Simple Summary
Cancers from the bile ducts and gall bladder are lethal. Cure by surgery is not possible in most of these tumors as they are often identified in later stages. Unlike other cancers, they have few chemotherapy treatment options. Multiple clinical trials in the past decade failed and could not replace the combination of two drugs, gemcitabine and cisplatin, as the preferred drug in new cases. Patients who fail to respond to this combination do not have reliable treatment options. The success of therapy directed at a specific genetic change (mutation) and activating the patient’s immune system (alone or in combination with chemotherapy) are encouraging. If we follow the current trials, the focus is on these newer treatments, with there being a high chance they may replace traditional chemotherapy in the future.
Abstract
Biliary tract cancers (BTC) are often diagnosed at advanced stages and have a grave outcome due to limited systemic options. Gemcitabine and cisplatin combination (GC) has been the first-line standard for more than a decade. Second-line chemotherapy (CT) options are limited. Targeted therapy or TT (fibroblast growth factor 2 inhibitors or FGFR2, isocitrate dehydrogenase 1 or IDH-1, and neurotrophic tyrosine receptor kinase or NTRK gene fusions inhibitors) have had reasonable success, but <5% of total BTC patients are eligible for them. The use of immune checkpoint inhibitors (ICI) such as pembrolizumab is restricted to microsatellite instability high (MSI-H) patients in the first line. The success of the TOPAZ-1 trial (GC plus durvalumab) is promising, with numerous trials underway that might soon bring targeted therapy (pemigatinib and infrigatinib) and ICI combinations (with CT or TT in microsatellite stable cancers) in the first line. Newer targets and newer agents for established targets are being investigated, and this may change the BTC management landscape in the coming years from traditional CT to individualized therapy (TT) or ICI-centered combinations. The latter group may occupy major space in BTC management due to the paucity of targetable mutations and a greater toxicity profile.
Keywords:
cholangiocarcinoma; gall bladder cancer; FGFR2; pemigatinib; infrigatinib; HER2; durvalumab; gemcitabine; NTRK; IDH 1. Introduction
Biliary tract cancers (BTC) comprise a group of malignancies originating in the epithelium of the biliary tract [1]. These include cholangiocarcinoma (CCA) and gallbladder carcinoma (GBC). Intrahepatic cholangiocarcinoma or iCCA refers to tumors proximal to the second-order ducts, while extrahepatic cholangiocarcinoma or eCCA refers to tumors arising more distally (perihilar CCA, between second-order ducts and cystic duct and distal CCA, distal to cystic duct) [2]. Perihilar CCA represents 50% of the total CCAs, with distal lesions comprising 40% and the final 10% being intrahepatic [3]. BTCs are relatively rare in developed countries, comprising approximately 3% of gastrointestinal malignancies with an incidence of 0.35 to 2 in 100,000 [4]. In developing countries such as China and Thailand, the incidence can be as high as 14–80 in 100,000. GBCs are less common, with an incidence of 1 in 100,000 in the USA but increasing as high as 27 in 100,000 in Chile [5,6]. Risk factors for CCAs include primary sclerosing cholangitis, choledochal cysts, cholelithiasis, hepatolithiasis, chronic liver disease, genetic conditions such as Lynch syndrome, BRCA mutations, cystic fibrosis, biliary papillomatosis, and liver fluke infection in endemic regions [7,8]. Risk factors for GBC include cholelithiasis, chronic infection with pathogens such as salmonella and Helicobacter pylori, obesity, and anatomical changes in the biliary tree [9]. The continued rise of CCAs, specifically iCCA, in the past four decades globally is concerning [10,11,12]. Its association with metabolic and infectious risk factors might be the primary reason for this dangerous trend.
