CD229 (Ly9) a Novel Biomarker for B-Cell Malignancies and Multiple Myeloma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of an Anti-CD229 mAb for Immunohistology
2.2. Ly9 Gene Inactivation Using CRISPR/Cas9 Technology
2.3. Western Blot
2.4. Human Tissues and Cell Lines
2.5. Immunohistochemistry
2.6. Scoring CD229 Expression
2.7. Immunofluorescence
2.8. Flow Cytometry
2.9. Myeloma Patients
2.10. Soluble CD229 ELISA
2.11. Statistical Analysis
3. Results
3.1. CD229 Tissue Expression is Restricted to Hematopoietic Cells
3.2. CD229 Expression on B Cell Linage Lymphomas and Myeloma
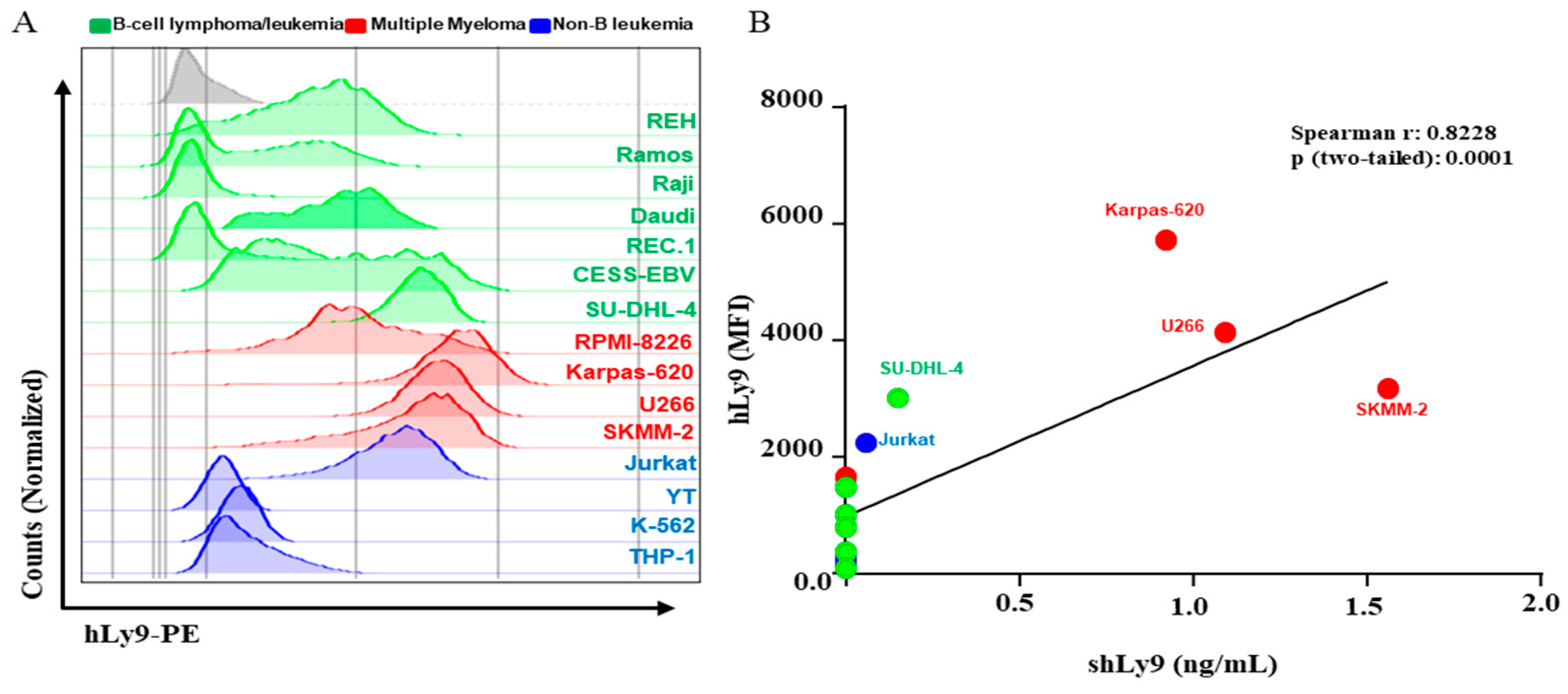
3.3. Soluble CD229 (sCD229) Is Secreted by B-Cell Lymphoma and Myeloma Cells Lines
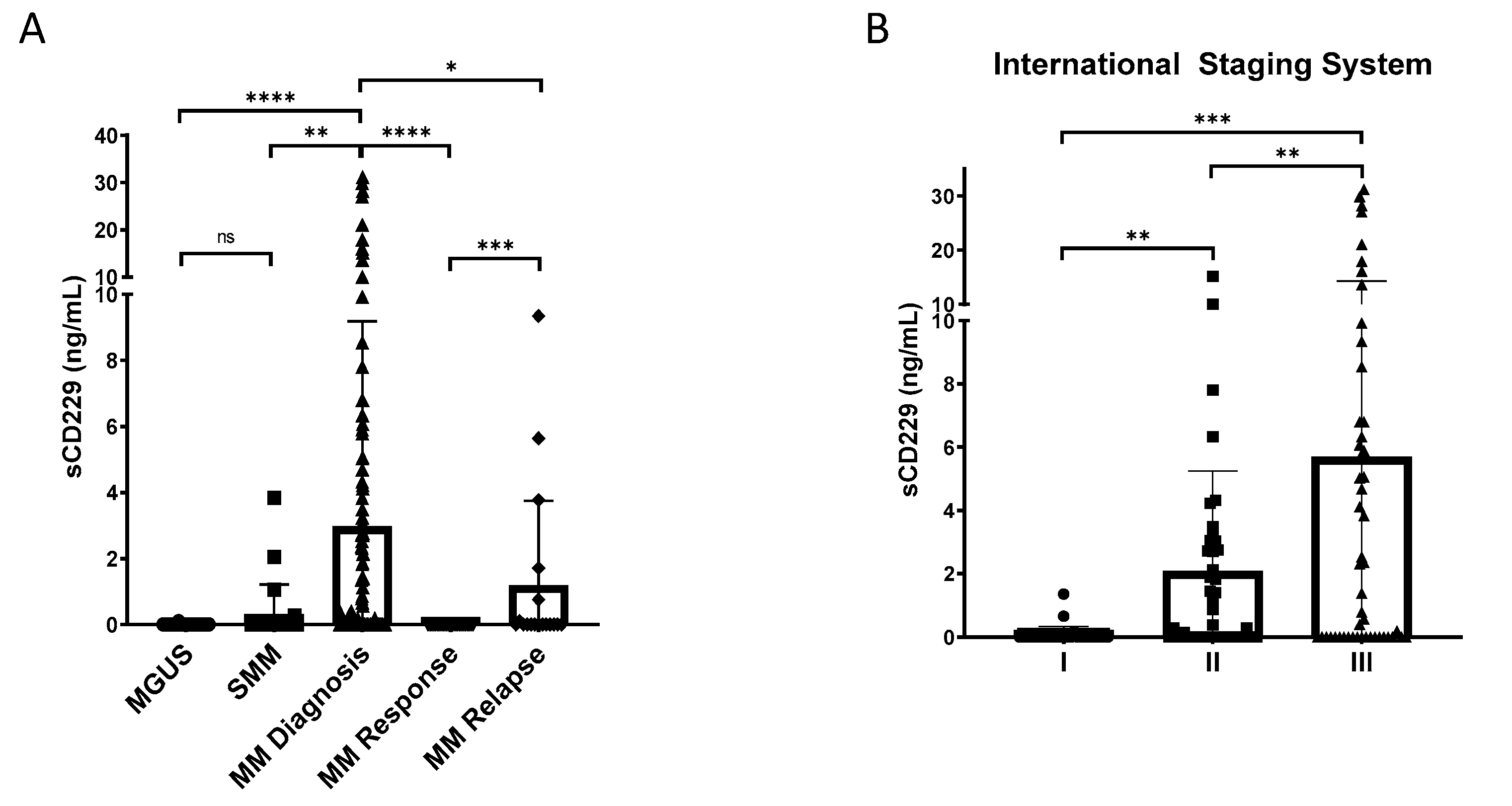
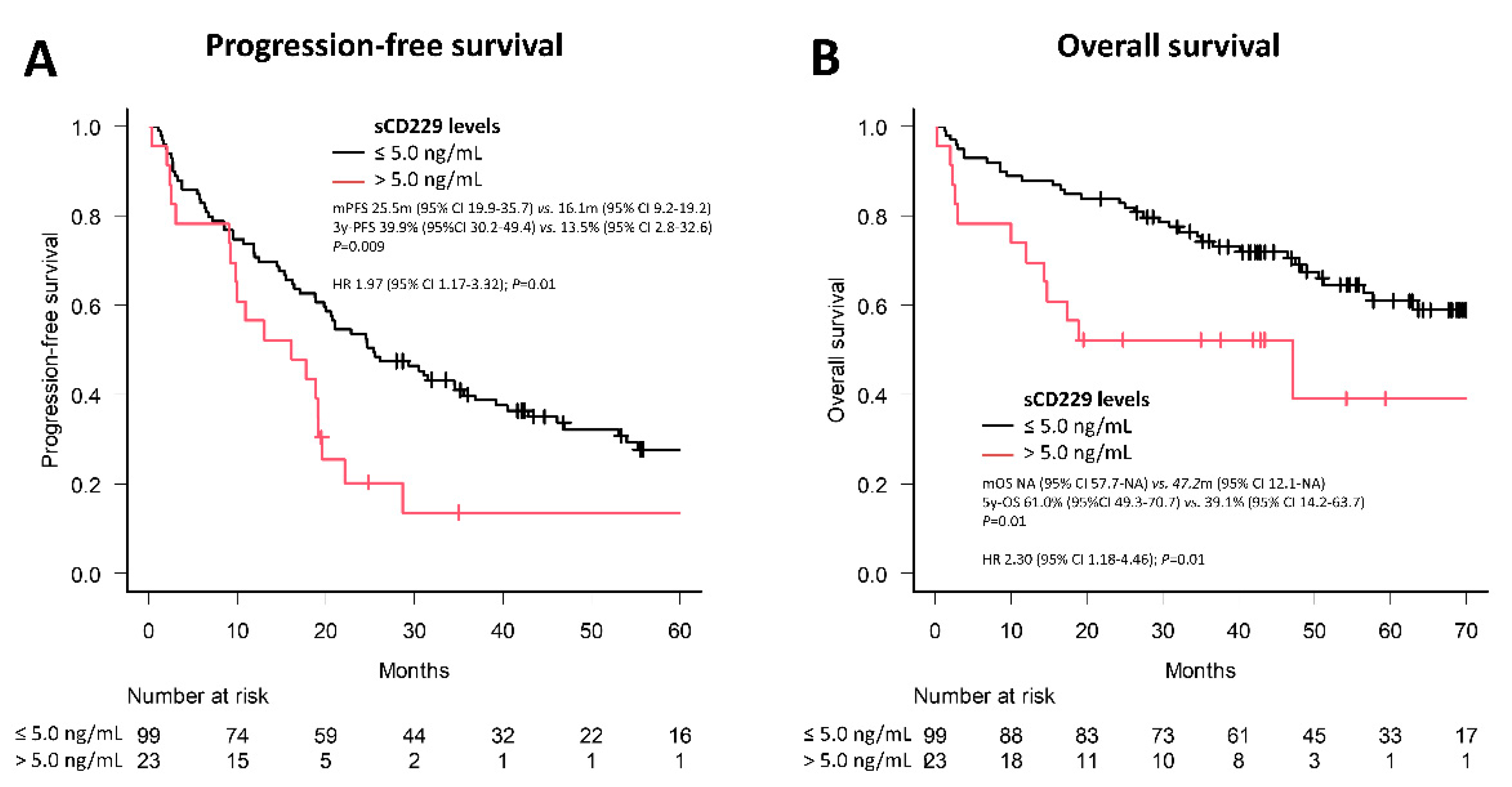
3.4. Soluble CD229 as a Biomarker in MM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engel, P.; Eck, M.J.; Terhorst, C. The SAP and SLAM families in immune responses and X-linked lymphoproliferative disease. Nat. Rev. Immunol. 2003, 3, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Cannons, J.L.; Tangye, S.G.; Schwartzberg, P.L. SLAM family receptors and SAP adaptors in immunity. Annu. Rev. Immunol. 2011, 29, 665–705. [Google Scholar] [CrossRef] [PubMed]
- Romero, X.; Sintes, J.; Engel, P. Role of SLAM family receptors and specific adapter SAP in innate-like lymphocytes. Crit. Rev. Immunol. 2014, 34, 263–299. [Google Scholar] [CrossRef] [PubMed]
- Romero, X.; Zapater, N.; Calvo, M.; Kalko, S.G.; De La Fuente, M.A.; Tovar, V.; Ockeloen, C.; Pizcueta, P.; Engel, P. CD229 (Ly9) lymphocyte cell surface receptor interacts homophilically through its N-terminal domain and relocalizes to the immunological synapse. J. Immunol. 