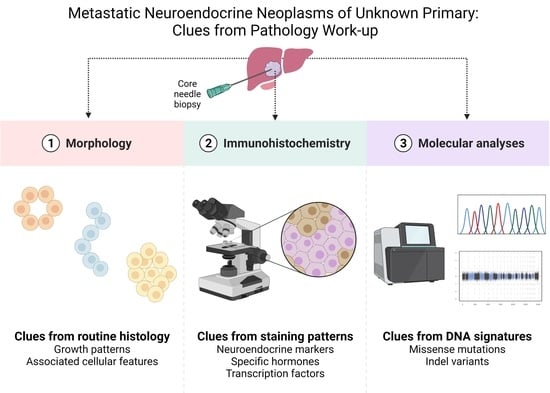
Metastatic Neuroendocrine Neoplasms of Unknown Primary: Clues from Pathology Workup
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. The Core-Needle Biopsy: Advantages to Fine-Needle Aspiration Cytology
1.2. Diagnostic Workup and Tumor Grading: Morphology Is Key
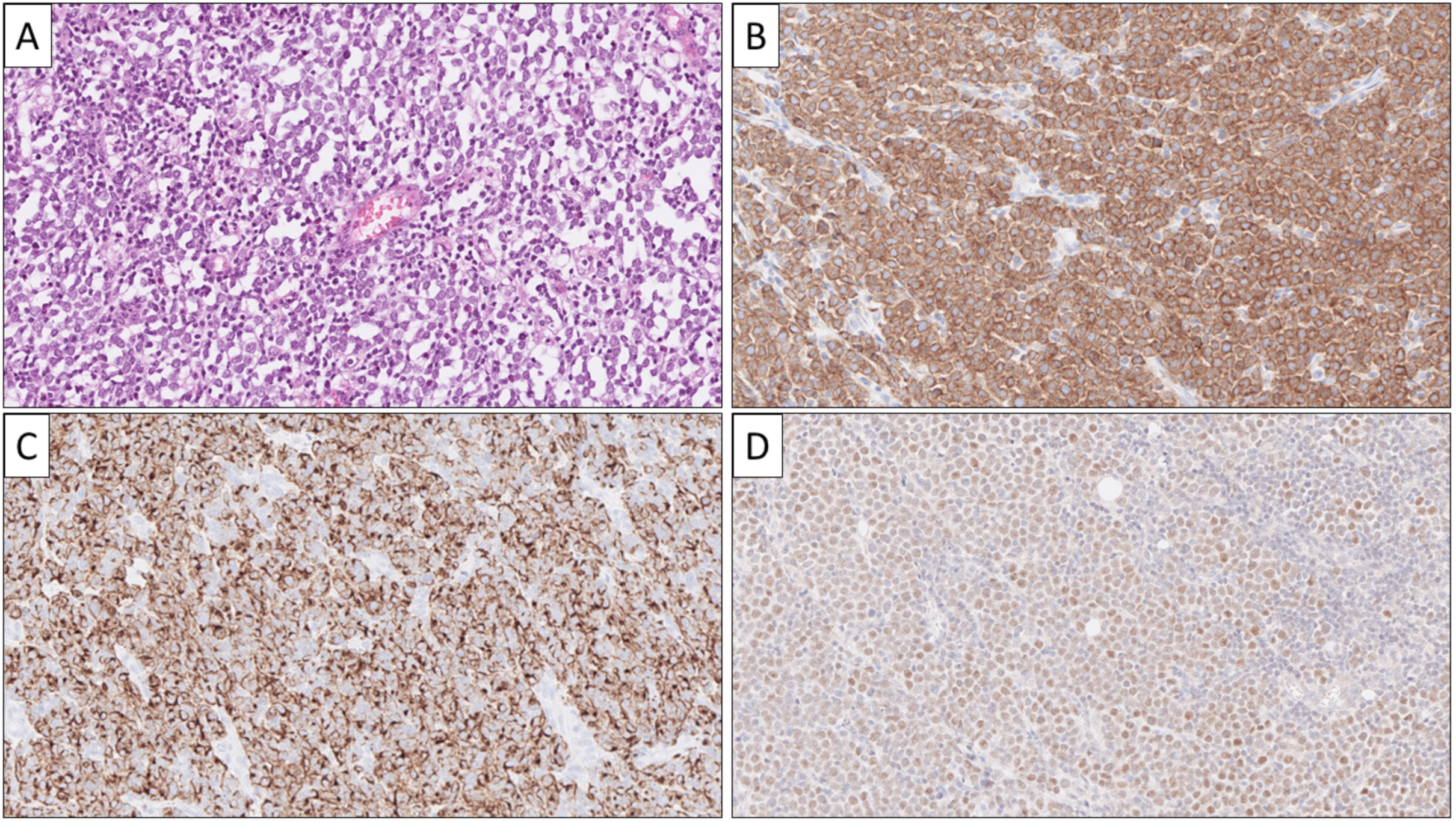
1.3. Diagnostic Workup: Immunohistochemistry
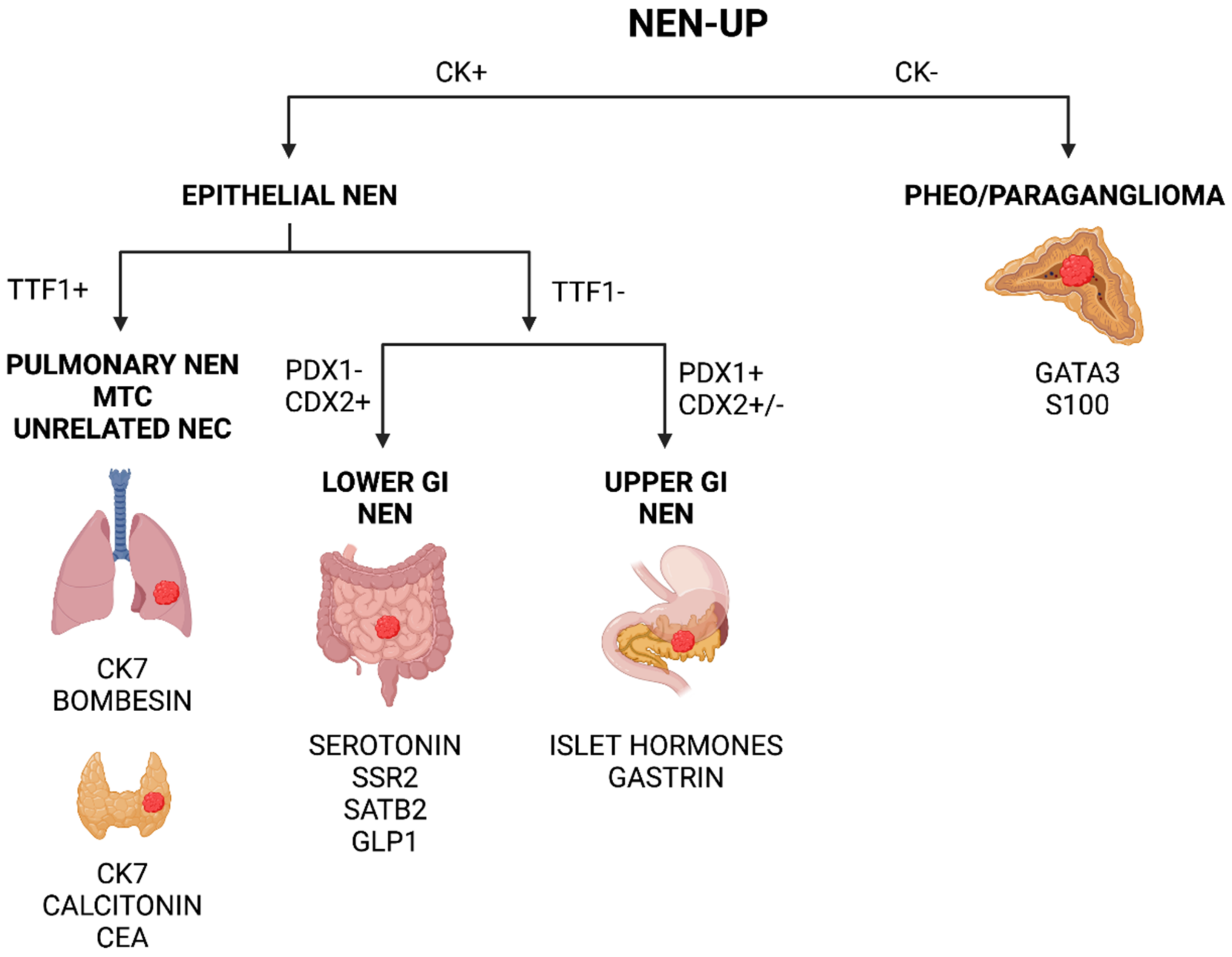
1.4. Pinpointing the Primary Origin: Clues from Immunohistochemistry
1.4.1. Metastatic Pancreatic Neuroendocrine Neoplasia (Pan-NEN)
1.4.2. Metastatic Neuroendocrine Neoplasia from Small Intestine and Appendix
1.4.3. Metastatic Colorectal NEN
1.4.4. Metastatic Pulmonary NEN
1.4.5. Metastatic Pheochromocytoma and Abdominal Paraganglioma
1.4.6. Metastatic Merkel Cell Carcinoma
1.4.7. A Word of Warning
1.5. Pinpointing the Primary Origin: Clues from Molecular Analyses
2. Discussion
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stoyianni, A.; Pentheroudakis, G.; Pavlidis, N. Neuroendocrine Carcinoma of Unknown Primary: A Systematic Review of the Literature and a Comparative Study with Other Neuroendocrine Tumors. Cancer Treat Rev. 2011, 37, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Kan, Y.; Yang, J.-G. Clinical Value of 68Ga-DOTA-SSTR PET/CT in the Diagnosis and Detection of Neuroendocrine Tumors of Unknown Primary Origin: A Systematic Review and Meta-Analysis. Acta Radiol. 2021, 62, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Berner, A.M.; Pipinikas, C.; Ryan, A.; Dibra, H.; Moghul, I.; Webster, A.; Luong, T.V.; Thirlwell, C. Diagnostic Approaches to Neuroendocrine Neoplasms of Unknown Primary Site. Neuroendocrinology 2020, 110, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Parekh, J.R.; Zuraek, M.B.; Venook, A.P.; Bergsland, E.K.; Warren, R.S.; Nakakura, E.K. Identification of Unknown Primary Tumors in Patients with Neuroendocrine Liver Metastases. Arch. Surg. 2010, 145, 276–280. [Google Scholar] [CrossRef] [Green Version]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.-F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef]
- Catena, L.; Bichisao, E.; Milione, M.; Valente, M.; Platania, M.; Pusceddu, S.; Ducceschi, M.; Zilembo, N.; Formisano, B.; Bajetta, E. Neuroendocrine Tumors of Unknown Primary Site: Gold Dust or Misdiagnosed Neoplasms? Tumori 2011, 97, 564–567. [Google Scholar] [CrossRef]
- Taal, B.G.; Visser, O. Epidemiology of Neuroendocrine Tumours. Neuroendocrinology 2004, 80 (Suppl. 1), 3–7. [Google Scholar] [CrossRef]
- Fraenkel, M.; Kim, M.; Faggiano, A.; de Herder, W.W.; Valk, G.D. Knowledge NETwork Incidence of Gastroenteropancreatic Neuroendocrine Tumours: A Systematic Review of the Literature. Endocr. Relat. Cancer 2014, 21, R153–R163. [Google Scholar] [CrossRef]
