Tumor Microenvironment in Mixed Neuroendocrine Non-Neuroendocrine Neoplasms: Interaction between Tumors and Immune Cells, and Potential Effects of Neuroendocrine Differentiation on the Tumor Microenvironment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissues and Patient Characteristics
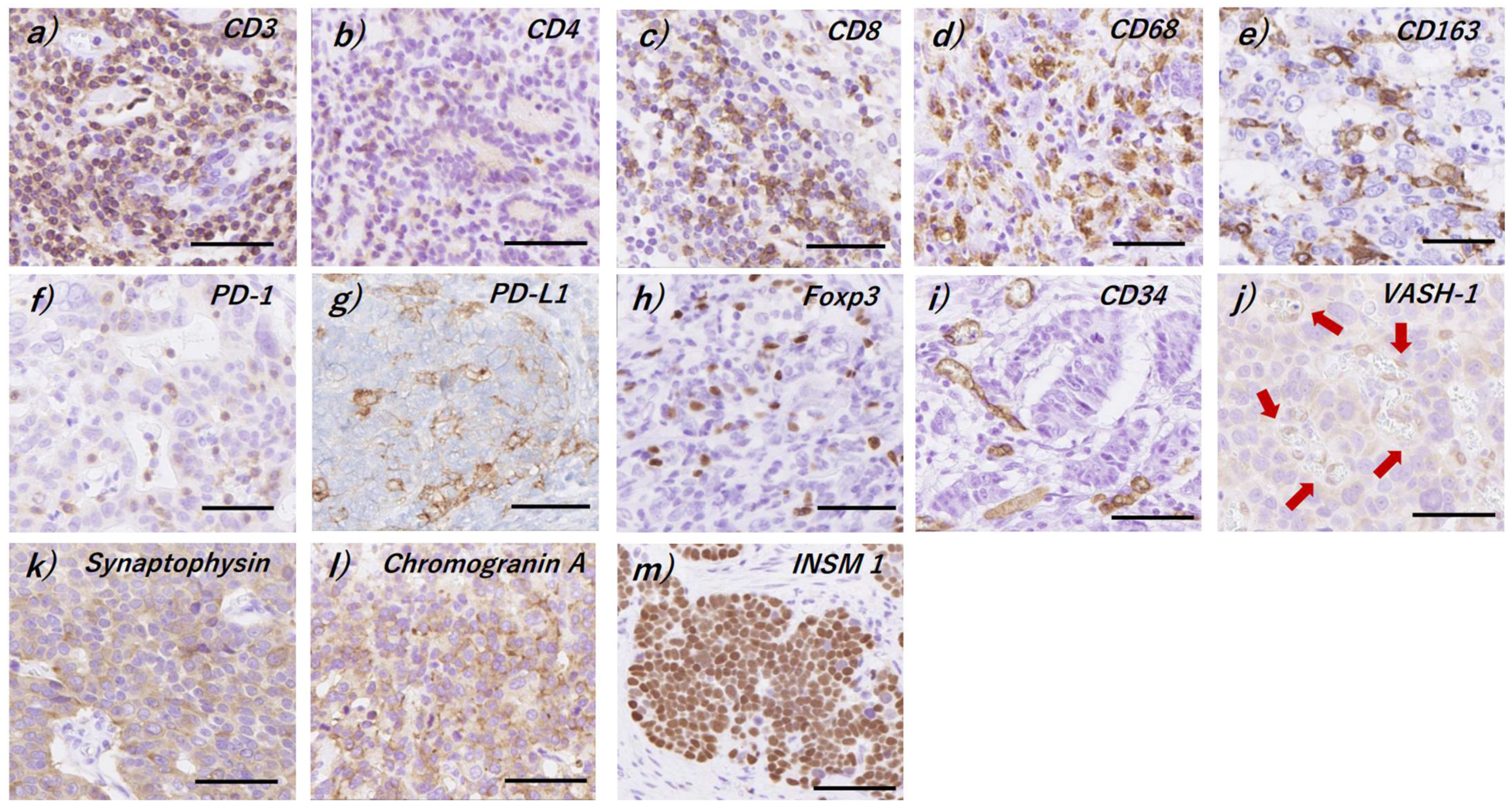
2.2. Immunohistochemistry
2.3. Evaluation of Immunoreactivity
2.4. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics in the Cases Examined
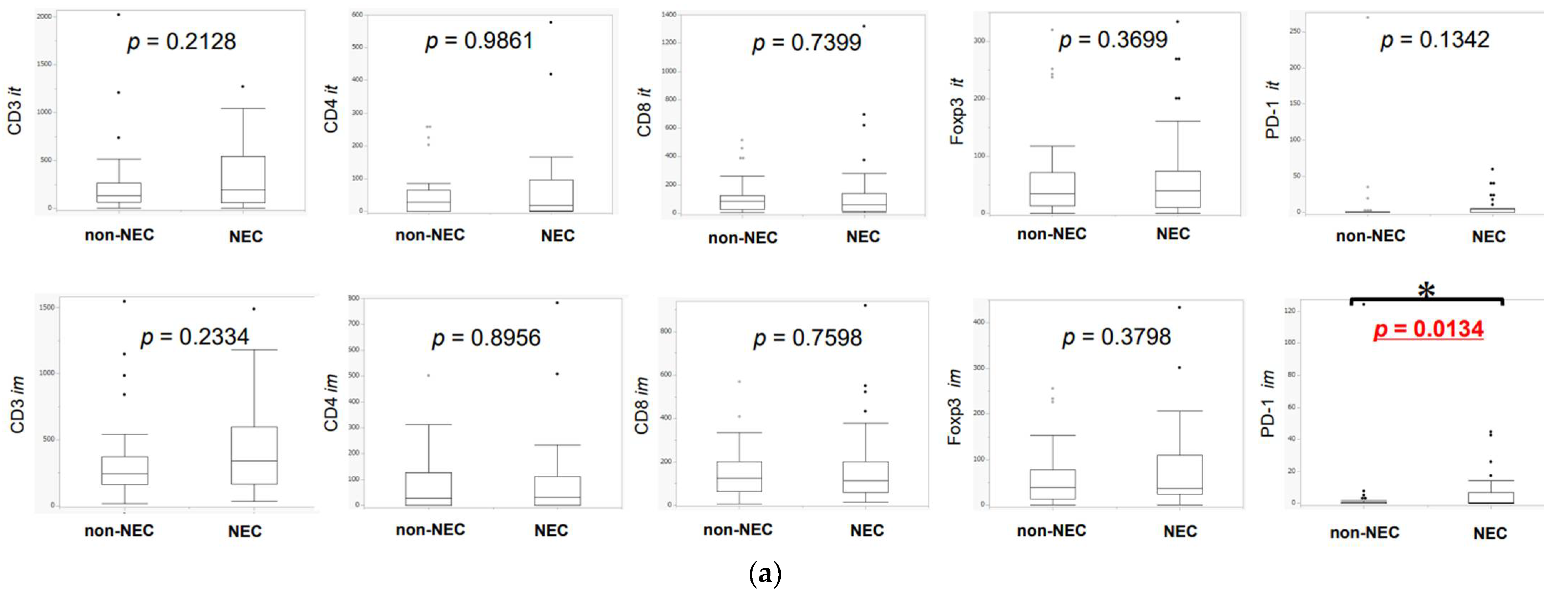
3.2. Difference in TILs between NEC and Non-NEC Components in Intra-Tumoral Areas and Invasive Margins
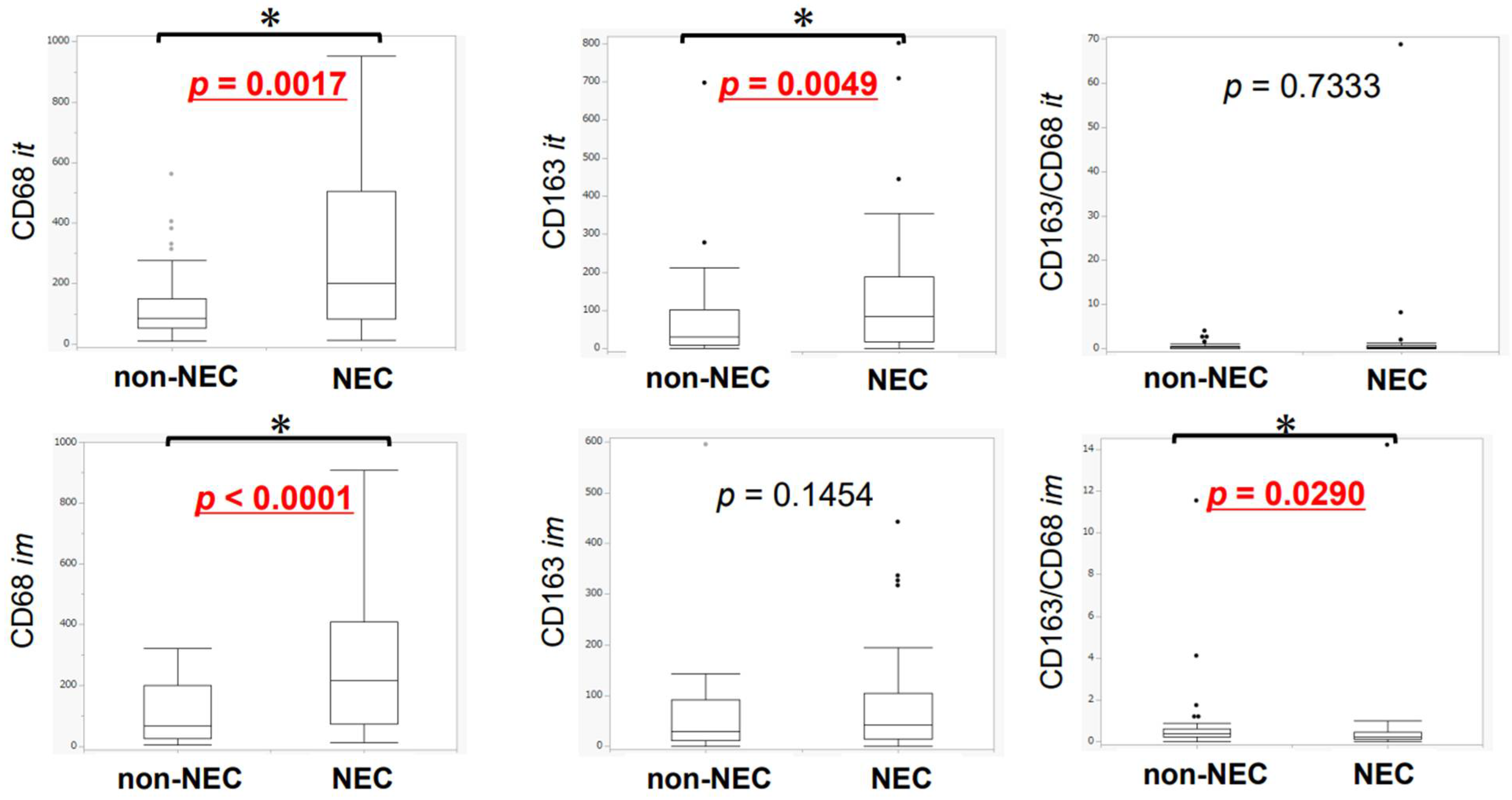
3.3. Difference in TAMs between NEC and Non-NEC Components in Intra-Tumoral Areas and Invasive Margins
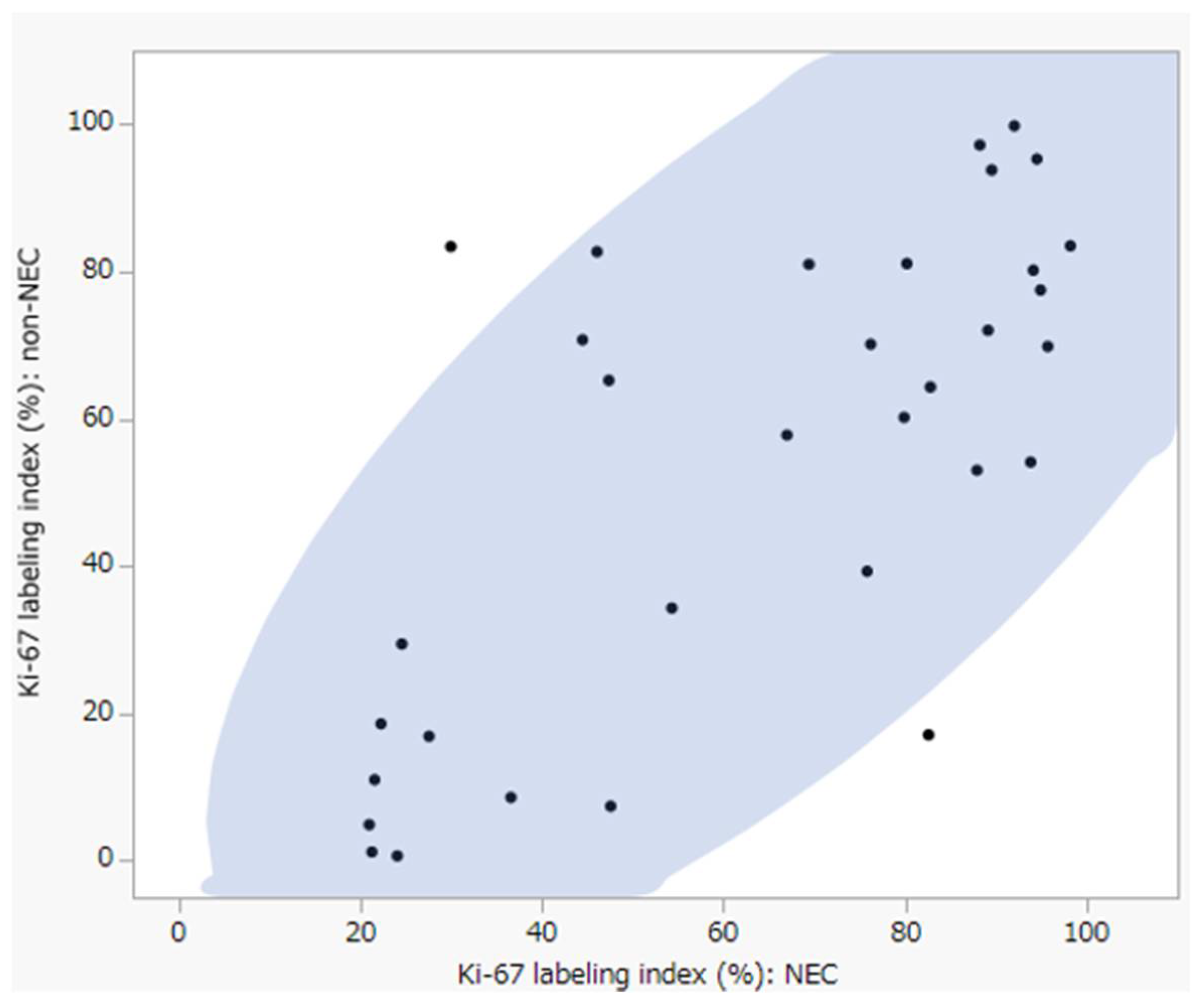
3.4. Differences in Neoangiogenesis between NEC and Non-NEC Components
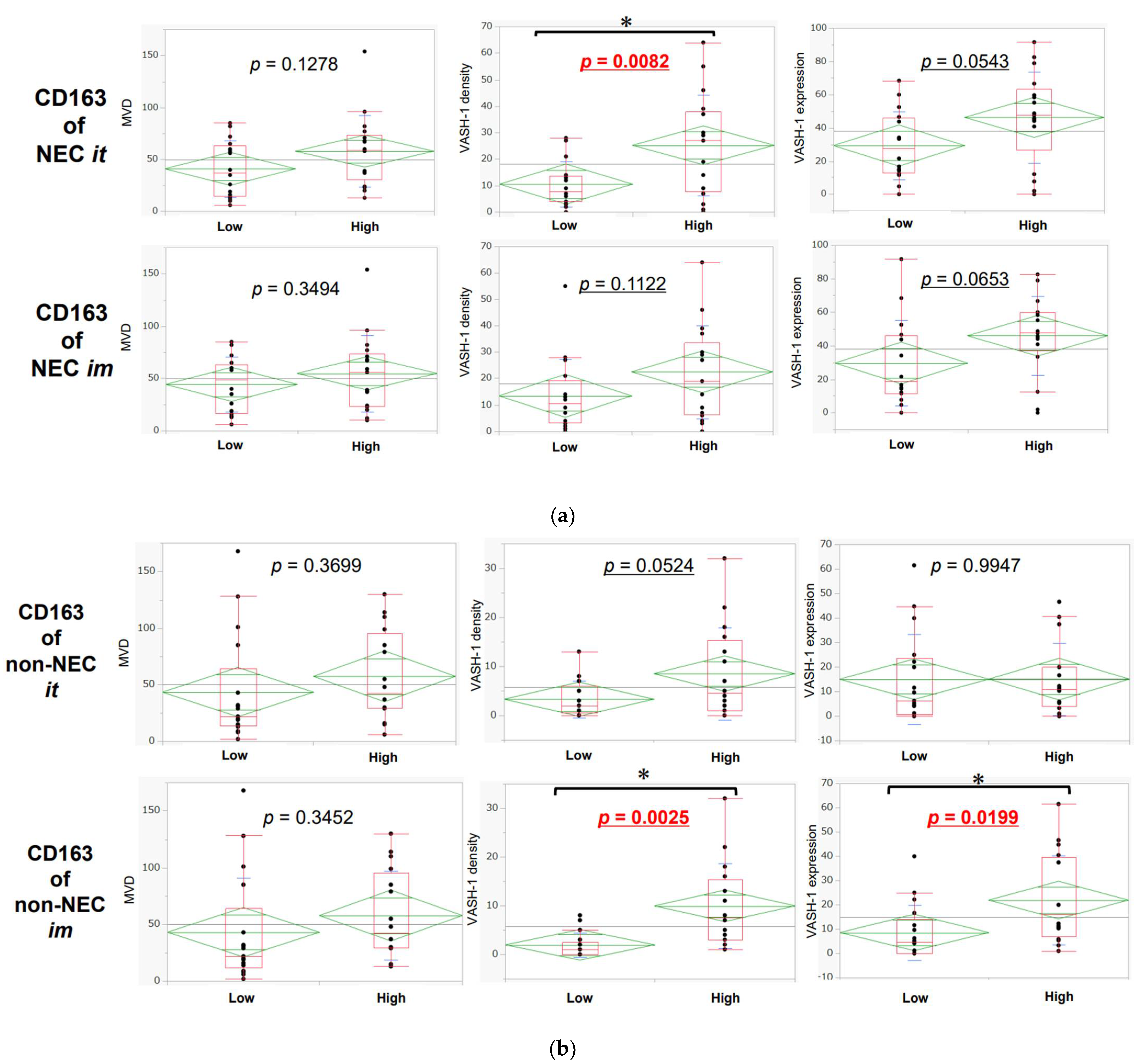
3.5. Association between Neoangiogenesis and CD163-Positive Macrophage Infiltration in Intra-Tumoral Areas and Invasive Margins in NEC and Non-NEC Components
3.6. Association of TILs or TAMs with Lymph Node Metastasis, Lymphatic Invasion, and Venous Invasion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Öberg, K.; Modlin, I.M.; De Herder, W.; Pavel, M.; Klimstra, D.; Frilling, A.; Metz, D.C.; Heaney, A.; Kwekkeboom, D.; Strosberg, J.; et al. Consensus on Biomarkers for Neuroendocrine Tumour Disease. Lancet Oncol. 2015, 16, e435–e446. [Google Scholar] [CrossRef] [Green Version]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Hong, S.M.; Ro, J.Y. Recent Updates on Grading and Classification of Neuroendocrine Tumors. Ann. Diagn. Pathol. 2017, 29, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Grosse, C.; Noack, P.; Silye, R. Accuracy of Grading Pancreatic Neuroendocrine Neoplasms with Ki-67 Index in Fine-Needle Aspiration Cellblock Material. Cytopathology 2019, 30, 187–193. [Google Scholar] [CrossRef]
- Hamilton, S.R.; Aaltonen, L.A. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System; International Agency for Research on Cancer (IARC) Press: Lyon, France, 2000. [Google Scholar]
- DeLellis, R.A.; Lloyd, R.V.; Heitz, P.U. World Health Organization of Tumours. Pathology and Genetics of Tumours of Endocrine Organs; International Agency for Research on Cancer (IARC) Press: Lyon, France, 2004. [Google Scholar]