2005, 174, 7033–7042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De La Fuente, M.A.; Tovar, V.; Villamor, N.; Zapater, N.; Pizcueta, P.; Campo, E.; Bosch, J.; Engel, P. Molecular characterization and expression of a novel human leukocyte cell-surface marker homologous to mouse Ly-9. Blood 2001, 97, 3513–3520. [Google Scholar] [CrossRef]
- Sayós, J.; Martín, M.; Chen, A.; Simarro, M.; Howie, D.; Morra, M.; Engel, P.; Terhorst, C. Cell surface receptors Ly-9 and CD84 recruit the X-linked lymphoproliferative disease gene product SAP. Blood 2001, 97, 3867–3874. [Google Scholar] [CrossRef] [PubMed]
- Morra, M.; Lu, J.; Poy, F.; Martin, M.; Sayos, J.; Calpe, S.; Gullo, C.; Howie, D.; Rietdijk, S.; Thompson, A.; et al. Structural basis for the interaction of the free SH2 domain EAT-2 with SLAM receptors in hematopoietic cells. EMBO J. 2001, 20, 5840–5852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morra, M.; Simarro-Grande, M.; Martin, M.; Chen, A.S.; Lanyi, A.; Silander, O.; Calpe, S.; Davis, J.; Pawson, T.; Eck, M.J.; et al. Characterization of SH2D1A missense mutations identified in X-linked lymphoproliferative disease patients. J. Biol. Chem. 2001, 276, 36809–36816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Iosef, C.; Jia, C.Y.; Han, V.K.; Li, S.S. Dual functional roles for the X-linked lymphoproliferative syndrome gene product SAP/SH2D1A in signaling through the signaling lymphocyte activation molecule (SLAM) family of immune receptors. J. Biol. Chem. 2003, 278, 3852–3859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sintes, J.; Cuenca, M.; Romero, X.; Bastos, R.; Terhorst, C.; Angulo, A.; Engel, P. Cutting edge: Ly9 (CD229), a SLAM family receptor, negatively regulates the development of thymic innate memory-like CD8+ T and invariant NKT cells. J. Immunol. 