- Sundin, A.; Vullierme, M.-P.; Kaltsas, G.; Plöckinger, U.; Mallorca Consensus Conference participants. European Neuroendocrine Tumor Society ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological Examinations. Neuroendocrinology 2009, 90, 167–183. [Google Scholar] [CrossRef]
- Kirshbom, P.M.; Kherani, A.R.; Onaitis, M.W.; Feldman, J.M.; Tyler, D.S. Carcinoids of Unknown Origin: Comparative Analysis with Foregut, Midgut, and Hindgut Carcinoids. Surgery 1998, 124, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, J.E.; Howe, J.R. Imaging in Neuroendocrine Tumors: An Update for the Clinician. Int. J. Endocr. Oncol. 2015, 2, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruthi, A.; Pankaj, P.; Verma, R.; Jain, A.; Belho, E.S.; Mahajan, H. Ga-68 DOTANOC PET/CT Imaging in Detection of Primary Site in Patients with Metastatic Neuroendocrine Tumours of Unknown Origin and Its Impact on Clinical Decision Making: Experience from a Tertiary Care Centre in India. J. Gastrointest. Oncol. 2016, 7, 449–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansquer, C.; Touchefeu, Y.; Faivre-Chauvet, A.; Leux, C.; Le Bras, M.; Régenet, N.; Fleury, V.; Maucherat, B.; Senellart, H.; Guyetant, S.; et al. Head-to-Head Comparison of 18F-DOPA PET/CT and 68Ga-DOTANOC PET/CT in Patients with Midgut Neuroendocrine Tumors. Clin. Nucl Med. 2021, 46, 181–186. [Google Scholar] [CrossRef]
- Pavel, M.; Baudin, E.; Couvelard, A.; Krenning, E.; Öberg, K.; Steinmüller, T.; Anlauf, M.; Wiedenmann, B.; Salazar, R. Barcelona Consensus Conference participants ENETS Consensus Guidelines for the Management of Patients with Liver and Other Distant Metastases from Neuroendocrine Neoplasms of Foregut, Midgut, Hindgut, and Unknown Primary. Neuroendocrinology 2012, 95, 157–176. [Google Scholar] [CrossRef]
- Jimenez-Heffernan, J.A.; Lopez-Ferrer, P.; Vicandi, B.; Mariño, A.; Tejerina, E.; Nistal, M.; Viguer, J.M. Fine-Needle Aspiration Cytology of Large Cell Neuroendocrine Carcinoma of the Lung: A Cytohistologic Correlation Study of 11 Cases. Cancer 2008, 114, 180–186. [Google Scholar] [CrossRef]
- Cai, G.; Ramdall, R.B.; Levine, P.; Yang, G.C.H. Fine-Needle Aspiration of Metastatic Prostatic Neuroendocrine Carcinomas: Cytomorphologic and Immunophenotypic Features. Diagn. Cytopathol. 2008, 36, 545–549. [Google Scholar] [CrossRef]
- Díaz Del Arco, C.; Esteban López-Jamar, J.M.; Ortega Medina, L.; Díaz Pérez, J.Á.; Fernández Aceñero, M.J. Fine-Needle Aspiration Biopsy of Pancreatic Neuroendocrine Tumors: Correlation between Ki-67 Index in Cytological Samples and Clinical Behavior. Diagn. Cytopathol. 2017, 45, 29–35. [Google Scholar] [CrossRef]