A lack of robust screening measures, late diagnosis (unresectable to metastatic), challenging histology at presentations combined with limited systemic options, the high recurrence rate after surgery, and unreliable biomarkers to monitor the treatment response contribute to poor outcomes in BTCs [13]. Surgical management is curative in early-stage BTC, but it is feasible in only a small fraction of cases (≈30%) [14,15]. Therefore, the majority of the patients must be treated with systemic therapy and palliative intent. Even with resection, 3-year recurrence rates can be as high as 80% [16]. Liver transplant is approved for certain unresectable hilar or perihilar eCCA (≤3 cm, absent nodal and intra or extrahepatic metastatic disease and no biopsy) only [17].
This literature review discusses systemic therapy’s current chemotherapy-centric landscape with a limited role of targeted therapy and immune checkpoint inhibitors (ICI). The status of newer targets and newer agents for established targets, ICI-based combinations on the horizon, and their impact on shaping the future of BTC management is also examined. The emphasis of this paper will be on palliative therapy. The adjuvant therapy (AT) and neoadjuvant therapy (NAT) options will be briefly discussed.
2. Chemotherapy in Biliary Tract Cancers
2.1. Chemotherapy in the First Line
Over 70% BTCs present in advanced stages or aBTC (unresectable or metastatic) and are only eligible to receive palliative therapy. The combination of gemcitabine (Gem) and cisplatin (Cis), or GC, is the current approved first-line therapy [18]. There were no positive first-line trials for over a decade. The standard approach to BTCs is illustrated in Figure 1.
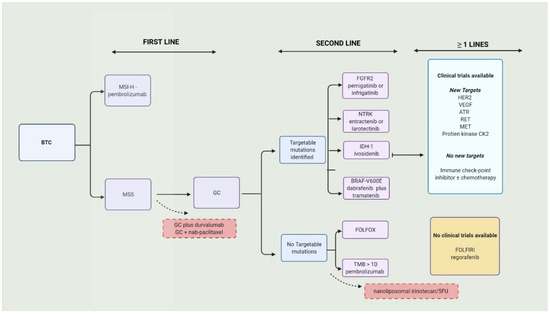
Figure 1.
Current approach to biliary tract cancers. BTC—biliary tract cancers; MSI-H—microsatellite instability; MSS—microsatellite stable; GC—gemcitabine/cisplatin; FGFR2—fibroblast growth factor 2; IDH—isocitrate dehydrogenase-1; NTRK—neurotrophic tyrosine receptor kinase; HER2—human epidermal growth factor receptor 2 inhibitors; VEGF—vascular endothelial growth factor; TMB—tumor mutational burden; ATR—ataxia telangiectasia mutated and Rad3-related.
In ABC-01, a phase II randomized trial, GC combination was compared to Gem alone in treatment-naïve aBTC patients [19]. The tumor response rates (28% vs. 23%), time to progression (8 months vs. 4 months), and 6-month progression-free survival or PFS rate (57% vs. 46%) were higher in the combination group. GC approval in the first line was based on the ABC-02 trial, a phase III randomized control trial in which GC was compared to Gem alone. The median overall survival or OS (11.7 months vs. 8.1 months; hazard ratio or HR = 0.64; p < 0.001) and the median PFS (8 months vs. 5 months; HR = 0.63; p < 0.001) was higher in the GC group. The tumor control (complete response (CR) or partial response (PR) or stable disease (SD)) was also higher in the GC group (81% vs. 72%; p = 0.04). The tolerance profile was comparable between both groups, except for neutropenia (higher with GC). More details are discussed in Supplementary Table S1.
The combination of oxaliplatin, irinotecan, and infusional fluorouracil (mFOLFIRINOX) was inferior to GC in the first-line setting, as evidenced by the PRODIGE 38 AMEBICA trial [20]. In this randomized phase II/III trial, the 6-month PFS rate (44.6% in mFOLFIRINOX vs. 47.3% in GC), PFS (6.2 m vs. 7.4 m), and OS (11.7 m vs. 13.8 m) were superior in the GC group. A partially activated monophosphorylated Gem compound, NUC-1031, that can overcome the resistance developed against Gem, was tested in the first line for aBTC [21]. This compound does not need a nucleoside transporter to enter the cell, has enzyme-mediated activation, and resists degradation by cytidine deaminase [22]. Although early trials with NUC-1031 plus Cis had a greater objective response rate or ORR over GC (44% vs. 26%), the phase III trial was discontinued as the interim analysis showed that it would be unlikely to meet its primary end-point of 2.2 months superiority in OS compared to GC [21]. In the BREGO trial, Regorafenib (Reg) and GEMOX (gemcitabine and oxaliplatin combination) were compared to GEMOX alone in aBTC [23]. The overall results were unsatisfactory (the Reg-GEMOX group was not superior to the GEMOX-only group for PFS or OS). Subgroup analysis showed a higher disease control rate (or DCR), PFS, and OS in patients who continued Reg beyond four cycles.