2013, 190, 21–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuenca, M.; Puñet-Ortiz, J.; Ruart, M.; Terhorst, C.; Engel, P. Ly9 (SLAMF3) receptor differentially regulates iNKT cell development and activation in mice. Eur. J. Immunol. 2018, 48, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Cuenca, M.; Romero, X.; Sintes, J.; Terhorst, C.; Engel, P. Targeting of Ly9 (CD229) Disrupts Marginal Zone and B1 B Cell Homeostasis and Antibody Responses. J. Immunol. 2016, 196, 726–737. [Google Scholar] [CrossRef] [PubMed]
- De Salort, J.; Cuenca, M.; Terhorst, C.; Engel, P.; Romero, X. Ly9 (CD229) Cell-Surface Receptor is Crucial for the Development of Spontaneous Autoantibody Production to Nuclear Antigens. Front. Immunol. 2013, 4, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sintes, J.; Vidal-Laliena, M.; Romero, X.; Tovar, V.; Engel, P. Characterization of mouse CD229 (Ly9), a leukocyte cell surface molecule of the CD150 (SLAM) family. Tissue Antigens 2007, 70, 355–362. [Google Scholar] [CrossRef]
- Romero, X.; Benítez, D.; March, S.; Vilella, R.; Miralpeix, M.; Engel, P. Differential expression of SAP and EAT-2-binding leukocyte cell-surface molecules CD84, CD150 (SLAM), CD229 (Ly9) and CD244 (2B4). Tissue Antigens 2004, 64, 132–144. [Google Scholar] [CrossRef] [PubMed]
- De Salort, J.; Sintes, J.; Llinàs, L.; Matesanz-Isabel, J.; Engel, P. Expression of SLAM (CD150) cell-surface receptors on human B-cell subsets: From pro-B to plasma cells. Immunol. Lett. 2011, 134, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Atanackovic, D.; Panse, J.; Hildebrandt, Y.; Jadczak, A.; Kobold, S.; Cao, Y.; Templin, J.; Meyer, S.; Reinhard, H.; Bartels, K.; et al. Surface molecule CD229 as a novel target for the diagnosis and treatment of multiple