- Crinò, S.F.; Ammendola, S.; Meneghetti, A.; Bernardoni, L.; Conti Bellocchi, M.C.; Gabbrielli, A.; Landoni, L.; Paiella, S.; Pin, F.; Parisi, A.; et al. Comparison between EUS-Guided Fine-Needle Aspiration Cytology and EUS-Guided Fine-Needle Biopsy Histology for the Evaluation of Pancreatic Neuroendocrine Tumors. Pancreatology 2021, 21, 443–450. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kim, Y.; An, S.; Kim, S.J.; Kim, J.Y.; Kim, S.-Y.; Hwang, D.W.; Park, D.H.; Lee, S.S.; Kim, S.C.; et al. Grading by the Ki-67 Labeling Index of Endoscopic Ultrasound-Guided Fine Needle Aspiration Biopsy Specimens of Pancreatic Neuroendocrine Tumors Can Be Underestimated. Pancreas 2018, 47, 1296–1303. [Google Scholar] [CrossRef]
- Ahmed, A.; VandenBussche, C.J.; Ali, S.Z.; Olson, M.T. The Dilemma of “Indeterminate” Interpretations of Pancreatic Neuroendocrine Tumors on Fine Needle Aspiration. Diagn. Cytopathol. 2016, 44, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Abi-Raad, R.; Lavik, J.-P.; Barbieri, A.L.; Zhang, X.; Adeniran, A.J.; Cai, G. Grading Pancreatic Neuroendocrine Tumors by Ki-67 Index Evaluated on Fine-Needle Aspiration Cell Block Material. Am. J. Clin. Pathol. 2020, 153, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours. Digestive System Tumours, 5th ed.; IARC Press: Lyon, France, 2021; ISBN 92-832-4499-0. [Google Scholar]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. (Eds.) WHO Classification of Tumours of Endocrine Organs; World Health Organization Classification of Tumours, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017; ISBN 978-92-832-4493-6. [Google Scholar]
- Juhlin, C.C. Challenges in Paragangliomas and Pheochromocytomas: From Histology to Molecular Immunohistochemistry. Endocr. Pathol. 2021, 32, 228–244. [Google Scholar] [CrossRef] [PubMed]
- Özşen, M.; Yalçinkaya, U.; Akyildiz, E.Ü.; Bayram, A.S.; Gökalp, G. Bronchial Carcinoid Tumors with Massive Osseous Metaplasia: A Case Report and Review of the Literature. Turk. Patoloji Derg. 2020, 36, 159–163. [Google Scholar] [CrossRef]
- Ljungberg, O. Cytologic Diagnosis of Medullary Carcinoma of the Thyroid Gland with Special Regard to the Demonstration of Amyloid in Smears of Fine Needle Aspirates. Acta Cytol. 1972, 16, 253–255. [Google Scholar]
- Dayal, Y.; Doos, W.G.; O’Brien, M.J.; Nunnemacher, G.; DeLellis, R.A.; Wolfe, H.J. Psammomatous Somatostatinomas of the Duodenum. Am. J. Surg. Pathol. 1983, 7, 653–665. [Google Scholar] [CrossRef]
- Duan, K.; Mete, O. Algorithmic Approach to Neuroendocrine Tumors in Targeted Biopsies: Practical Applications of Immunohistochemical Markers. Cancer Cytopathol. 2016, 124, 871–884. [Google Scholar] [CrossRef] [Green Version]
