How Compatible Are Immune Checkpoint Inhibitors and Thermal Ablation for Liver Metastases?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Background
2.1. Immune Resistance and Tumor Antigens
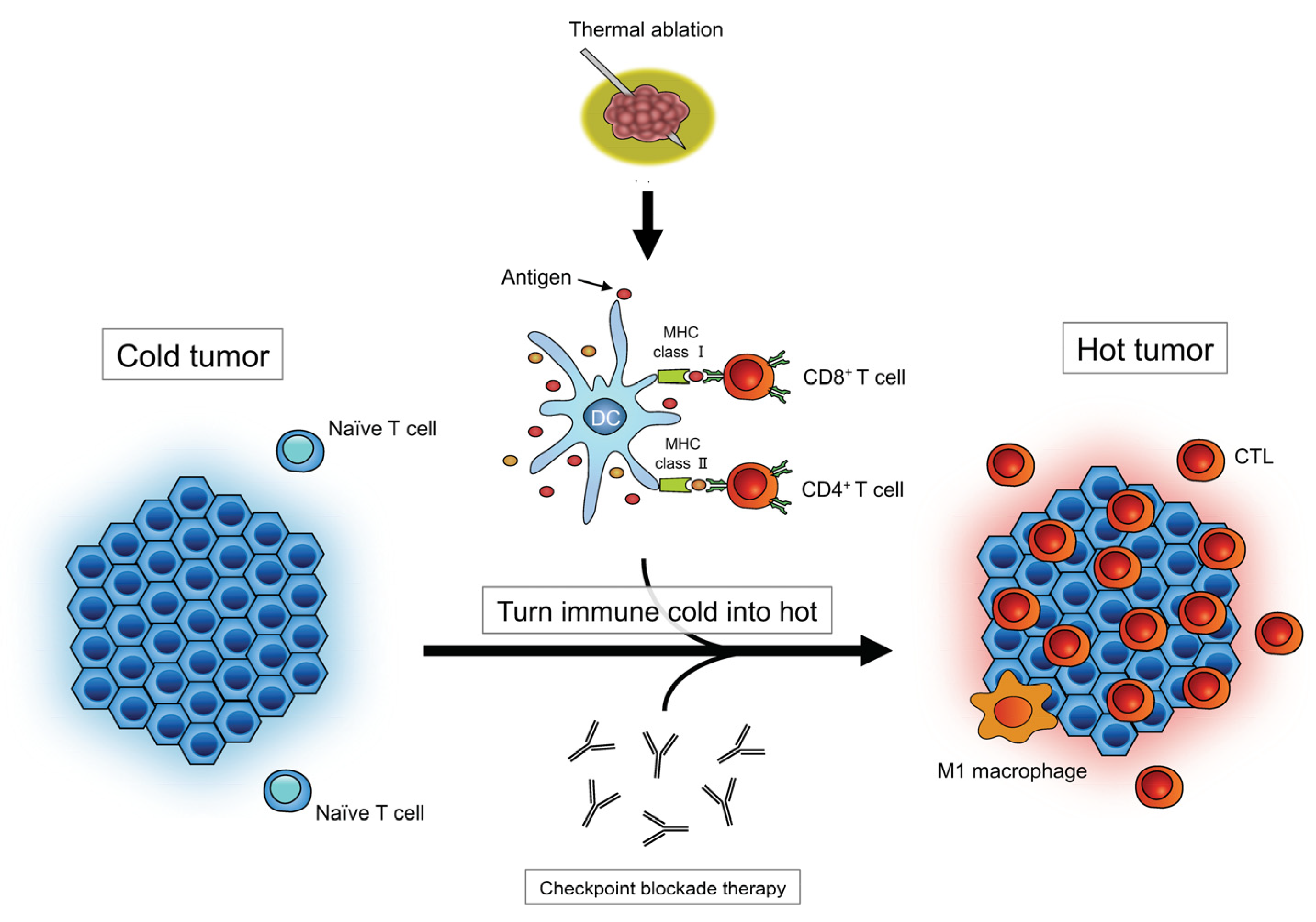
2.2. Impact of Thermally Induced Cell Death on Immune Responses
3. Outcomes
3.1. Preclinical Studies
3.1.1. Ablation-Induced Immunomodulation
3.1.2. Combination of Ablation and Immunotherapy in Mice
3.2. Clinical Studies
3.2.1. Oncological Outcomes
3.2.2. Immune-Related Adverse Events (irAEs)
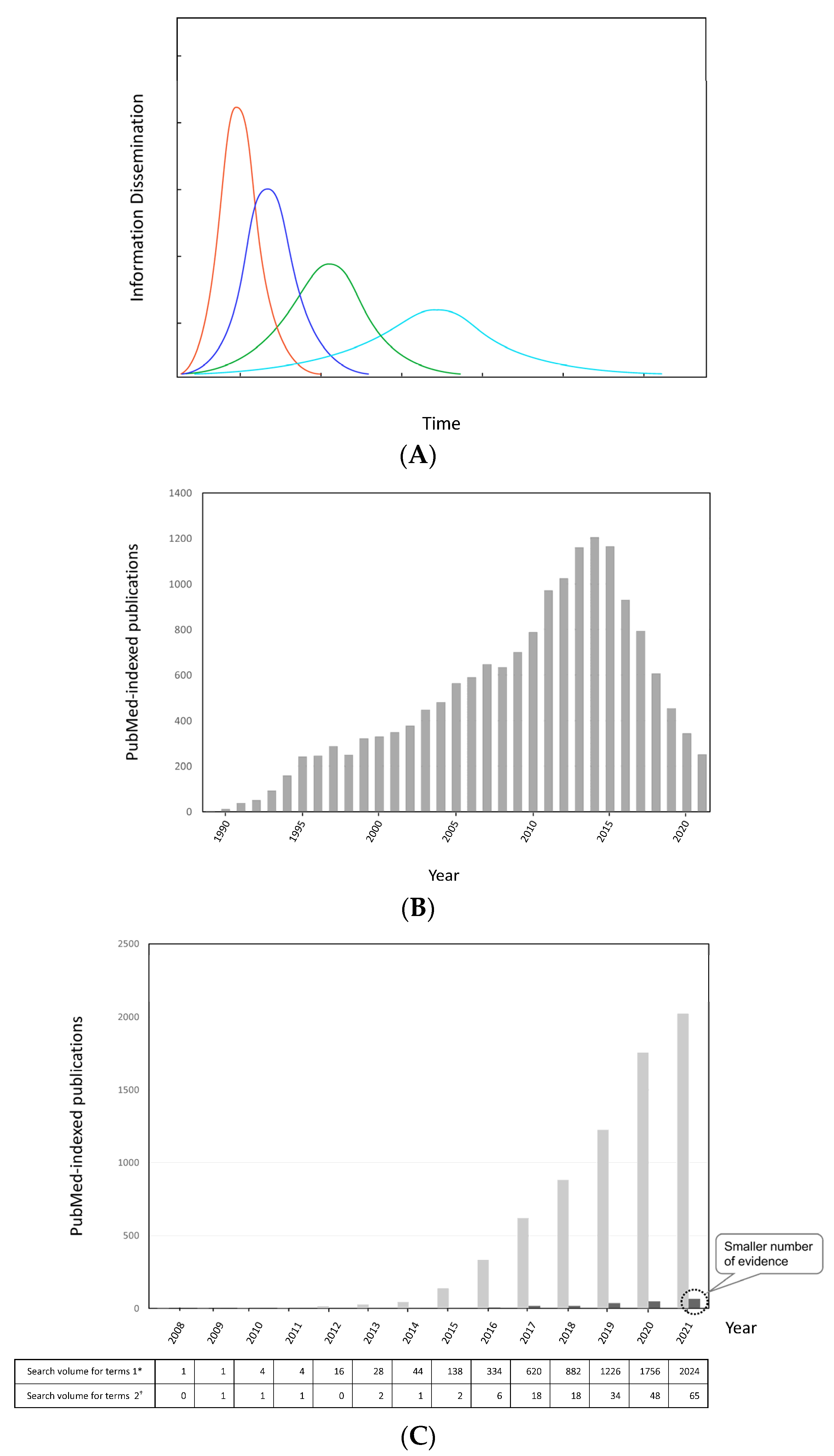
3.2.3. Information Dissemination Considering Scientific Credibility
4. Summary
Author Contributions
Funding
Conflicts of Interest
Ethics Approval
Abbreviations
| CEA | carcinoembryonic antigen |
| CTL | cytotoxic T lymphocyte |
| CTLA | cytotoxic T lymphocyte-associated antigen |
| ICIs | immune checkpoint inhibitors |
| IO | immuno-oncology |
| irAEs | immune-related adverse events |
| PD-1 | programmed death-1 |
| PD-L1 | programmed death-ligand 1 |
| TILs | tumor-infiltrating lymphocytes |
| TME | tumor microenvironment |
References
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, E.D.; Hurwitz, A.A.; Foster, B.A.; Madias, C.; Feldhaus, A.L.; Greenberg, N.M.; Burg, M.B.; Allison, J.P. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc. Natl. Acad. Sci. USA 1997, 94, 8099–8103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Kudo, M. Limited impact of anti-PD-1/PD-L1 monotherapy for hepatocellular carcinoma. Liver Cancer 2020, 9, 629–639. [Google Scholar] [CrossRef]
- Hirsch, L.; Zitvogel, L.; Eggermont, A.; Marabelle, A. PD-Loma: A cancer entity with a shared sensitivity to the PD-1/PD-L1 pathway blockade. Br. J. Cancer 2019, 120, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Dempke, W.C.M.; Fenchel, K.; Uciechowski, P.; Dale, S.P. Second- and third-generation drugs for immuno-oncology treatment-The more the better? Eur. J. Cancer 2017, 74, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Peng, H.; Wang, Y.; Xu, M.; Lin, M.; Xie, X.; Peng, B.; Kuang, M. Screening for immune-potentiating antigens