- Bosman, T.; Carneiro, F.; Hruban, N.D. World Health Organization of Tumours. WHO Classification of Tumours of the Digestive System; International Agency for Research on Cancer (IARC) Press: Lyon, France, 2010. [Google Scholar]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G. World Health Organization of Tumours. WHO Classification of Tumours of Endocrine Organs, 4th ed; International Agency for Research on Cancer (IARC) Press: Lyon, France, 2017. [Google Scholar]
- Frizziero, M.; Wang, X.; Chakrabarty, B.; Childs, A.; Luong, T.V.; Walter, T.; Khan, M.S.; Morgan, M.; Christian, A.; Elshafie, M.; et al. Retrospective Study on Mixed Neuroendocrine Non-Neuroendocrine Neoplasms from Five European Centres. World J. Gastroenterol. 2019, 25, 5991–6005. [Google Scholar] [CrossRef]
- La Rosa, S.; Marando, A.; Sessa, F.; Capella, C. Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update. Cancers 2012, 4, 11–30. [Google Scholar] [CrossRef] [Green Version]
- La Rosa, S.; Marando, A.; Furlan, D.; Sahnane, N.; Capella, C. Colorectal Poorly Differentiated Neuroendocrine Carcinomas and Mixed Adenoneuroendocrine Carcinomas: Insights into the Diagnostic Immunophenotype, Assessment of Methylation Profile, and Search for Prognostic Markers. Am. J. Surg. Pathol. 2012, 36, 601–611. [Google Scholar] [CrossRef]
- Jiang, M.; Tan, Y.; Li, X.; Fu, J.; Hu, H.; Ye, X.; Cao, Y.; Xu, J.; Yuan, Y. Clinicopathological Features and Prognostic Factors of Colorectal Neuroendocrine Neoplasms. Gastroenterol. Res. Pract. 2017, 2017, 4206172. [Google Scholar] [CrossRef]
- Shi, H.; Qi, C.; Meng, L.; Yao, H.; Jiang, C.; Fan, M.; Pang, S.; Zhang, Q.; Lin, R. Do Neuroendocrine Carcinomas and Mixed Neuroendocrine-Non-Neuroendocrine Neoplasm of the Gastrointestinal Tract Have the Same Prognosis? A SEER Database Analysis of 12,878 Cases. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820938304. [Google Scholar] [CrossRef]
- Naito, Y.; Saito, K.; Shiiba, K.; Ohuchi, A.; Saigenji, K.; Nagura, H.; Ohtani, H. CD8+ T Cells Infiltrated within Cancer Cell Nests as a Prognostic Factor in Human Colorectal Cancer. Cancer Res. 1998, 58, 3491–3494. [Google Scholar]
- Eiichi, S.; Sara, H.; Olson, J.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; et al. Intraepithelial CD8 Tumor-Infiltrating Lymphocytes and a High CD8/Regulatory T Cell Ratio Are Associated with Favorable Prognosis in Ovarian Cancer. Proc. Natl Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef] [Green Version]