The addition of nab-paclitaxel (NP) to GC (GC/NP) in the first line had encouraging results in a single-arm phase II trial [24]. The hematological toxicity was very high in the first 32 (of 60) patients enrolled in the trial who received Gem (1000 mg/m2), Cis (25 mg/m2), and NP (125 mg/m2) on days 1 and 8 of 21-day cycles. The doses of Gem and NP were dropped to 800 and 100 mg/m2, respectively, for the next 28 patients. The median PFS was 11.8 months and the median OS was 19.2 months. DCR (PR plus SD) was superior in the high-dose group (90% vs. 78% in reduced dose). Comparing GC and GC/NP is not ideal (no head–head trials), but GC/NP seems to have a better OS and PFS, and worse neutropenia and anemia, based on observations from the respective published trial data (please refer to Supplementary Table S1 for more details) [18,24].
In a Korean retrospective review from four medical centers, the safety and efficacy of GC/NP in treating aBTC was reported last year [25]. The authors looked at the outcomes (ORR, DCR, PFS, and OS) in two groups of patients based on when they received GC/NP: a) in the first line; b) NP was added to GC before or after disease progression (PD). The former group’s ORR (48% vs. 31%) and DCR (90% vs. 75%) were superior. The ORR (40% vs. 16%) and DCR (86% vs. 60%) were greater when NP was added before PD in the latter group. The safety profile was acceptable in these patients and, as expected, Grade 3/4 events were lower in patients who received a reduced dose of GC/NP. A phase III randomized trial (SWOG1815, NCT03768414) is underway to examine the benefit of adding NP to GC in aBTC (GC/NP vs. GC). GC plus S-1 (an oral fluoropyrimidine derivative) combination has a survival benefit over GC in treating aBTCs [26]. The preliminary data of KHBO1401-MITSUBA, a phase III randomized trial, showed improved OS (13.5 months vs. 12.6 months), PFS (7.4 months vs. 5.5 months), and response rates (41% vs. 15%) in the triplet group compared to the GC group.
In the TOPAZ-1 trial, phase III randomized, double-blind, placebo-controlled GC plus durvalumab (ICI) or GC-D was compared to GC plus a placebo [27]. Patients received GC-D for eight cycles (days 1 and 8, Q3W) followed by durvalumab only or placebo Q4W. The mOS 12.8 months vs. 11.5 months (hazard ratio [HR], 0.80; 95% confidence interval [CI], 0.66–0.97; p = 0.021), mPFS 7.2 months vs. 5.7 months (HR, 0.75; 95% CI, 0.64–0.89; p = 0.001), and ORR (26.7% vs. 18.7%) was superior in GC-D compared to the GC group. G3/4 AEs were similar in both groups. While the results of the GC-D combination are promising, we need to wait for the full study data to make reliable conclusions. The results of other clinical trials are discussed in Table 1. Although it is not ideal to compare the results from the ABC-02, TOPAZ-1, and GC/NP trials, we attempted to compare the survival data and toxicity profile (of few AEs) in Supplementary Table S1.