from hepatocellular carcinoma patients after radiofrequency ablation by serum proteomic analysis. BMC Cancer 2018, 18, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Chen, L.; Wu, C.; Zhu, Y.; Xu, B.; Zheng, X.; Sun, M.; Wen, W.; Dai, X.; Yang, M.; et al. PD-1 blockade boosts radiofrequency ablation-elicited adaptive immune responses against tumor. Clin. Cancer Res. 2016, 22, 1173–1184. [Google Scholar] [CrossRef] [Green Version]
- Minami, Y.; Nishida, N.; Kudo, M. Radiofrequency ablation of liver metastasis: Potential impact on immune checkpoint inhibitor therapy. Eur. Radiol. 2019, 29, 5045–5051. [Google Scholar] [CrossRef]
- Takaki, H.; Cornelis, F.; Kako, Y.; Kobayashi, K.; Kamikonya, N.; Yamakado, K. Thermal ablation and immunomodulation: From preclinical experiments to clinical trials. Diagn. Interv. Imaging 2017, 98, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fares, C.M.; Van Allen, E.M.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 147–164. [Google Scholar] [CrossRef]
- Mpakali, A.; Stratikos, E. The role of antigen processing and presentation in cancer and the efficacy of immune checkpoint inhibitor immunotherapy. Cancers 2021, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S.; Long, G.V.; Scolyer, R.A.; Teng, M.W.L.; Smyth, M.J. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat. Rev. 2017, 52, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Ton, N.; Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar]
- Pitt, J.M.; Vétizou, M.; Daillère, R.; Roberti, M.P.; Yamazaki, T.; Routy, B.; Lepage, P.; Boneca, I.G.; Chamaillard, M.; Kroemer, G.; et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity 2016, 44, 1255–1269. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Brace, C.L.; Lee, F.T., Jr.; Goldberg, S.N. Principles of and advances in percutaneous ablation. Radiology 2011, 258, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Kudo, M. Radiofrequency ablation of liver metastases from colorectal cancer: A literature review. Gut Liver 2013, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Vanagas, T.; Gulbinas, A.; Pundzius, J.; Barauskas, G. Radiofrequency