- Madahian, S.; Judelson, R.; Zhu, X.; Meng, X.; Dresser, K.; Hutchinson, L.; Bledsoe, J.R. CD56 Expression in Basaloid Anal Squamous Cell Carcinoma—A Potential Diagnostic Pitfall. Ann. Diagn. Pathol. 2021, 53, 151758. [Google Scholar] [CrossRef]
- Kriegsmann, K.; Zgorzelski, C.; Muley, T.; Christopoulos, P.; Thomas, M.; Winter, H.; Eichhorn, M.; Eichhorn, F.; von Winterfeld, M.; Herpel, E.; et al. Role of Synaptophysin, Chromogranin and CD56 in Adenocarcinoma and Squamous Cell Carcinoma of the Lung Lacking Morphological Features of Neuroendocrine Differentiation: A Retrospective Large-Scale Study on 1170 Tissue Samples. BMC Cancer 2021, 21, 486. [Google Scholar] [CrossRef]
- Juhlin, C.C.; Zedenius, J.; Höög, A. Clinical Routine Application of the Second-Generation Neuroendocrine Markers ISL1, INSM1, and Secretagogin in Neuroendocrine Neoplasia: Staining Outcomes and Potential Clues for Determining Tumor Origin. Endocr. Pathol. 2020, 31, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Juhlin, C.C. Second-Generation Neuroendocrine Immunohistochemical Markers: Reflections from Clinical Implementation. Biology 2021, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Juhlin, C.C.; Zedenius, J.; Haglund, F. Metastatic Malignant Melanoma with Neuroendocrine Differentiation: A Case Report and Review of the Literature. J. Med. Case Rep. 2020, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Mete, O.; Asa, S.L.; Gill, A.J.; Kimura, N.; de Krijger, R.R.; Tischler, A. Overview of the 2022 WHO Classification of Paragangliomas and Pheochromocytomas. Endocr. Pathol. 2022, 33, 90–114. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.S.; Alexander, J.; Swanson, P.E.; Jain, D.; Yeh, M.M. PDX-1, CDX-2, TTF-1, and CK7: A Reliable Immunohistochemical Panel for Pancreatic Neuroendocrine Neoplasms. Am. J. Surg. Pathol. 2012, 36, 737–743. [Google Scholar] [CrossRef]
- Long, K.B.; Srivastava, A.; Hirsch, M.S.; Hornick, J.L. PAX8 Expression in Well-Differentiated Pancreatic Endocrine Tumors: Correlation with Clinicopathologic Features and Comparison with Gastrointestinal and Pulmonary Carcinoid Tumors. Am. J. Surg. Pathol. 2010, 34, 723–729. [Google Scholar] [CrossRef]
- Yu, S.; Hornick, J.L.; Gonzalez, R.S. An Algorithmic Approach Utilizing CK7, TTF1, Beta-Catenin, CDX2, and SSTR2A Can Help Differentiate between Gastrointestinal and Pulmonary Neuroendocrine Carcinomas. Virchows Arch. 2021, 10, 874. [Google Scholar] [CrossRef]
- Barbareschi, M.; Roldo, C.; Zamboni, G.; Capelli, P.; Cavazza, A.; Macri, E.; Cangi, M.G.; Chilosi, M.; Doglioni, C. CDX-2 Homeobox Gene Product Expression in Neuroendocrine Tumors: Its Role as a Marker of Intestinal Neuroendocrine Tumors. Am. J. Surg. Pathol. 2004, 28, 1169–1176. [Google Scholar] [CrossRef]