2.2. Chemotherapy in the Second Line
In aBTC (and ampullary cancers), patients who progressed on GC with a preserved performance status (Eastern Cooperative Oncology Group or ECOG scale of 0–1), FOLFOX had a small OS benefit (6.2 months vs. 5.3 months; adjusted hazard ratio = 0.69 [95% CI 0.50–0.97]; p = 0.031) compared to supportive care [28]. The survival rate was higher in the FOLFOX group at 6 months (51% vs. 36%) and 1 year (26% vs. 11%). Subgroup analysis in this trial produced some interesting results. The OS (not PFS) was superior with FOLFOX among the platinum-sensitive (PD after 90 days of completion of first-line chemotherapy) and platinum-resistant/refractory (PD on the first line or in less than 90 days after completion of first-line chemotherapy). Expectedly, high-grade AE were more prevalent in the FOLFOX group (69% vs. 52%). A retrospective study in Italy examined the differences in outcomes after second-line chemotherapy (post-GC) between elderly (≥70 years) and younger (<70 years) patients. There were no significant differences in the outcomes (OS or PFS) between the two groups. The most-used second-line agents in the elderly population were Gem alone or capecitabine alone or a combination of both. Treatment-related toxicity was very high in the elderly population compared to the younger group (48.5% vs. 8.2%; OR 6.31; p < 0.001) [29].
A combination of nanoliposomal irinotecan (Nan-Iri) and 5FU was compared to 5FU alone in the NIFTY trial [30]. It was a multicenter, open-label, randomized, phase IIb trial in which patients progressed on GC. The combination group had a superior PFS (7.1 m vs. 1.4 m; HR = 0.56; 95% CI 0.39–0.81; p = 0.0019) and ORR (19.3% vs. 2.1%) compared to the 5FU group. G3-4 neutropenia (24% vs. 1%) and serious adverse events (42% vs. 24%) occurred more in the combination group than the 5FU-only group. It was concluded that Nan-Iri plus 5-FU could be considered for second-line treatment in patients with BTC who formerly progressed on GC, especially in patients who cannot tolerate platinum agents. On the other hand, mFOLFIRINOX had reasonable efficacy and safety for patients who progressed on GC (≥3 cycles) and is an option for patients with no targetable mutations [31].
3. Targeted Therapy in Biliary Tract Cancers
Second-line options in patients who progressed on GC are limited. In the subset of patients with targetable mutations, fibroblast growth factor 2 (FGFR2) inhibitors such as those with pemigatinib and infrigatinib [32], neurotrophic tyrosine receptor kinase (NTRK) gene fusions such as larotrectinib and entrectinib [33,34], and isocitrate dehydrogenase 1 (IDH-1) with ivosidenib [35], are suitable agents which are preferred over chemotherapy in the second line (preferably after GC). Individual targeted therapy options will be discussed in the following text. The reported results of trials and ongoing trials with targeted therapy are summarized in Table 1 and Table 2.

Table 2.
Ongoing trials with targeted therapy in biliary tract cancer.

Table 1.
Results of recent trials in biliary tract cancer.
Table 1.
Results of recent trials in biliary tract cancer.
| Line | Phase (N) | Clinical Trial Identifier | Treated Cancer Group | Experimental Arm | Target of the Drug (If Applicable) | Comparative Arm | Primary Outcome Studied in the Trial | Top 3 Treatment-Related Adverse Events | Notes |
|---|---|---|---|---|---|---|---|---|---|