ablation of liver tumors (I): Biological background. Medicina 2010, 46, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Leber, B.; Mayrhauser, U.; Leopold, B.; Koestenbauer, S.; Tscheliessnigg, K.; Stadlbauer, V.; Stiegler, P. Impact of temperature on cell death in a cell-culture model of hepatocellular carcinoma. Anticancer Res. 2012, 32, 915–921. [Google Scholar]
- Ghanamah, M.; Berber, E.; Siperstein, A. Pattern of carcinoembryonic antigen drop after laparoscopic radiofrequency ablation of liver metastasis from colorectal carcinoma. Cancer 2006, 107, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Q.; Muktiali, M.; Ren, B.; Hu, Y.; Li, D.; Li, Z.; Li, D.; Xie, Y.; Tao, M.; et al. Effect of microwave ablation treatment of hepatic malignancies on serum cytokine levels. BMC Cancer 2020, 20, 812. [Google Scholar] [CrossRef]
- van den Bijgaart, R.J.E.; Schuurmans, F.; Fütterer, J.J.; Verheij, M.; Cornelissen, L.A.M.; Adema, G.J. Immune modulation plus tumor ablation: Adjuvants and antibodies to prime and boost anti-tumor immunity in situ. Front. Immunol. 2021, 12, 617365. [Google Scholar] [CrossRef] [PubMed]
- den Brok, M.H.M.G.M.; Sutmuller, R.P.M.; Nierkens, S.; Bennink, E.J.; Frielink, C.; Toonen, L.W.J.; Boerman, O.C.; Figdor, C.G.; Ruers, T.J.M.; Adema, G.J. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br. J. Cancer 2006, 95, 896–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toraya-Brown, S.; Sheen, M.R.; Zhang, P.; Chen, L.; Baird, J.R.; Demidenko, E.; Turk, M.J.; Hoopes, P.J.; Conejo-Garcia, J.R.; Steven Fiering, S. Local hyperthermia treatment of tumors induces CD8(+) T cell-mediated resistance against distal and secondary tumors. Nanomedicine 2014, 10, 1273–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.Q.; Lu, P.; Xu, Z.L.; Zhou, Q.; Zhang, J.; Wang, Z.B.; Wu, F. Alterations in Immune Response Profile of Tumor-Draining Lymph Nodes after High-Intensity Focused Ultrasound Ablation of Breast Cancer Patients. Cells 2021, 10, 3346. [Google Scholar] [CrossRef] [PubMed]