- Erickson, L.A.; Papouchado, B.; Dimashkieh, H.; Zhang, S.; Nakamura, N.; Lloyd, R.V. Cdx2 as a Marker for Neuroendocrine Tumors of Unknown Primary Sites. Endocr. Pathol. 2004, 15, 247–252. [Google Scholar] [CrossRef]
- Bellizzi, A.M. SATB2 in Neuroendocrine Neoplasms: Strong Expression Is Restricted to Well-Differentiated Tumours of Lower Gastrointestinal Tract Origin and Is Most Frequent in Merkel Cell Carcinoma among Poorly Differentiated Carcinomas. Histopathology 2020, 76, 251–264. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Tiwari, A.; Bhardwaj, N.; Chuang, F.; Kim, E.; Lugo, H.; Yuan, X.; Diffalha, S.A.; Peralta-Venturina, M.; Balzer, B.; et al. Positivity for SATB2 Distinguishes Islet1 Positive Rectal Neuroendocrine Tumours from Pancreaticoduodenal Neuroendocrine Tumours. J. Clin. Pathol. 2021, 74, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, K.-S.; Kim, K.-J.; Park, I.J.; Lee, J.L.; Myung, S.-J.; Park, Y.; Park, Y.S.; Yu, C.S.; Kim, J.C.; et al. Non-L-Cell Immunophenotype and Large Tumor Size in Rectal Neuroendocrine Tumors Are Associated with Aggressive Clinical Behavior and Worse Prognosis. Am. J. Surg. Pathol. 2015, 39, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Zhou, X.; Moschiano, E.; De Peralta-Venturina, M.; Mertens, R.B.; Dhall, D. The Immunohistochemical Expression of Islet 1 and PAX8 by Rectal Neuroendocrine Tumors Should Be Taken into Account in the Differential Diagnosis of Metastatic Neuroendocrine Tumors of Unknown Primary Origin. Endocr. Pathol. 2013, 24, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, D.C.; Srirajaskanthan, R.; Vincent, R.P.; Guy, C.; Drakou, E.E.; Aylwin, S.J.B.; Grossman, A.B.; Ramage, J.K.; Dimitriadis, G.K. Calcitonin-Secreting Neuroendocrine Neoplasms of the Lung: A Systematic Review and Narrative Synthesis. Endocr. Connect. 2021, 10, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Bonato, M.; Cerati, M.; Pagani, A.; Papotti, M.; Bosi, F.; Bussolati, G.; Capella, C. Differential Diagnostic Patterns of Lung Neuroendocrine Tumours. A Clinico-Pathological and Immunohistochemical Study of 122 Cases. Virchows Arch. A Pathol. Anat. Histopathol 1992, 420, 201–211. [Google Scholar] [CrossRef]
- Haynes, C.M.; Sangoi, A.R.; Pai, R.K. PAX8 Is Expressed in Pancreatic Well-Differentiated Neuroendocrine Tumors and in Extrapancreatic Poorly Differentiated Neuroendocrine Carcinomas in Fine-Needle Aspiration Biopsy Specimens. Cancer Cytopathol. 2011, 119, 193–201. [Google Scholar] [CrossRef]
- Asa, S.L.; Ezzat, S.; Mete, O. The Diagnosis and Clinical Significance of Paragangliomas in Unusual Locations. J. Clin. Med. 2018, 7, 280. [Google Scholar] [CrossRef] [Green Version]
- Mete, O.; Pakbaz, S.; Lerario, A.M.; Giordano, T.J.; Asa, S.L. Significance of Alpha-Inhibin Expression in Pheochromocytomas and Paragangliomas. Am. J. Surg. Pathol. 2021, 45, 1264–1273. [Google Scholar] [CrossRef]