| First line | III | NCT03875235 [27] | BTC | Durvalumab (D) + GC | PD-1 | GC + placebo (Pbo) | OS—12.8 m vs. 11.5 m (D vs. Pbo, HR = 0.80; 95% CI, 0.66–0.97; p = 0.021) | Anemia Low neutrophil count Low platelet count | PFS-7.2 m vs. 5.7 m (D vs. Pbo, HR, 0.75; 95% CI, 0.64–0.89; p = 0.001); ORR—26.7% vs. 18.7% (D vs. Pbo); Grade 3/4—62.7% vs. 64.9% (D vs. Pbo) |
| II | NCT03796429 [36] | BTC | Toripalimab + GC | PD-1 | Single arm | PFS—6.7 m OS—NR | Leukopenia Anemia Rash | ORR—21 DCR—85% G3/4, non-hematological in 20% and hematological—69% | |
| II | NCT03951597 [37] | iCCA | Toripalimab + lenvatinib + GemOx + | PD-1 + TKI | Single arm | ORR—80% (1CR and three patients obtained enough control to allow for resection) | Jaundice Rash Proteinuria | DCR—93.3%, PFS—10 m OS—NR DOR—9.8 m | |
| II | NCT04361331 [38] | iCCA | Lenvatinib + GemOx | TKI | Single arm | ORR—30% 1/30 was down staged to have resection | Fatigue Jaundice Vomiting | PFS and OS—NR DCRc—87% No G5, ≥G3 in 40% | |
| Ib II | NCT02992340 | BTC | Varlitinib + GC | Pan-HER 2 | Single arm | DLT—1/11 (200 mg); 1/12 (300 mg) | blood and lymphatic system disorders | PR = 8/23; SD = 12/23 ORR—35%, DCR—87%, DoR—4 m, PFS—6.8 m | |
| Ib II | NCT02128282 [39] | CCA | Silmitasertib (CX-4945) + GC | Casein kinase 2 (CK2) | Single arm | PFS 11 m | Diarrhea Neutropenia Nausea | Compared to GC—Better PFS Lesser neutropenia | |
| I | NCT02375880 [40] | BTC | DKN-01 + GC | Dickkopf-1 (DKK1) | Single arm | Safety—no DLT | Neutropenia Thrombocytopenia Leukopenia | ORR—21.3% PFS—8.7 m | |
| Subsequent lines | III | NCT02989857 (ClarIDHy) [41] | CCA | Ivosidenib (IVO) | IDH-1 | IVO alone vs. placebo | PFS—2.7 m vs. 1.4 m (HR = 0.37; 95% CI 0.25–0.54; p < 0.0001). | Ascites Fatigue Anemia | OS in updated analysis 10.3 m IVO vs. 7.5 m (HR = 0.79; 95% CI 0.56–1.12; p = 0.093) |
| II | NCT02966821 [42] | BTC | Surufatinib | VEGF | Single arm | PFS rate at 16 wks—46.33% (95%, 24.38–65.73) | Elevated bilirubin Hypertension Proteinuria | PFS—3.7 m OS—6.9 m | |
| II | ChiCTR1900022003 [43]. | BTC | Anlotinib + sintlimab | TKI + PD-1 | Single arm | OS—NR | Hypertension ** Diarrhea Hypothyroidism | PFS—6.5 m ORR—40% DCR—87% | |
| II | NCT02052778 [44]. | iCCA # | Futibatinib | FGFR2 | Single arm | ORR 37% | Hyperphosphatemia Diarrhea * Dry mouth * | DoR—8.3 m and DCR = 82% | |
| II | NCT03230318 [45] | iCCA | Derazantinib | FGFR2—mutations and amplifications | Single arm | 3-month PFS rate—76% | Not specified | DCR = 80% PFS = 7.3 m 6-month PFS rate = 50% | |
| II | NCT03797326 [46] | BTC # | Pembrolizumab + lenvatinib | PD-1 + TKI | Single arm | ORR—10% Safety—TRAE in 97% (>G354%) | Hypertension Dysphonia Diarrhea | DCR—68% PFS—6.1 m OS—8.6 m | |
| II | NCT02265341 [47] | BTC | Ponatinib | FGFR2 | Single arm | ORR—9% | Lymphopenia, Rash Fatigue (50%) | CR = 0, PR—8%, SD = 36%. PFS—2.4 m and OS—15.7 m | |
| II | NCT03834220 [48] | CCA among Solid tumors | Debio 1347 | FGFR Fusion | Single arm | ORR—2/5 (40%) of CCA | Fatigue Hyperphosphatemia Anemia | DoR and PFS were 16.1 weeks and 18.3 weeks (in all patients), respectively. | |
| II | NCT01953926 [49] | BTC + AC # | Neratinib | HER2 or EGFR Exon 18 | Single arm | ORR—12% | Diarrhea * Vomiting * | PSS—2.8 m OS—5.4 m | |
| I/ II | NCT01752920 [50] | iCCA | Derazantinib | FGFR2—fusions | Single arm | Safety—all-grade TRAE in 93% | Fatigue Eye-toxicity Hyperphospatemia | ≥3 Grade TRAE in 28% ORR—27% DCR—83% | |