- Rangamuwa, K.; Leong, T.; Weeden, C.; Asselin-Labat, M.L.; Bozinovski, S.; Christie, M.; John, T.; Antippa, P.; Irving, L.; Steinfort, D. Thermal ablation in non-small cell lung cancer: A review of treatment modalities and the evidence for combination with immune checkpoint inhibitors. Transl. Lung Cancer Res. 2021, 10, 2842–2857. [Google Scholar] [CrossRef]
- Mizukoshi, E.; Yamashita, T.; Arai, K.; Sunagozaka, H.; Ueda, T.; Arihara, F.; Kagaya, T.; Yamashita, T.; Fushimi, K.; Kaneko, S. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology 2013, 57, 1448–1457. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, S.; Castven, D.; Galle, P.R.; Marquardt, J.U. Translational considerations to improve response and overcome therapy resistance in immunotherapy for hepatocellular carcinoma. Cancers 2020, 12, 2495. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yu, M.; Chen, L.; Kong, P.; Li, L.; Ma, G.; Ge, H.; Cui, Y.; Li, Z.; Pan, H.; et al. Enhanced antitumor efficacy through microwave ablation in combination with immune checkpoints blockade in breast cancer: A pre-clinical study in a murine model. Diagn. Interv. Imaging 2018, 99, 135–142. [Google Scholar] [CrossRef] [PubMed]
- McArthur, H.L.; Diab, A.; Page, D.B.; Yuan, J.; Solomon, S.B.; Sacchini, V.; Comstock, C.; Durack, J.C.; Maybody, M.; Sung, J.; et al. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling. Clin. Cancer Res. 2016, 22, 5729–5737. [Google Scholar] [CrossRef] [Green Version]
- Segal, N.; Kemeny, N.; Cercek, A.; Reidy, D.; Raasch, P.; Warren, P.; Hrabovsky, A.E.; Campbell, N.; Shia, J.; Goodman, K.A.; et al. Non-randomized phase II study to assess the efficacy of pembrolizumab (Pem) plus radiotherapy (RT) or ablation in mismatch repair proficient (pMMR) metastatic colorectal cancer (mCRC) patients. J. Clin. Oncol. 2016, 34, 3539. [Google Scholar] [CrossRef]