myeloma. Haematologica 2011, 96, 1512–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muccio, V.E.; Saraci, E.; Gilestro, M.; Gattei, V.; Zucchetto, A.; Astolfi, M.; Ruggeri, M.; Marzanati, E.; Passera, R.; Palumbo, A.; et al. Multiple myeloma: New surface antigens for the characterization of plasma cells in the era of novel agents. Cytometry B Clin Cytom 2016, 90, 81–90. [Google Scholar] [CrossRef]
- Pojero, F.; Flores-Montero, J.; Sanoja, L.; Pérez, J.J.; Puig, N.; Paiva, B.; Bottcher, S.; van Dongen, J.J.; Orfao, A.; EuroFlow group. Utility of CD54, CD229, and CD319 for the identification of plasma cells in patients with clonal plasma cell diseases. Cytometry B Clin. Cytom. 2016, 90, 91–100. [Google Scholar] [CrossRef]
- Sintes, J.; Romero, X.; Marin, P.; Terhorst, C.; Engel, P. Differential expression of CD150 (SLAM) family receptors by human hematopoietic stem and progenitor cells. Exp. Hematol. 2008, 36, 1199–1204. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Lage, M.; Torres-Ruiz, R. Rodriguez-Perales, S. CRISPR/Cas9 Technology: Applications and Human Disease Modeling. Prog. Mol. Biol. Transl. Sci. 2017, 152, 23–48. [Google Scholar] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Hans, C.P.; Weisenburger, D.D.; Greiner, T.C.; Gascoyne, R.D.; Delabie, J.; Ott, G.; Müller-Hermelink, H.K.; Campo, E.; Braziel, R.M.; Jaffe, E.S.; et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004, 103, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Chtanova, T.; Tangye, S.G.; Newton, R.; Frank, N.; Hodge, M.R.; Rolph, M.S.; Mackay, C.R. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J. Immunol. 2004, 173, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.; Radhakrishnan, S.V.; Luetkens, T.; Atanackovic, D. The role of surface molecule CD229 in Multiple Myeloma. Clin. Immunol. 2019, 204, 69–73. [Google Scholar] [CrossRef]