| I | NCT02699515 [51] | BTC # | Bintrafusp alfa, | TGF-β and PD-L1 | Single arm | Safety—emergent and all adverse events | Rash Fever Increased lipase | 63% had TRAE 37% ≥ G3 | |
| I | NCT02892123 [52] | BTC # | ZW25 (Zanidatamab) | bispecific HER2 | Single arm | Safety/tolerability—only G1–G2 reported in 70% | Fatigue ** Diarrhea Infusion reaction | ORR—47 DCR—65% DoR—6.6 m | |
| Ib | NCT03996408 [53] | BTC | Anlotinib TQB2450 | TKI + PDL1 | Single arm | DLT/ MTD in first 3 weeks (one cycle)—none RP2D—25 mg ORR—42% | * Hypertension Leukopenia Increased total bilirubin Neutropenia | PFS—240 days DCR—75% |
# Part of a basket trial but these results are from the BTC cohort; * All grade AE, ** G1-G2 AE; BTC—biliary tract cancers include gall bladder cancers and CCA; iCCA—intrahepatic cholangiocarcinoma; eCCA—extra-hepatic cholangiocarcinoma; CCA—cholangiocarcinoma includes iCCA and eCCA; AC—ampullary cancer; GC—gemcitabine/cisplatin; Gem/Ox—gemcitabine/oxaliplatin; OS—median overall survival; PFS—median progression free survival; m—months; wks—weeks; HR—hazard ratio; CI—confidence interval; TRAEs—treatment-related adverse events; NR—not reached; DCR—disease control rate; ORR—objective response rate; CR—complete response; PR—partial response; DOR—duration of response; IDH—isocitrate dehydrogenase-1; VEGF—vascular endothelial growth factor; FGFR2—fibroblast growth factor 2; HER2—human epidermal growth factor receptor 2 inhibitors; EGFR—epidermal growth factor receptor; mab—monoclonal antibody; TGF—transforming growth factor; PD-1—programmed cell death protein 1; PDL1—programmed cell death ligand protein; TKI—tyrosine kinase inhibitor; DLT—dose limiting toxicity; MTD—maximum tolerated dose; R2PD—recommended phase II dose.
3.1. Fibroblastic Growth Factors Receptor Inhibitors (FGFRis)
Fibroblast growth factors (FGF) are protein ligands that play a vital role in regulating cell proliferation, differentiation, migration, and tissue repair/angiogenesis [54]. FGFRs are transmembrane proteins with three extracellular domains (D1–D3), a transmembrane domain, and an intracellular tyrosine kinase domain 13 [55]. There are 18 types of FGF (FGF1–10 and 16–23) that can bind to a family of 4 FGFRs (FGFR1–4) [56]. The effects of ligand (FGF) binding on FGFR can be simplified as follows (in order): dimerization of FGFR, transphosphorylation of TK domains, attachment of which adaptor proteins at the phosphorylated site (docking site), phosphorylation of adaptor proteins, activation of a cascade of downstream signaling pathways, Ras-Raf-MAPK, PI3K-AKT, Stat, and PLCγ, and gene transcription [57]. Any alterations in the FGFRs, such as amplification, mutation, and fusion/rearrangement, can activate the above-mentioned pathway constitutively, promoting uncontrolled cell growth, migration, and survival, ultimately leading to malignant transformation [58].
The prevalence of FGFR alterations among solid tumors (tissues) is approximately 7% and, when detected, are common in the lung, colon, breast, endometrial adenocarcinoma, and glioblastoma multiforme [59,60]. The majority were in FGFR1 (49%), followed by FGFR3 (26%), FGFR2 (19%), and FGFR4 (7%) [60]. Approximately 5% of tissues had >1 FGFR alterations. When classified by the kind of alterations, two-thirds had amplifications, a quarter had mutations, and only 8% had fusions. The frequency of FGFR fusions is greater in CCA, specifically in iCCA (14%), compared to other solid tumors (colorectal, hepatocellular and gastric) [61].