- Kuhajda, F.P.; Olson, J.L.; Mann, R.B. Merkel Cell (Small Cell) Carcinoma of the Skin: Immunohistochemical and Ultrastructural Demonstration of Distinctive Perinuclear Cytokeratin Aggregates and a Possible Association with B Cell Neoplasms. Histochem. J. 1986, 18, 239–244. [Google Scholar] [CrossRef]
- Haneke, E.; Schulze, H.J.; Mahrle, G. Immunohistochemical and Immunoelectron Microscopic Demonstration of Chromogranin A in Formalin-Fixed Tissue of Merkel Cell Carcinoma. J. Am. Acad. Derm. 1993, 28, 222–226. [Google Scholar] [CrossRef]
- Shuda, M.; Arora, R.; Kwun, H.J.; Feng, H.; Sarid, R.; Fernández-Figueras, M.-T.; Tolstov, Y.; Gjoerup, O.; Mansukhani, M.M.; Swerdlow, S.H.; et al. Human Merkel Cell Polyomavirus Infection I. MCV T Antigen Expression in Merkel Cell Carcinoma, Lymphoid Tissues and Lymphoid Tumors. Int. J. Cancer 2009, 125, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Wick, M.R.; Millns, J.L.; Sibley, R.K.; Pittelkow, M.R.; Winkelmann, R.K. Secondary Neuroendocrine Carcinomas of the Skin. An Immunohistochemical Comparison with Primary Neuroendocrine Carcinoma of the Skin (“Merkel Cell” Carcinoma). J. Am. Acad. Derm. 1985, 13, 134–142. [Google Scholar] [CrossRef]
- Snow, S.N.; Larson, P.O.; Hardy, S.; Bentz, M.; Madjar, D.; Landeck, A.; Oriba, H.; Olansky, D. Merkel Cell Carcinoma of the Skin and Mucosa: Report of 12 Cutaneous Cases with 2 Cases Arising from the Nasal Mucosa. Derm. Surg. 2001, 27, 165–170. [Google Scholar] [CrossRef]
- Lee, H.; Fu, Z.; Koo, B.H.; Sheehan, C.E.; Young, G.Q.; Lin, J.; Patil, D.T.; Yang, Z. The Expression of TTF1, CDX2 and ISL1 in 74 Poorly Differentiated Neuroendocrine Carcinomas. Ann. Diagn. Pathol. 2018, 37, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Gucer, H.; Caliskan, S.; Kefeli, M.; Mete, O. Do You Know the Details of Your PAX8 Antibody? Monoclonal PAX8 (MRQ-50) Is Not Expressed in a Series of 45 Medullary Thyroid Carcinomas. Endocr. Pathol. 2020, 31, 33–38. [Google Scholar] [CrossRef]
- Ciobanu, O.A.; Martin, S.; Fica, S. Perspectives on the Diagnostic, Predictive and Prognostic Markers of Neuroendocrine Neoplasms (Review). Exp. Med. 2021, 22, 1479. [Google Scholar] [CrossRef]
- Venizelos, A.; Elvebakken, H.; Perren, A.; Nikolaienko, O.; Deng, W.; Lothe, I.M.B.; Couvelard, A.; Hjortland, G.O.; Sundlöv, A.; Svensson, J.; et al. The Molecular Characteristics of High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms. Endocr. Relat. Cancer 2021, 29, 1–14. [Google Scholar] [CrossRef]