- Marchi, F.; Missale, F.; Incandela, F.; Filauro, M.; Mazzola, F.; Mora, F.; Paderno, A.; Parrinello, G.; Piazza, C.; Peretti, G. Prognostic Significance of Peripheral T-Cell Subsets in Laryngeal Squamous Cell Carcinoma. Laryngoscope Investig. Otolaryngol. 2019, 4, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Delayre, T.; Guilbaud, T.; Resseguier, N.; Mamessier, E.; Rubis, M.; Moutardier, V.; Fara, R.; Berdah, S.V.; Garcia, S.; Birnbaum, D.J. Prognostic Impact of Tumour-Infiltrating Lymphocytes and Cancer-Associated Fibroblasts in Patients with Pancreatic Adenocarcinoma of the Body and Tail Undergoing Resection. Br. J. Surg. 2020, 107, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Kim, H.; Cho, H.W.; Kim, S.Y.; Song, K.J.; Hyung, W.J.; Park, C.G.; Kim, C.B. The Ratio of Intra-Tumoral Regulatory T Cells (Foxp3+)/Helper T Cells (CD4+) Is a Prognostic Factor and Associated with Recurrence Pattern in Gastric Cardia Cancer. J. Surg. Oncol. 2011, 104, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Sung, J.Y.; Lee, J.; Park, Y.K.; Kim, Y.W.; Kim, G.Y.; Won, K.Y.; Lim, S.J. Polarized CD163+ Tumor-Associated Macrophages Are Associated with Increased Angiogenesis and CXCL12 Expression in Gastric Cancer. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 357–365. [Google Scholar] [CrossRef]
- Zheng, X.; Weigert, A.; Reu, S.; Guenther, S.; Mansouri, S.; Bassaly, B.; Gattenlöhner, S.; Grimminger, F.; Pullamsetti, S.; Seeger, W.; et al. Spatial Density and Distribution of Tumor-Associated Macrophages Predict Survival in Non-Small Cell Lung Carcinoma. Cancer Res. 2020, 80, 4414–4425. [Google Scholar] [CrossRef]
- Lee, J.; Jung, Y.Y.; Lee, J.H.; Hong, M.; Hwang, H.W.; Hong, S.A.; Hong, S.H. The Prognostic Value of Sex-Determining Region Y-Box 2 and CD8+ Tumor-Infiltrating Lymphocytes in Limited-Stage Small-Cell Lung Cancer. Oncology 2021, 99, 528–538. [Google Scholar] [CrossRef]
- Usó, M.; Jantus-Lewintre, E.; Bremnes, R.M.; Calabuig, S.; Blasco, A.; Pastor, E.; Borreda, I.; Molina-Pinelo, S.; Paz-Ares, L.; Guijarro, R.; et al. Analysis of the Immune Microenvironment in Resected Non-Small Cell Lung Cancer: The Prognostic Value of Different T Lymphocyte Markers. Oncotarget 2016, 7, 52849–52861. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Michelakos, T.; Deshpande, V.; Arora, K.S.; Yamada, T.; Ting, D.T.; Taylor, M.S.; Castillo, C.F.; Warshaw, A.L.; Lillemoe, K.D.; et al. Role of Tumor-Associated Macrophages in the Clinical Course of Pancreatic Neuroendocrine Tumors (PanNETs). Clin. Cancer Res. 2019, 25, 2644–2655. [Google Scholar] [CrossRef]
