 2 | 2 | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, O.I.; Regenauer, M.; Czogalla, B.; Brambs, C.; Burges, A.; Mayer, B. Interpatient Heterogeneity in Drug Response and Protein Biomarker Expression of Recurrent Ovarian Cancer. Cancers 2022, 14, 2279. https://doi.org/10.3390/cancers14092279
Hoffmann OI, Regenauer M, Czogalla B, Brambs C, Burges A, Mayer B. Interpatient Heterogeneity in Drug Response and Protein Biomarker Expression of Recurrent Ovarian Cancer. Cancers. 2022; 14(9):2279. https://doi.org/10.3390/cancers14092279
Chicago/Turabian StyleHoffmann, Oliver Ingo, Manuel Regenauer, Bastian Czogalla, Christine Brambs, Alexander Burges, and Barbara Mayer. 2022. "Interpatient Heterogeneity in Drug Response and Protein Biomarker Expression of Recurrent Ovarian Cancer" Cancers 14, no. 9: 2279. https://doi.org/10.3390/cancers14092279
APA StyleHoffmann, O. I., Regenauer, M., Czogalla, B., Brambs, C., Burges, A., & Mayer, B. (2022). Interpatient Heterogeneity in Drug Response and Protein Biomarker Expression of Recurrent Ovarian Cancer. Cancers, 14(9), 2279. https://doi.org/10.3390/cancers14092279