- Kovacsovics, S.; Kovacsovics-Bankowski, M.; Salama, M.E.; Bhardwaj, N.; Steinbach, M.; Langemo, A.; Kovacsovics, T.; Marvin, J.; Binder, M.; Panse, J.; et al. CD229 is expressed on the surface of plasma cells carrying an aberrant phenotype and chemotherapy-resistant precursor cells in multiple myeloma. Hum. Vaccin. Immunother. 2015, 11, 1606–1611. [Google Scholar]
- Ishibashi, M.; Takahashi, R.; Tsubota, A.; Sasaki, M.; Handa, H.; Imai, Y.; Tanaka, N.; Tsukune, Y.; Tanosaki, S.; Ito, S.; et al. SLAMF3-Mediated Signaling via ERK Pathway Activation Promotes Aggressive Phenotypic Behaviors in Multiple Myeloma. Mol. Cancer Res. MCR. 2020, 18, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Brudno, J.N.; Maric, I.; Hartman, S.D.; Rose, J.J.; Wang, M.; Lam, N.; Kovacsovics, T.; Marvin, J.; Binder, M.; Panse, J.; et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J. Clin. Oncol. 2018, 36, 2267–2280. [Google Scholar] [CrossRef]
- Cohen, A.D.; Garfall, A.L.; Stadtmauer, E.A.; Melenhorst, J.J.; Lacey, S.F.; Lancaster, E.; Vogl, D.T.; Weiss, B.M.; Dengel, K.; Nelson, A.; et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Invest. 2019, 129, 2210–2221. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Lobato, L.G.; Ganzetti, M.; Fernández de Larrea, C.; Hudecek, M.; Einsele, H.; Danhof, S. CAR T-Cells in Multiple Myeloma: State of the Art and Future Directions. Front. Oncol. 2020, 10, 1243. [Google Scholar] [CrossRef] [PubMed]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, M.; Xu, N.; Ng, N.; Sanchez, E.; Soof, C.M.; Patil, S.; Udd, K.; Bujarski, S.; Cao, J.; et al. Serum B-cell maturation antigen (BCMA) reduces binding of anti-BCMA antibody to multiple myeloma