- Vigano, L.; Soldani, C.; Franceschini, B.; Cimino, M.; Lleo, A.; Donadon, M.; Roncalli, M.; Aghemo, A.; Di Tommaso, L.; Torzilli, G. Tumor-Infiltrating Lymphocytes and Macrophages in Intrahepatic Cholangiocellular Carcinoma. Impact on Prognosis after Complete Surgery. J. Gastrointest. Surg. 2019, 23, 2216–2224. [Google Scholar] [CrossRef]
- Da Silva, A.; Bowden, M.; Zhang, S.; Masugi, Y.; Thorner, A.R.; Herbert, Z.T.; Zhou, C.W.; Brais, L.; Chan, J.A.; Hodi, F.S.; et al. Characterization of the Neuroendocrine Tumor Immune Microenvironment. Pancreas 2018, 47, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, Y.; Wang, N.; Zhang, X.; Tan, B.; Zhang, G.; Cheng, Y. The Clinical Significance of Tumor-Infiltrating Neutrophils and Neutrophil-to-CD8+ Lymphocyte Ratio in Patients with Resectable Esophageal Squamous Cell Carcinoma. J. Transl. Med. 2014, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Steele, K.E.; Tan, T.H.; Korn, R.; Dacosta, K.; Brown, C.; Kuziora, M.; Zimmermann, J.; Laffin, B.; Widmaier, M.; Rognoni, L.; et al. Measuring Multiple Parameters of CD8+ Tumor-Infiltrating Lymphocytes in Human Cancers by Image Analysis. J. Immunother. Cancer 2018, 6, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, I.; Zhang, D.; Velez, M.; Kovar, S.; Liao, X. Poorly differentiated neuroendocrine carcinomas of the gastrointestinal tract: A single-institute study of 43 cases. Pathol Res Pract. 2021, 226, 153614. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immunooncology Biomarkers Working Group: Part 1: Assessing the Host Immune Response, TILs in Invasive Breast Carcinoma and Ductal Carcinoma in Situ, Metastatic Tumor Deposits and Areas for Further Research. Adv. Anat. Pathol. 2017, 24, 235–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Challoner, B.R.; von Loga, K.; Woolston, A.; Griffiths, B.; Sivamanoharan, N.; Semiannikova, M.; Newey, A.; Barber, L.J.; Mansfield, D.; Hewitt, L.C.; et al. Computational Image Analysis of T-Cell Infiltrates in Resectable Gastric Cancer: Association with Survival and Molecular Subtypes. J. Natl Cancer Inst. 2021, 113, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Heo, Y.J.; Lee, T.; Byeon, S.J.; Kim, E.J.; Shin, H.C.; Kim, B.; Kang, S.Y.; Ha, S.Y.; Kim, K.M. Digital Image Analysis in Pathologist-Selected Regions of Interest Predicts Survival More Accurately than Whole-Slide Analysis: A Direct Comparison Study in 153 Gastric Carcinomas. J. Pathol. Clin. Res. 2021, 7, 42–51. [Google Scholar] [CrossRef]