- Uccella, S.; La Rosa, S.; Metovic, J.; Marchiori, D.; Scoazec, J.-Y.; Volante, M.; Mete, O.; Papotti, M. Genomics of High-Grade Neuroendocrine Neoplasms: Well-Differentiated Neuroendocrine Tumor with High-Grade Features (G3 NET) and Neuroendocrine Carcinomas (NEC) of Various Anatomic Sites. Endocr. Pathol. 2021, 32, 192–210. [Google Scholar] [CrossRef]
- Sun, T.Y.; Hendifar, A.; Padda, S.K. Lung Neuroendocrine Tumors: How Does Molecular Profiling Help? Curr. Oncol. Rep. 2022, 1–6. [Google Scholar] [CrossRef]
- Jiao, Y.; Shi, C.; Edil, B.H.; de Wilde, R.F.; Klimstra, D.S.; Maitra, A.; Schulick, R.D.; Tang, L.H.; Wolfgang, C.L.; Choti, M.A.; et al. DAXX/ATRX, MEN1, and MTOR Pathway Genes Are Frequently Altered in Pancreatic Neuroendocrine Tumors. Science 2011, 331, 1199–1203. [Google Scholar] [CrossRef] [Green Version]
- Samsom, K.G.; Levy, S.; van Veenendaal, L.M.; Roepman, P.; Kodach, L.L.; Steeghs, N.; Valk, G.D.; Wouter Dercksen, M.; Kuhlmann, K.F.D.; Verbeek, W.H.M.; et al. Driver Mutations Occur Frequently in Metastases of Well-Differentiated Small Intestine Neuroendocrine Tumours. Histopathology 2021, 78, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Simbolo, M.; Vicentini, C.; Mafficini, A.; Fassan, M.; Pedron, S.; Corbo, V.; Mastracci, L.; Rusev, B.; Pedrazzani, C.; Landoni, L.; et al. Mutational and Copy Number Asset of Primary Sporadic Neuroendocrine Tumors of the Small Intestine. Virchows Arch. 2018, 473, 709–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Görtz, B.; Roth, J.; Krähenmann, A.; de Krijger, R.R.; Muletta-Feurer, S.; Rütimann, K.; Saremaslani, P.; Speel, E.J.; Heitz, P.U.; Komminoth, P. Mutations and Allelic Deletions of the MEN1 Gene Are Associated with a Subset of Sporadic Endocrine Pancreatic and Neuroendocrine Tumors and Not Restricted to Foregut Neoplasms. Am. J. Pathol. 1999, 154, 429–436. [Google Scholar] [CrossRef] [Green Version]
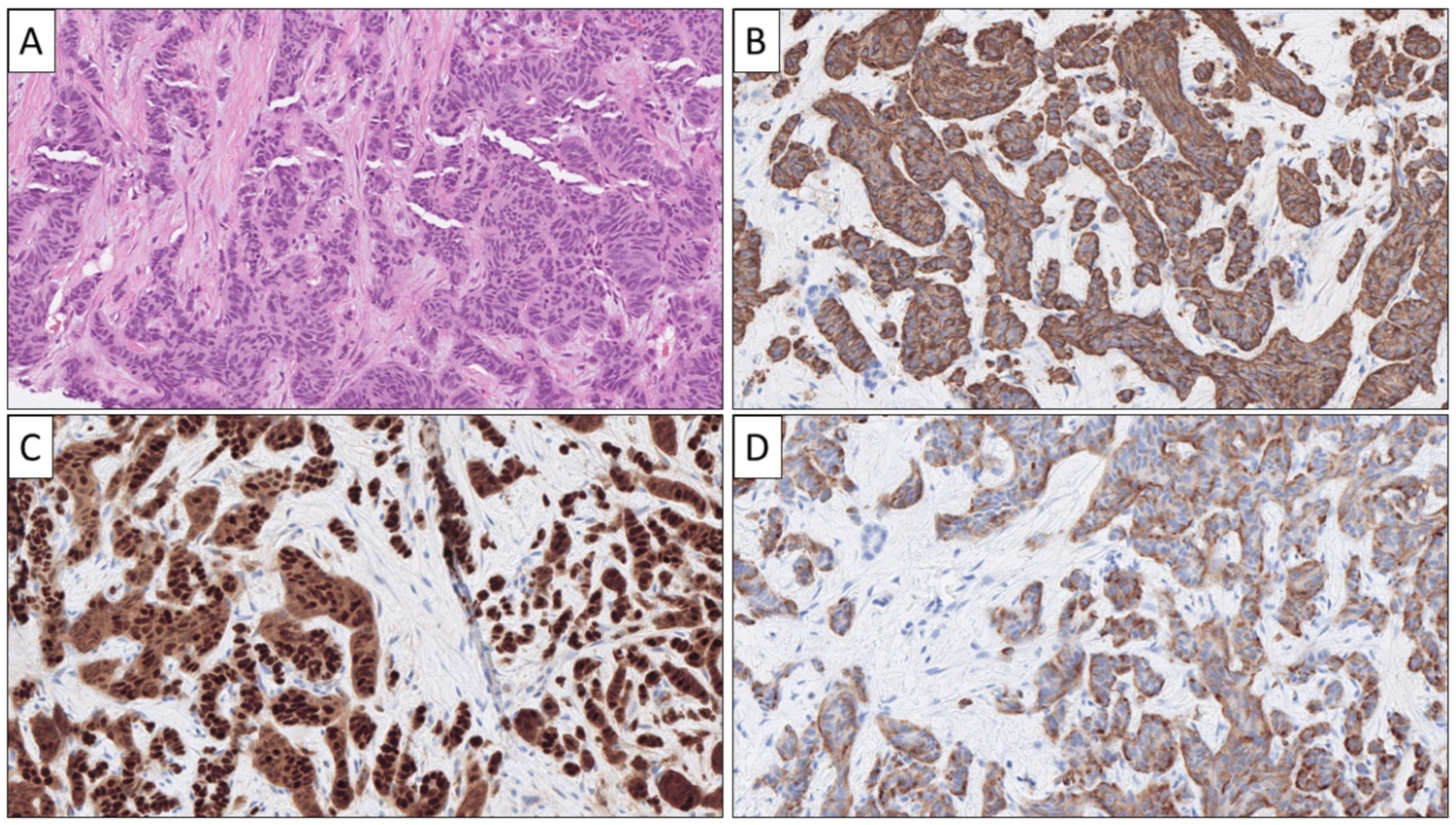
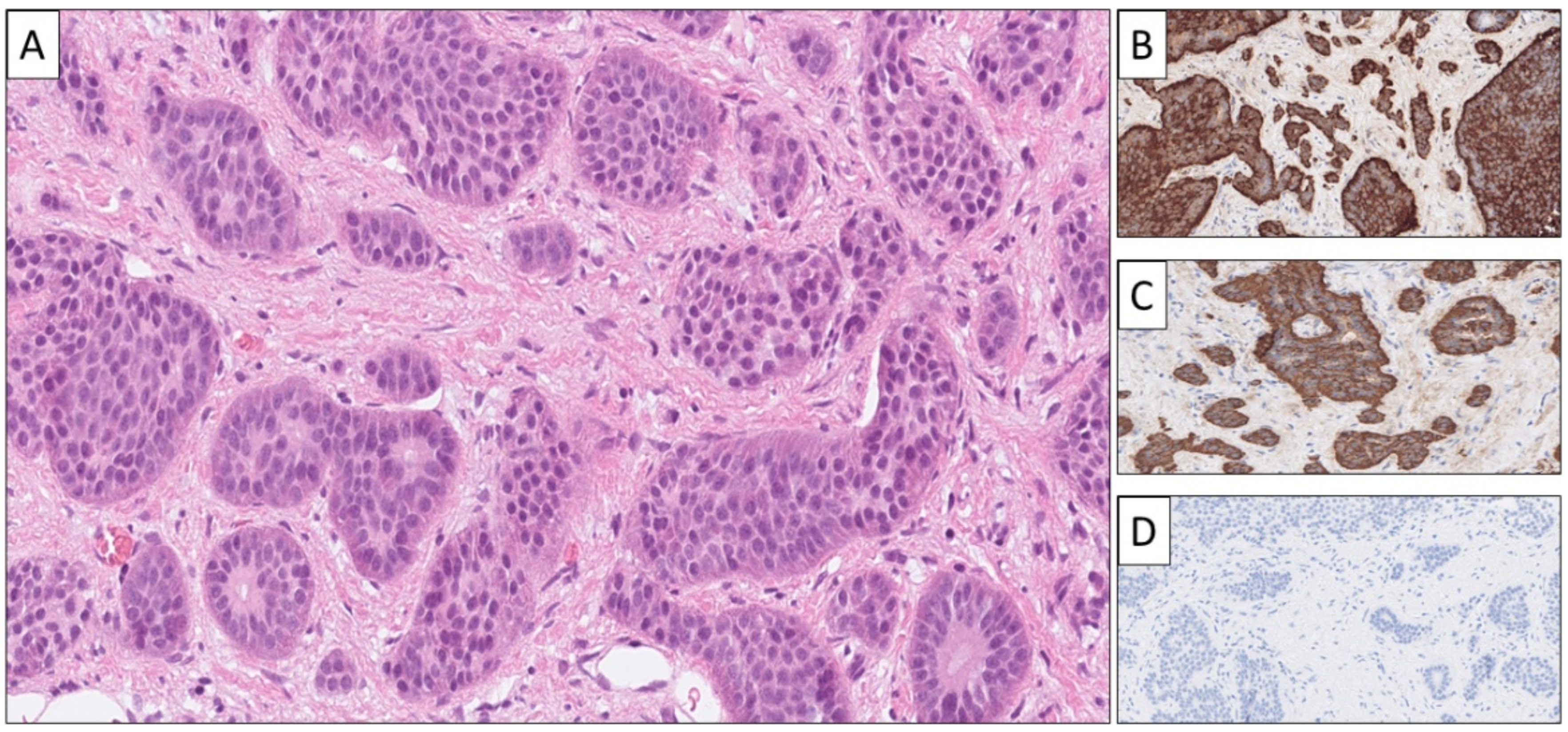

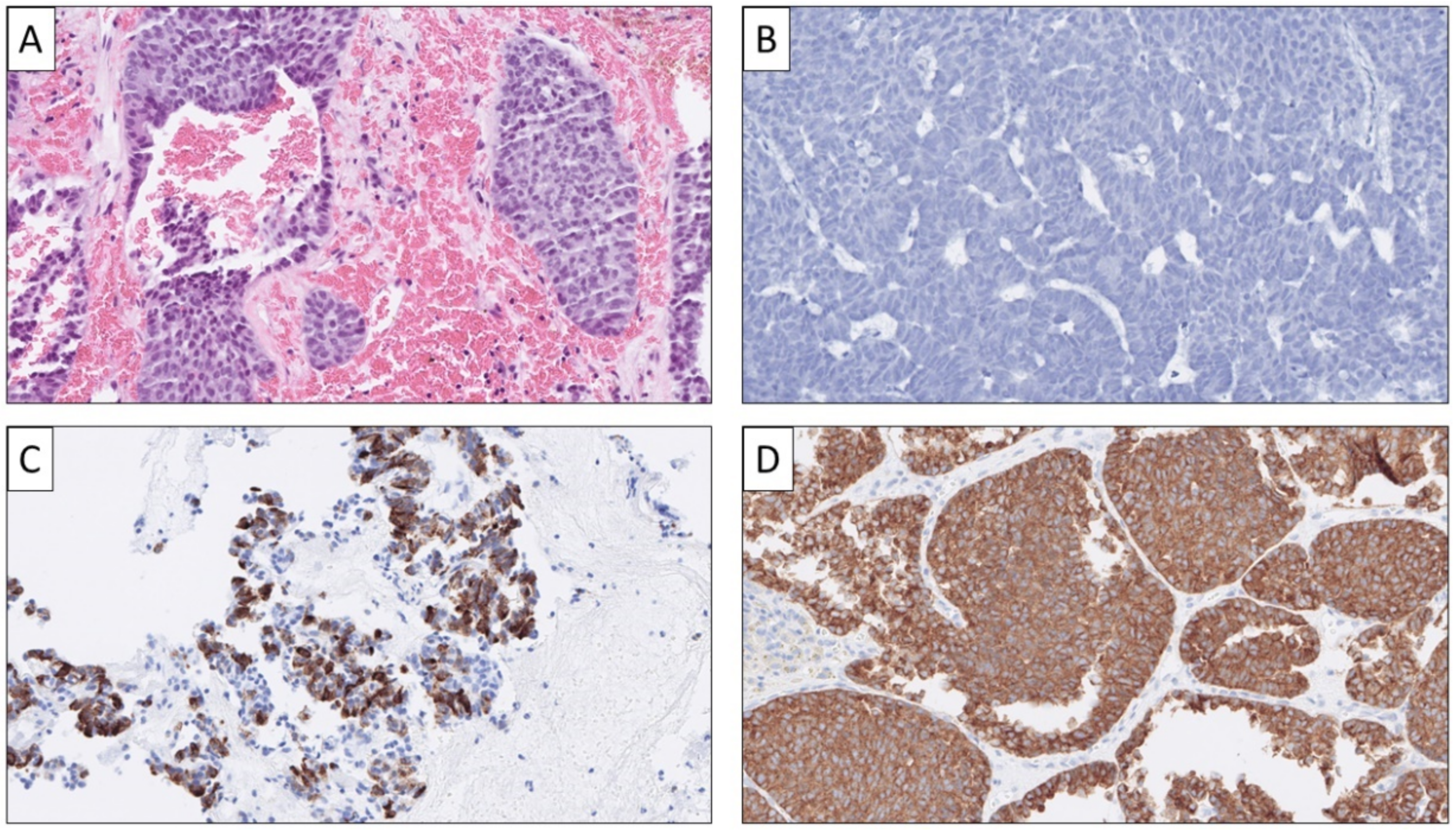


| Primary Site | Common Growth Patterns * | Associated Histological Features |
|---|---|---|
| Lung | Various | Rarely with stromal ossification |
| Thyroid-medullary carcinoma | Various | Amyloid deposits, amphophilic cytoplasm |
| Thymus | Nested, trabecular, cord-like | - |
| Stomach | Pseudo-glandular, trabecular, nested | - |
| Duodenum-somatostatinoma | Pseudo-glandular, nested | Psammoma bodies |
| Duodenum, Pancreas-gastrinoma | Trabecular, pseudo-glandular | - |
| Pancreas-insulinoma | Trabecular, nested, solid | Hyalinized stroma |
| Pancreas-glucagonoma | Nested, cord-like | - |
| Pancreas-somatostatinoma | Nested, cord-like | Psammoma bodies |
| Pancreas-VIPoma | Nested, cord-like | - |
| Pancreas-non-producing | Nested, cord-like | - |
| Small intestine | Nested, organoid | Peripheral cytoplasmic granularity |
| Appendix-enterochromaffin | Nested, cord-like | - |