Among the FGFR alterations, FGFR2 fusions/rearrangements have a favorable prognostic impact (even with chemotherapy) in BTCs and are more sensitive to FGFR inhibitors (FGFRi), as reported in retrospective studies [58,62,63]. Currently, the indication for using pemigatinib and infrigatinib (FGFRis) is BTC patients with FGFR2 fusion/rearrangement who progressed on chemotherapy (GC) [64,65]. FLIGHT-202 is a single-arm phase II trial where CCA patients with FGFR2 rearrangement or fusions were treated with pemigatinib in the second line (N = 107) [64]. Although there were small cohorts of patients with other (N = 20) or no (N = 18) FGF/FGFR alterations, the primary objective was to study the ORR in patients with fusions/rearrangements. With an ORR of 36% (CR in 3%, PR in 33%, SD in 47%), DCR of 82%, a 1-year PFS rate, and an OS rate of 29% and 68%, pemigatinib earned approval as an ideal second-line agent for patients with FGFR fusions/rearrangements. Alternatively, pemigatinib did not show any efficacy benefit in the other two cohorts (other or no FGF/FGFR alterations). Infrigatinib, a selective, ATP-competitive FGFRi, was also studied in a similar population [65]. The ORR was 25% (CR in 1%, PR in 22%, SD in 61%), DCR was 84%, and the median DOR was 5 months. The median PFS and OS were 7.3 months and 12.2 months, respectively. Both drugs could cause severe hyperphosphatemia requiring aggressive management. Fatigue, stomatitis, hyponatremia, palmar-plantar erythrodysesthesia syndrome, and alopecia are some of the other important AEs with these drugs.
Other FGFRis with early success include derazantinib (ARQ 087), futibatinib, and Debio1347 [50,66,67]. The interim results of BTCs with FGFR2 mutations or amplifications (not fusions/rearrangements) treated with dezaratinib in FIDES-01 trial were reported recently [45]. Of 28 patients enrolled in this trial, 78% had missense point mutations and the remainder were other short variants and amplifications. The DCR was 74% (PR in 8.7% and SD in 65%), with the PFS rate at 3 months and 6 months being 76% and 50%, respectively. The response was seen across all types of alterations. Erdafitinib, another FGFRi, showed durable responses (ORR of 41%, median DOR of 7.3 months) and an acceptable safety profile in CCA with FGFR fusions/rearrangements and mutations in the second line [68].
3.2. Isocitrate Dehydrogenase Inhibitors
Isocitrate dehydrogenase (IDH) is a key enzyme in the TCA (tricarboxylic acid) cycle and helps in converting isocitrate to α-ketoglutarate [69]. A mutant IDH-1 produces an abnormal enzyme that further converts isocitrate to α-ketoglutarate to a metabolite with malignant potential, 2-hydroxyglutarate (2-HG) [70]. The prevalence of IDH-mutations (IDH-1/IDH-2) is <5% among BTCs, while IDH-mutant tumors typically have lower tumor mutation burden (TMB) and rarely have microsatellite instability or PDL-1 positivity compared to IDH-wild type tumors [71,72,73].
Ivosidenib (IVO) is one of the first IDH-1 inhibitors that showed benefit in treating CCA [35]. The first phase III results (ClarIDHy) published last year showed a statistically significant improvement in the PFS (2.7 months in IVO vs. 1.4 months in placebo; HR = 0.37; p < 0.0001) in refractory CCAs treated with IVO (compared to placebo). This trial allowed crossover from placebo to IVO group after progression. Only 30% (vs. 22% in placebo) had drug-related serious AEs. An updated analysis of this trial reported higher OS in IVO group (10.3 months vs. 7.5 months; HR = 0.79; p = 0.09), but when adjusted to crossover (derived using rank-preserving structural failure time), there was a 2-month survival advantage in the IVO group (7.5 m in IVO vs. 5.1 in placebo; p = 0.0001) [41]. LY3410738 is a mutated-IDH1 inhibitor that is different from commonly used IDH-1 inhibitors such as ivosidinib in that it binds covalently to the mutant enzyme and at a different site, thereby reducing the risk of secondary mutations [74]. It is being studied in a phase I basket trial in the second line [75]. There is also a group for CCA where it is combined with GC.