- Jäntti, T.; Luhtala, S.; Mäenpää, J.; Staff, S. Characterization of Immunoreactivity with Whole-Slide Imaging and Digital Analysis in High-Grade Serous Ovarian Cancer. Tumour Biol. 2020, 42, 1010428320971404. [Google Scholar] [CrossRef]
- Silva, M.A.; Triltsch, N.; Leis, S.; Kanchev, I.; Tan, T.H.; Van Peel, B.; Van Kerckhoven, M.; Deschoolmeester, V.; Zimmermann, J. Biomarker Recommendation for PD-1/PD-L1 Immunotherapy Development in Pediatric Cancer Based on Digital Image Analysis of PD-L1 and Immune Cells. J. Pathol. Clin. Res. 2020, 6, 124–137. [Google Scholar] [CrossRef]
- Ninomiya, Y.; Ozawa, S.; Oguma, J.; Kazuno, A.; Nitta, M.; Kajiwara, H.; Sato, Y. Expression of vasohibin-1 and −2 Predicts Poor Prognosis among Patients with Squamous Cell Carcinoma of the Esophagus. Oncol. Lett. 2018, 16, 5265–5274. [Google Scholar] [CrossRef]
- Konno-Kumagai, T.; Fujishima, F.; Nakamura, Y.; Nakano, T.; Nagai, T.; Kamei, T.; Sasano, H. Programmed death-1 Ligands and Tumor Infiltrating T Lymphocytes in Primary and Lymph Node Metastasis of Esophageal Cancer Patients. Dis. Esophagus. 2019, 32, doy063. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.C.; Bamboat, Z.M.; Maker, A.V.; Shia, J.; Pillarisetty, V.G.; Yopp, A.C.; Hedvat, C.V.; Gonen, M.; Jarnagin, W.R.; Fong, Y.; et al. Regulatory T cell infiltration predicts outcome following resection of colorectal cancer liver metastases. Ann. Surg. Oncol. 2013, 20, 946–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tartour, E.; Pere, H.; Maillere, B.; Terme, M.; Merillon, N.; Taieb, J.; Sandoval, F.; Quintin-Colonna, F.; Lacerda, K.; Karadimou, A.; et al. Angiogenesis and Immunity: A Bidirectional Link Potentially Relevant for the Monitoring of Antiangiogenic Therapy and the Development of Novel Therapeutic Combination with Immunotherapy. Cancer Metastasis Rev. 2011, 30, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Koh, J.; Kim, K.; Chie, E.K.; Kim, B.; Lee, K.B.; Jang, J.Y.; Kim, S.W.; Oh, D.Y.; Bang, Y.J.; et al. High ratio of programmed cell death protein 1 (PD-1) (+)/CD8(+) tumor-infiltrating lymphocytes identifies a poor prognostic subset of extrahepatic bile duct cancer undergoing surgery plus adjuvant chemoradiotherapy. Radiother. Oncol. 2015, 117, 165–170. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Prakhar, P.; Alvarez-Delvalle, J.; Keller, H.; Crossman, A.; Tai, X.; Park, Y.K.; Park, J.H. The Small Intestine Epithelium Exempts Foxp3+ Tregs from Their IL-2 Requirement for Homeostasis and Effector Function. JCI Insight 2021, 6, e149656. [Google Scholar] [CrossRef]