- Leppelmann, K.S.; Mooradian, M.J.; Ganguli, S.; Uppot, R.N.; Yamada, K.; Irani, Z.; Wehrenberg-Klee, E.P.; Zubiri, L.; Reynolds, K.L.; Arellano, R.S.; et al. Thermal ablation, embolization, and selective internal radiation therapy combined with checkpoint inhibitor cancer immunotherapy: Safety analysis. J. Vasc. Interv. Radiol. 2021, 32, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Centora, D. The Spread of Behavior in an Online Social Network Experiment. Science 2010, 329, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, N. Rumor spreading model considering rumor credibility, correlation and crowd classification based on personality. Sci. Rep. 2020, 10, 5887. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Tan, Z.X.; Ye, Y.; Wang, L.; Cheong, K.H.; Xie, N.G. A rumor spreading model based on information entropy. Sci. Rep. 2017, 7, 9615. [Google Scholar] [CrossRef]
- Salehinejad, S.; Jangipour Afshar, P.; Borhaninejad, V. Rumor surveillance methods in outbreaks: A systematic literature review. Health Promot. Perspect. 2021, 11, 12–19. [Google Scholar] [CrossRef]
- Künzli, B.M.; Abitabile, P.; Maurer, C.A. Radiofrequency ablation of liver tumors: Actual limitations and potential solutions in the future. World J. Hepatol. 2011, 3, 8–14. [Google Scholar] [CrossRef]
- Petre, E.N.; Sofocleous, C. Thermal ablation in the management of colorectal cancer patients with oligometastatic liver disease. Visc. Med. 2017, 33, 62–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minami, Y.; Takaki, H.; Yamakado, K.; Kudo, M. How Compatible Are Immune Checkpoint Inhibitors and Thermal Ablation for Liver Metastases? Cancers 2022, 14, 2206. https://doi.org/10.3390/cancers14092206
Minami Y, Takaki H, Yamakado K, Kudo M. How Compatible Are Immune Checkpoint Inhibitors and Thermal Ablation for Liver Metastases? Cancers. 2022; 14(9):2206. https://doi.org/10.3390/cancers14092206
Chicago/Turabian StyleMinami, Yasunori, Haruyuki Takaki, Koichiro Yamakado, and Masatoshi Kudo. 2022. "How Compatible Are Immune Checkpoint Inhibitors and Thermal Ablation for Liver Metastases?" Cancers 14, no. 9: 2206. https://doi.org/10.3390/cancers14092206
APA StyleMinami, Y., Takaki, H., Yamakado, K., & Kudo, M. (2022). How Compatible Are Immune Checkpoint Inhibitors and Thermal Ablation for Liver Metastases? Cancers, 14(9), 2206. https://doi.org/10.3390/cancers14092206