| Appendix-L-cell | Trabecular, pseudo-glandular | - |
| Appendix-tubular | Tubular | - |
| Adrenal-pheochromocytoma | Nested, “zell-ballen” | Hyaline globules, basophilic cytoplasm |
| Paraganglia-paraganglioma | Nested, “zell-ballen” | Basophilic cytoplasm |
| Merkel cell carcinoma | Variable | Small round blue cell tumor |
| Prostate | Small cell phenotype | - |
| Colon | Nested, trabecular, nested | - |
| Rectum | Nested, trabecular, cord-like | - |
| Anal canal | Small cell phenotype | - |
| Primary Site | CGA * | SYP * | ISL1 | INSM1 | SECG | Other Markers of Importance |
|---|---|---|---|---|---|---|
| Lung | + | + | + | + | + | TTF1, bombesin/GRP |
| Thyroid-medullary carcinoma | + | + | + | + | N/A | TTF1, PAX8, Calcitonin, CEA |
| Thymus | + | + | N/A | N/A | N/A | - |
| Stomach | + | + | N/A | N/A | N/A | PDX1 |
| Duodenum-somatostatinoma | + | + | + | N/A | N/A | PDX1, somatostatin |
| Duodenum, Pancreas-gastrinoma | + | + | + | N/A | N/A | Gastrin |
| Pancreas-insulinoma | + | + | + | + | + | Insulin |
| Pancreas-glucagonoma | + | + | + | + | + | Glucagon |
| Pancreas-somatostatinoma | + | + | + | + | + | Somatostatin |
| Pancreas-VIPoma | + | + | + | + | + | VIP |
| Pancreas-non-producing | + | + | + | + | + | Pancreatic polypeptide |
| Small intestine | + | + | − | + | + | Serotonin, CDX2 |
| Appendix-enterochromaffin | + | + | + | + | + | Serotonin |
| Appendix-L-cell | − | + | N/A | N/A | N/A | GLP1, PP, CEA |
| Appendix-tubular | + | + | N/A | N/A | N/A | Serotonin |
| Adrenal-pheochromocytoma | + | + | + | + | − | GATA3, S100 |
| Paraganglia-paraganglioma | + | + | + | + | − | GATA3, S100 |
| Merkel cell carcinoma | + | + | + | N/A | N/A | Dot-like CK20, MCV-polyoma |
| Prostate | + | + | N/A | N/A | N/A | NKX3.1 |
| Colon | + | + | + | + | + | CDX2, SATB2 |
| Rectum | − | + | + | + | + | GLP1, SATB2 |
| Anal canal | + | + | N/A | N/A | N/A | P16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhlin, C.C.; Zedenius, J.; Höög, A. Metastatic Neuroendocrine Neoplasms of Unknown Primary: Clues from Pathology Workup. Cancers 2022, 14, 2210. https://doi.org/10.3390/cancers14092210
Juhlin CC, Zedenius J, Höög A. Metastatic Neuroendocrine Neoplasms of Unknown Primary: Clues from Pathology Workup. Cancers. 2022; 14(9):2210. https://doi.org/10.3390/cancers14092210
Chicago/Turabian StyleJuhlin, Carl Christofer, Jan Zedenius, and Anders Höög. 2022. "Metastatic Neuroendocrine Neoplasms of Unknown Primary: Clues from Pathology Workup" Cancers 14, no. 9: 2210. https://doi.org/10.3390/cancers14092210
APA StyleJuhlin, C. C., Zedenius, J., & Höög, A. (2022). Metastatic Neuroendocrine Neoplasms of Unknown Primary: Clues from Pathology Workup. Cancers, 14(9), 2210. https://doi.org/10.3390/cancers14092210