- Watson Ng, W.S.; Hampartzoumian, T.; Lloyd, A.R.; Grimm, M.C. A Murine Model of Appendicitis and the Impact of Inflammation on Appendiceal Lymphocyte Constituents. Clin. Exp. Immunol. 2007, 150, 169–178. [Google Scholar] [CrossRef]
- Garcia, C.R.; Sorbye, H.; Baudin, E.; Raymond, E.; Wiedenmann, B.; Niederle, B.; Sedlackova, E.; Toumpanakis, C.; Anlauf, M.; Cwikla, J.B.; et al. Enets Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology 2017, 103, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Sawa-Wejksza, K.; Dudek, A.; Lemieszek, M.; Kaławaj, K.; Kandefer-Szerszeń, M. Colon Cancer-Derived Conditioned Medium Induces Differentiation of THP-1 Monocytes into a Mixed Population of M1/M2 Cells. Tumour Biol. 2018, 40, 1010428318797880. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.-J.; Lai, W.; Wu, H.; Liu, L.; Xu, H.Y.; Wang, J.; Chu, Z.H. Neuroendocrine-Like Cells -Derived CXCL10 and CXCL11 Induce the Infiltration of Tumor-Associated Macrophage Leading to the Poor Prognosis of Colorectal Cancer. Oncotarget 2016, 7, 27394–27407. [Google Scholar] [CrossRef] [Green Version]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 Polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yu, T.T.; Zhang, D.M.; Hou, X.M.; Liu, X.J.; Zhao, D.; Shan, L. Vasohibin-1 expression detected by immunohistochemistry correlates with prognosis in non-small cell lung cancer. Med. Oncol. 2014, 31, 963. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, T.; Kimura, H.; Heishi, T.; Suzuki, Y.; Miyashita, H.; Ohta, H.; Sonoda, H.; Moriya, T.; Suzuki, S.; Kondo, T.; et al. Vasohibin-1 Expression in Endothelium of Tumor Blood Vessels Regulates Angiogenesis. Am. J. Pathol. 2009, 175, 430–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Alvarez, A.; Hernando Cubero, J.; Capdevila, J. Drug Development in Neuroendocrine Tumors: What Is on the Horizon? Curr. Treat. Options Oncol. 2021, 22, 43. [Google Scholar] [CrossRef]
- Kawasaki, K.; Toshimitsu, K.; Matano, M.; Fujita, M.; Fujii, M.; Togasaki, K.; Ebisudani, T.; Shimokawa, M.; Takano, A.; Takahashi, S.; et al. An Organoid Biobank of Neuroendocrine Neoplasms Enables Genotype-Phenotype Mapping. Cell 2020, 183, 1420–1435.e21. [Google Scholar] [CrossRef]



| Number of Patients | GEP | 33 |
| Age (Years) | Mean (Range) | 72 (56-86) |
| Sex (%) | Male (%) | 26 (79) |
| Female (%) | 7 (21) | |
| Tumor Location (%) | Foregut (%) | 27 (82) |
| Midgut (%) | 2 (6) | |
| Hindgut (%) | 4 (12) | |
| Treatment (%) | Operation (%) | 31 (94) |