cells. Leuk. Res. 2019, 81, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Ghermezi, M.; Li, M.; Vardanyan, S.; Harutyunyan, N.M.; Gottlieb, J.; Berenson, A.; Andreu-Vieyra, C.; Petraki, S.; Sanchez, E.; Udd, K.; et al. Serum B-cell maturation antigen: A novel biomarker to predict outcomes for multiple myeloma patients. Haematologica 2017, 102, 785–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radhakrishnan, S.V.; Luetkens, T.; Scherer, S.D.; Davis, P.; Mause, E.R.V.; Olson, M.L.; Yousef, S.; Panse, J.; Abdiche, Y.; Li, K.D.; et al. CD229 CAR T cells eliminate multiple myeloma and tumor propagating cells without fratricide. Nat. Commun. 2020, 11, 798. [Google Scholar] [CrossRef] [PubMed]
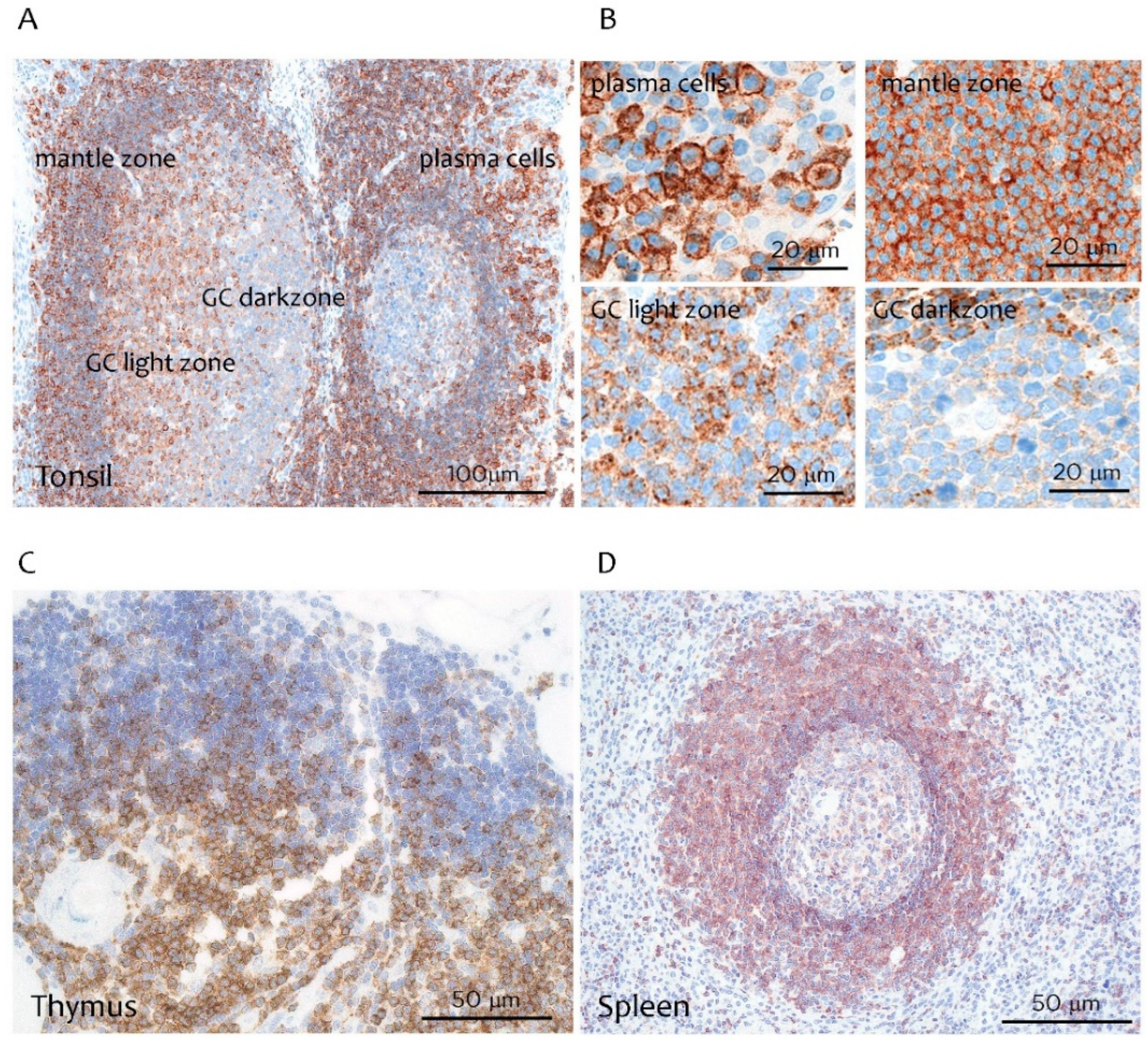
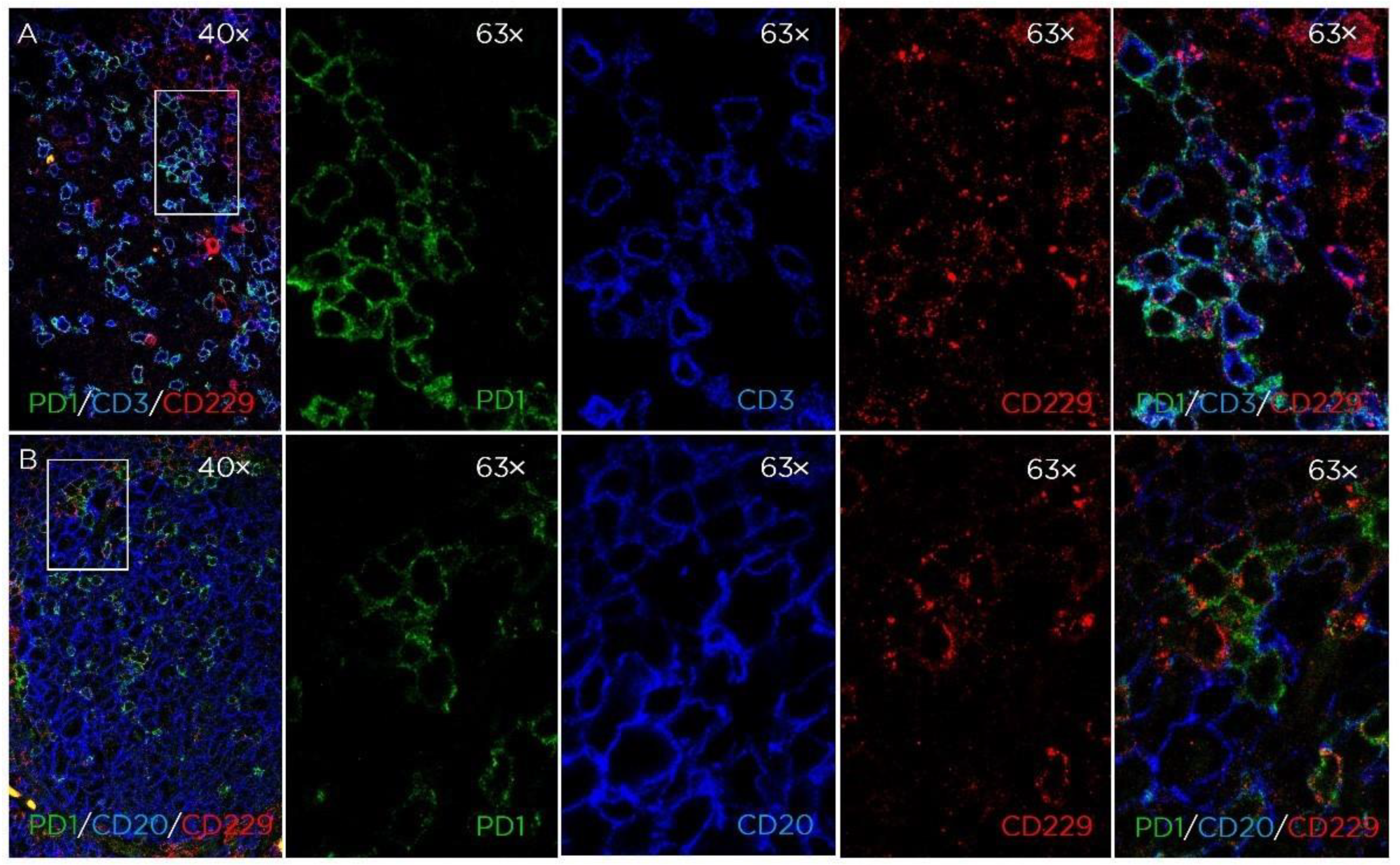
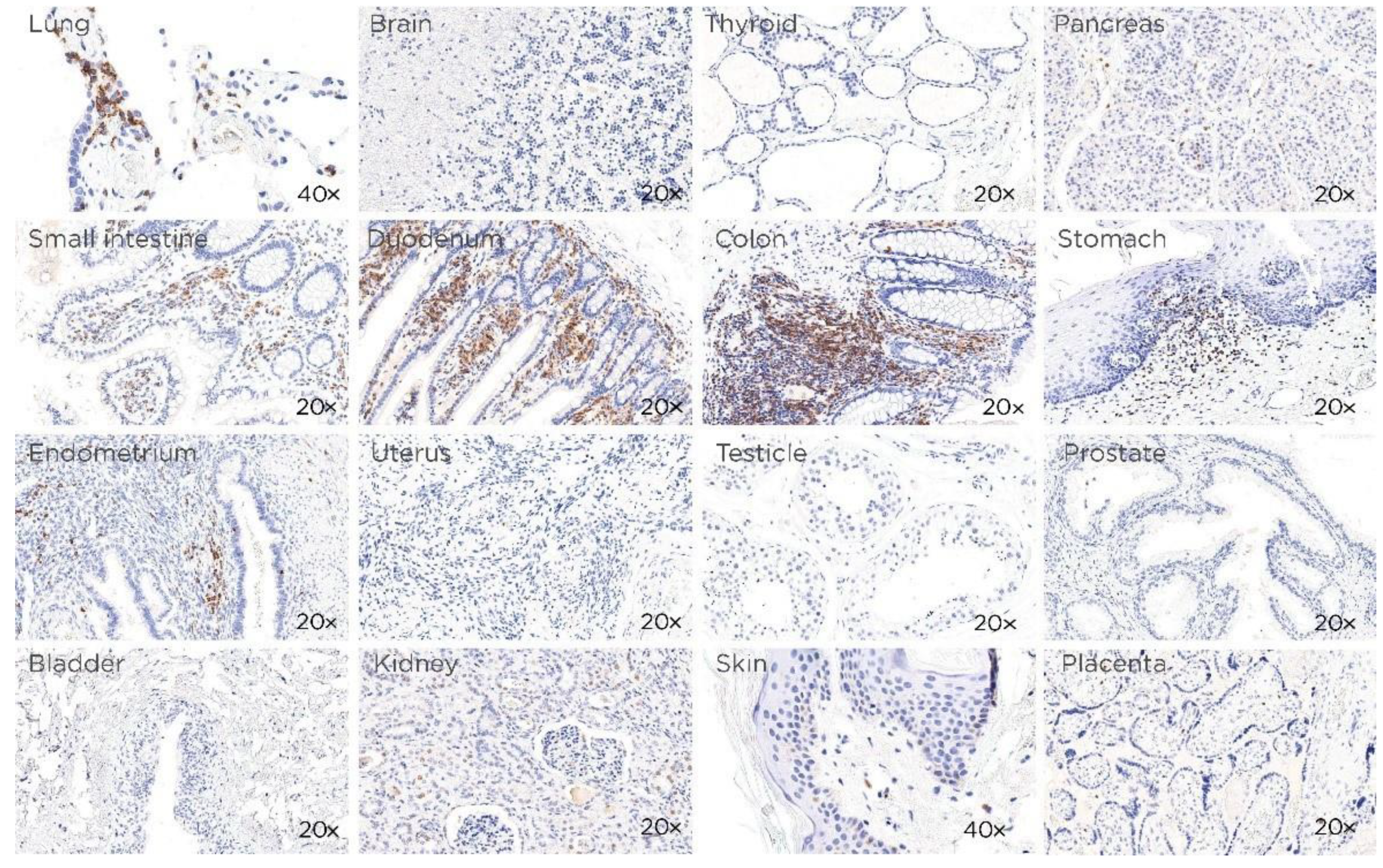
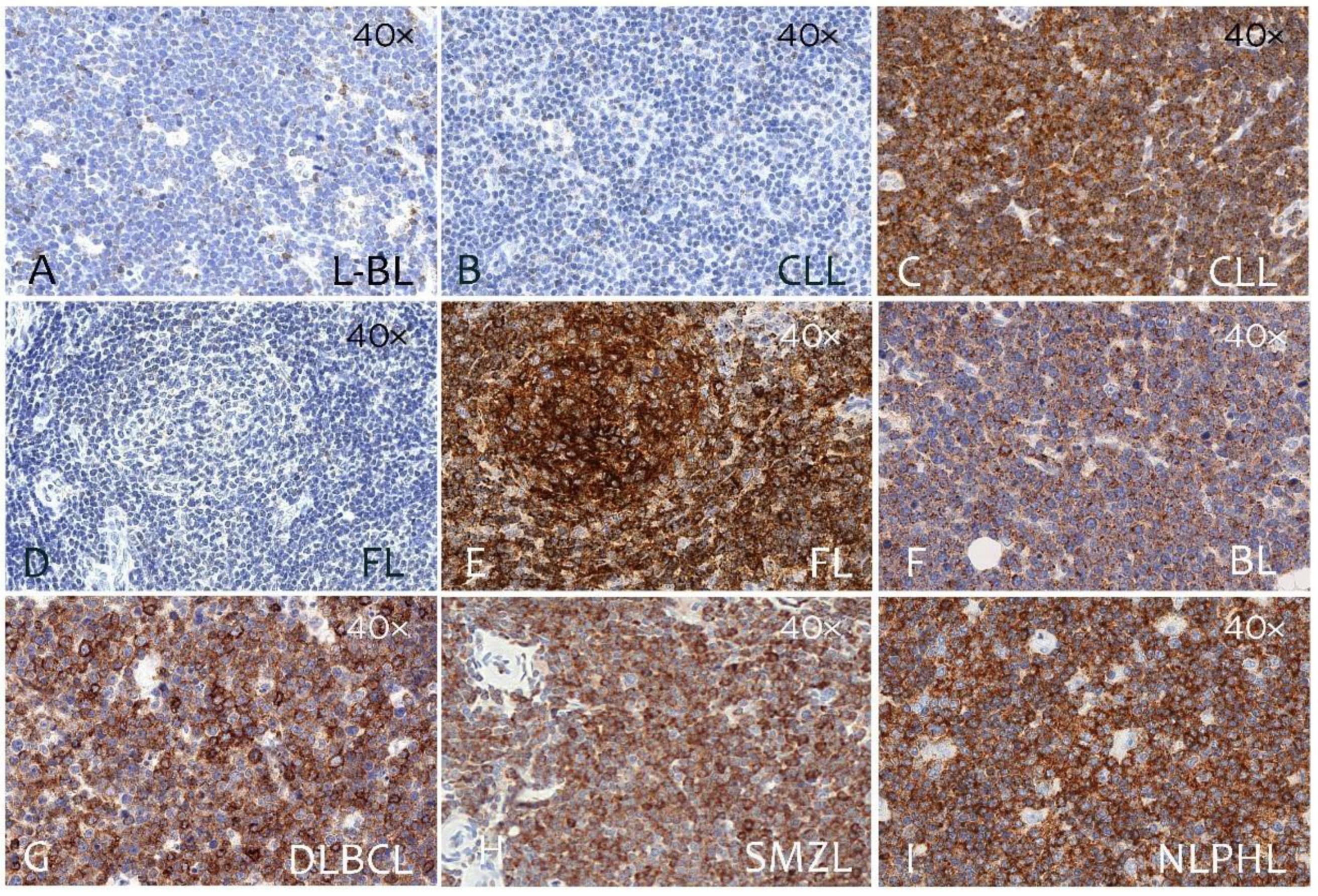

| Types of Lymphoma | No. Cases | Positive Cases | Negative Cases | % Positive Cases |
|---|---|---|---|---|
| Precursor neoplasms | ||||
| Precursor B lymphoblastic lymphoma | 7 | 2 | 5 | 28% |
| Mature B-Cell Neoplasms | ||||
| Chronic lymphocytic leukemia | 20 | 14 | 6 | 70% |
| Follicular lymphoma | 20 | 12 | 8 | 60% |
| Classic mantle-cell lymphoma | 20 | 14 | 6 | 70% |
| Diffuse large B-cell lymphoma GCB Type | 29 | 19 | 10 | 66% |
| Diffuse large B-cell lymphoma Non-GCB type | 19 | 10 | 9 | 52% |
| Burkitt lymphoma | 20 | 4 | 16 | 20% |
| Nodal marginal-zone lymphoma | 34 | 28 | 6 | 82% |
| MALT lymphoma | 28 | 24 | 4 | 86% |
| Splenic marginal-zone lymphoma | 15 | 13 | 2 | 87% |
| Multiple myeloma 1 | 20 | 20 | 0 | 100% |
| Hodgkin Lymphomas | ||||
| Nodular lymphocyte-predominant HL | 7 | 0 | 7 | 0% |
| Lymphocyte-rich cHL | 3 | 0 | 3 | 0% |
| Nodular sclerosis cHL | 10 | 0 | 10 | 0% |