| ESD (%) | 2 (6) | |
| Histopathological Type (NEC) (%) | Small cell type (%) | 20 (60) |
| Large cell type (%) | 13 (40) | |
| Histopathological Type (non-NEC) (%) | well differentiated (%) | 14 (42) |
| moderately differentiated (%) | 13 (40) | |
| poorly differentiated (%) | 5 (15) | |
| mucinous (%) | 1 (3) | |
| Lymphatic Invasion (%) | ly− (%) | 13 (40) |
| ly+ (%) | 20 (60) | |
| Venous Invasion (%) | v− (%) | 13 (40) |
| v+ (%) | 20 (60) | |
| pT (%) | T1-2 (%) | 22 (67) |
| T3-4 (%) | 11 (33) | |
| pN (%) | pN− (%) | 17 (52) |
| pN+ (%) | 16 (48) |
| Antibody | Vendor | Host | Antigen Retrieval | Dilution | Buffer pH | Reaction Time of Primary Antibody | Secondary Antibody |
|---|---|---|---|---|---|---|---|
| CD3 | DAKO | Rabbit | AC, 121 °C, 5 min | Ready to use | 9.0 | 4 °C, overnight | EnVision FLEX |
| CD4 | Nichirei | Mouse | AC, 121 °C, 5 min | 1/80 | 9.0 | 4 °C, overnight | Histofine |
| CD8 | DAKO | Mouse | AC, 121 °C, 5 min | 1/50 | 7.0 | 4 °C, overnight | EnVision FLEX |
| CD34 | Nichirei | Mouse | none | 1/200 | none | 4 °C, overnight | Histofine |
| CD68 | DAKO | Mouse | Trypsin (37 °C, 15 min) | 1/200 | none | 4 °C, overnight | Histofine |
| CD163 | Leica microsystem | Mouse | AC, 121 °C, 5 min | 1/600 | 6.0 | 4 °C, overnight | Histofine |
| Foxp3 | Abcam | Mouse | AC, 121 °C, 5 min | 1/200 | 6.0 | 4 °C, overnight | Histofine |
| PD-1 | Abcam | Mouse | AC, 121 °C, 5 min | 1/100 | 6.0 | 4 °C, overnight | Histofine |
| PD-L1 | Roche, sp263 | Rabbit | unknown | RTV | unknown | unknown | VENTANA, Optiview DAB Universal Kit |
| SYN | DAKO | Mouse | AC, 121 °C, 5 min | 1/300 | 6.0 | 4 °C, overnight | Histofine |
| ChgA | Agilent technologies | Rabbit | Microwave (210 W, 15 min) | 1/1500 | 6.0 | 4 °C, overnight | Histofine |
| INSM1 | Santacruz | Mouse | AC, 121 °C, 5 min | 1/200 | 6.0 | 4 °C, overnight | Histofine |
| Ki-67 | DAKO | Mouse | PT Link (97 °C, 20 min), Target Retrieval Solution | Ready to use | High pH | 20 min, room temperature | EnVision FLEX |
| VASH-1 | Donated | Mouse | AC, 121 °C, 5 min | 1/400 | 8.0 | 4 °C, overnight | Histofine |
| Antibodies | NEC-Non-NEC | |||
|---|---|---|---|---|
| Intra-Tumoral * | NEC > Non-NEC (%) | Invasive Margin * | NEC > Non-NEC (%) | |
| CD3 | 9.33 ± 359.25 | 54.5 | 43.33 ± 332.71 | 57.6 |
| CD4 | −0.67 ± 142.78 | 42.4 | 0 ± 108.94 | 39.4 |
| CD8 | −9.67 ± 277.47 | 36.4 | 0.67 ± 199.06 | 51.5 |
| Foxp3 | 5.00 ± 75.63 | 54.5 | 0.33 ± 68.18 | 48.5 |
| PD-1 | 0 ± 38.43 | 36.4 | 0 ± 23.76 | 45.5 |