| Mixed cellularity cHL | 8 | 0 | 8 | 0% |
| Characteristics | sCD229 ≤ 5.0 ng/mL (n = 99) | sCD229 > 5.0 ng/mL (n = 23) | p |
|---|---|---|---|
| Gender, male, n (%) | 58 (58.6) | 11 (47.8) | 0.36 |
| Age, median (IQR) | 65 (56–73) | 69 (66.5–78.5) | 0.01 |
| Immunological subtype, n (%) | 0.14 | ||
| IgG | 60 (60.6) | 10 (43.5) | |
| IgA | 20 (20.2) | 9 (39.1) | |
| Bence Jones | 16 (16.2) | 3 (13.0) | |
| Light chain isotype, n (%) | 0.09 | ||
| Kappa | 65 (65.7) | 10 (43.5) | |
| Lambda | 30 (30.3) | 12 (52.2) | |
| Hemoglobin g/L, median (IQR) | 10.8 (10–12.1) | 9.6 (8.85–11.4) | 0.01 |
| Platelets 109/L, median (IQR) | 214 (175–171) | 177 (129–207) | 0.005 |
| Calcium mg/dL, median (IQR) | 9.3 (8.85–9.8) | 9.8 (9.1–10.7) | 0.10 |
| Creatinine mg/dL, median (IQR) | 0.88 (0.70–1.26) | 1.16 (1.04–1.42) | 0.003 |
| Albumin g/L, median (IQR) | 37 (33–42) | 32 (28–37) | 0.007 |
| β2-microglobulin mg/L, median (IQR) | 3.85 (2.8–6.1) | 8.3 (5.8–11.1) | <0.001 |
| Lactate dehydrogensase ≥ ULN, n (%) | 11 (11.1) | 5 (21.7) | 0.18 |
| Bone lytic lesions, n (%) | 69 (70.4) | 12 (54.5) | 0.35 |
| Bone marrow plasma cells, median (IQR) | 28 (13–49.5) | 63 (50–73) | <0.001 |
| ECOG PS > 2, n (%) | 15 (15.3) | 6 (26.1) | 0.23 |
| International Staging System, n (%) | <0.001 | ||
| I | 32 (32.7) | 0 (0) | |
| II | 35 (35.7) | 4 (17.4) | |
| III | 31 (31.6) | 19 (82.6) | |
| Cytogenetic abnormalities | 0.47 | ||
| t(4;14) | 3 (3.0) | 1 (4.3) | |
| t(11;14) | 12 (12.1) | 2 (8.7) | |
| t(14;16) | 2 (2.0) | 1 (4.3) | |
| gain(1q+) | 3 (3.0) | 0 (0) | |
| del(17p) | 0 (0) | 0 (0) | |
| Response, n (%) | 0.80 | ||
| Overall response rate | 73 (73.7) | 16 (69.6) | |
| Complete remission rate | 19 (19.2) | 4 (17.4) | 1.00 |
| Induction treatment, n (%) | 0.32 | ||