| CD68 | 66.00 ± 224.67 | 60.6 | 58.67 ± 237.92 | 75.8 |
| CD163 | 16.67 ± 154.52 | 66.7 | 2.33 ± 103.05 | 51.5 |
| MVD | 4.00 ± 44.38 | 57.6 | - | - |
| VASH-1 density | 9.00 ± 16.77 | 72.7 | - | - |
| VASH-1 expression (%) | 15.00 ± 26.71 | 72.7 | - | - |
| CD4/CD3 | −0.0057 ± 1.18 | 45.5 | −0.0017 ± 0.50 | 33.3 |
| CD8/CD3 | −0.077 ± 0.84 | 39.4 | −0.052 ± 1.33 | 45.5 |
| Foxp3/CD4 | 0 ± 34.15 | 45.5 | 0.074 ± 26.88 | 51.5 |
| Foxp3/CD8 | 0.32 ± 2.82 | 57.6 | 0.034 ± 0.77 | 57.6 |
| CD163/CD68 | −0.041 ± 11.63 | 39.4 | −0.14 ± 0.86 | 30.3 |
| PD-1/CD4 | 0 ± 2.50 | 36.4 | 0 ± 7.55 | 45.5 |
| PD-1/CD8 | 0 ± 0.27 | 42.4 | 0 ± 0.058 | 42.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsunokake, J.; Fujishima, F.; Watanabe, H.; Sato, I.; Miura, K.; Sakamoto, K.; Suzuki, H.; Sawai, T.; Itakura, Y.; Hoshi, T.; et al. Tumor Microenvironment in Mixed Neuroendocrine Non-Neuroendocrine Neoplasms: Interaction between Tumors and Immune Cells, and Potential Effects of Neuroendocrine Differentiation on the Tumor Microenvironment. Cancers 2022, 14, 2152. https://doi.org/10.3390/cancers14092152
Tsunokake J, Fujishima F, Watanabe H, Sato I, Miura K, Sakamoto K, Suzuki H, Sawai T, Itakura Y, Hoshi T, et al. Tumor Microenvironment in Mixed Neuroendocrine Non-Neuroendocrine Neoplasms: Interaction between Tumors and Immune Cells, and Potential Effects of Neuroendocrine Differentiation on the Tumor Microenvironment. Cancers. 2022; 14(9):2152. https://doi.org/10.3390/cancers14092152
Chicago/Turabian StyleTsunokake, Junichi, Fumiyoshi Fujishima, Hirofumi Watanabe, Ikuro Sato, Koh Miura, Kazuhiro Sakamoto, Hiroyoshi Suzuki, Takashi Sawai, Yuko Itakura, Tatsuya Hoshi, and et al. 2022. "Tumor Microenvironment in Mixed Neuroendocrine Non-Neuroendocrine Neoplasms: Interaction between Tumors and Immune Cells, and Potential Effects of Neuroendocrine Differentiation on the Tumor Microenvironment" Cancers 14, no. 9: 2152. https://doi.org/10.3390/cancers14092152
APA StyleTsunokake, J., Fujishima, F., Watanabe, H., Sato, I., Miura, K., Sakamoto, K., Suzuki, H., Sawai, T., Itakura, Y., Hoshi, T., Kunimitsu, A., Yamauchi, T., Akaishi, R., Ozawa, Y., Fukutomi, T., Okamoto, H., Sato, C., Taniyama, Y., Kamei, T., & Sasano, H. (2022). Tumor Microenvironment in Mixed Neuroendocrine Non-Neuroendocrine Neoplasms: Interaction between Tumors and Immune Cells, and Potential Effects of Neuroendocrine Differentiation on the Tumor Microenvironment. Cancers, 14(9), 2152. https://doi.org/10.3390/cancers14092152