| Chemotherapy-based | 9 (9.1) | 3 (13.6) | |
| PI-based | 32 (32.3) | 7 (31.8) | |
| IMID-based | 9 (9.1) | 5 (22.7) | |
| PI + IMI-based | 40 (40.4) | 5 (22.7) | |
| Daratumumab-based | 6 (6.1) | 1 (4.5) | |
| Elotuzumab-based | 3 (3.0) | 1 (4.5) | |
| Autologous stem cell transplantation, n (%) | 49 (49.5) | 3 (13) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roncador, G.; Puñet-Ortiz, J.; Maestre, L.; Rodríguez-Lobato, L.G.; Jiménez, S.; Reyes-García, A.I.; García-González, Á.; García, J.F.; Piris, M.Á.; Montes-Moreno, S.; et al. CD229 (Ly9) a Novel Biomarker for B-Cell Malignancies and Multiple Myeloma. Cancers 2022, 14, 2154. https://doi.org/10.3390/cancers14092154
Roncador G, Puñet-Ortiz J, Maestre L, Rodríguez-Lobato LG, Jiménez S, Reyes-García AI, García-González Á, García JF, Piris MÁ, Montes-Moreno S, et al. CD229 (Ly9) a Novel Biomarker for B-Cell Malignancies and Multiple Myeloma. Cancers. 2022; 14(9):2154. https://doi.org/10.3390/cancers14092154
Chicago/Turabian StyleRoncador, Giovanna, Joan Puñet-Ortiz, Lorena Maestre, Luis Gerardo Rodríguez-Lobato, Scherezade Jiménez, Ana Isabel Reyes-García, Álvaro García-González, Juan F. García, Miguel Ángel Piris, Santiago Montes-Moreno, and et al. 2022. "CD229 (Ly9) a Novel Biomarker for B-Cell Malignancies and Multiple Myeloma" Cancers 14, no. 9: 2154. https://doi.org/10.3390/cancers14092154
APA StyleRoncador, G., Puñet-Ortiz, J., Maestre, L., Rodríguez-Lobato, L. G., Jiménez, S., Reyes-García, A. I., García-González, Á., García, J. F., Piris, M. Á., Montes-Moreno, S., Rodríguez-Justo, M., Mena, M.-P., Fernández de Larrea, C., & Engel, P. (2022). CD229 (Ly9) a Novel Biomarker for B-Cell Malignancies and Multiple Myeloma. Cancers, 14(9), 2154. https://doi.org/10.3390/cancers14092154