3.3. Neurotrophic Tyrosine Receptor Kinase Fusion Inhibitors
Fusions in neurotrophic tyrosine receptor kinase (NTRK) genes that encode tropomyosin receptor kinases (TRK) promote carcinogenesis and were identified as driver mutations in many cancers, including BTCs [76]. The prevalence of NTRK fusions among BTCs is very low (0.75%) [77]. Successful basket trials gave two NTRK inhibitors, larotrectinib (Lt) and entrectinib (Et), that have very high response rates and are well-tolerated [33,34]. It should be noted that these basket trials had very limited CCAs in their study population (2/55 for Lt and 1/54 for Et).
3.4. Vascular Endothelial Growth Factor Inhibitors
The overexpression of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptors (VEGFR) is common among BTCs (54% in CCA) and contributes to their poor outcomes [78,79]. In a phase II study (NCT02520141) published recently, the benefit of ramucirumab, a fully human, IgG1 monoclonal antibody direct inhibitor of VEGFR-2, was studied in treatment-refractory BTCs [80]. The response achieved was in line with other agents used in the similar population (PR in 2%, SD in 43%, mPFS of 3.2 months, and OS of 9.5 months). Suraftinib, a small-molecule inhibitor of VEGFR 1-3, was studied in the second-line (phase II) for BTC [42]. Patients received 300 mg daily in 28-day cycles. The primary end-point was a 16-week PFS rate that was 46.33% (95%, 24.38–65.73). The mPFS (3.7 months) and mOS (6.9 months) were reasonable. Interestingly, it was more effective in patients with disease in the liver and lower baseline CA 19-9 (≤1000 IU/mL). As expected, hypertension and proteinuria were frequent in the study population. A Chinese trial looked at the combination of a camrelizumab, ICI (anti-PD-1), and apatinib, a VEGFR2 inhibitor in the neoadjuvant setting for aBTC [81]. Efficacy, safety, and the exploration of biomarkers were the study’s aims. Patients received 200 mg carmelizumab/2 weeks with VEGFR2 inhibitor 250 mg/day for two cycles. Among 17 subjects (13 GBC and 4 CCA), the ORR was 71%, including 2 patients with CR. In a prospective trial with 22 patients with aBTCs, this combination had a DCR of 71% (PR—19% + 50% SD). G3/G4 TRAEs were reported in 64% of patients [82].
Anlotinib (AL3818) is a novel oral receptor tyrosine-kinase inhibitor (TKI) that works on VEGFR-2 and -3, FGFR1-4, platelet-derived growth factors (PDGFR)-α and -β, c-Kit and Ret, and inhibits tumor growth and angiogenesis [83]. It has shown some promise in lung cancer (NSCLC/SCLC), RCC, esophageal, and other solid tumors [84,85,86,87]. It was studied in combination with TQB2450, a PD-L1 inhibiting ICI in an open-label phase Ib trial in refractory aBTC [53]. Anolitinib at 12 mg dose with ICI was considered a safe dose. HTN, elevated bilirubin, and leukopenia/neutropenia were the top AEs. In evaluable patients, the ORR was 42% with DCR of 75%. In another phase II study, it was used in the second line along with another novel ICI, sintlimab (PD-1 inhibitor at 200 mg/Q3 weeks). When reported, primary end-point OS was not reached after a median follow-up of approximately 9 m. As was the case with other trials, HTN was seen in most patients (70%). The ORR (40%) was similar to other trials, but DCR was slightly higher (87%) [43].
3.5. Human Epidermal Growth Factor Receptor 2 Inhibitors
HER2 (human epidermal growth factor receptor 2) belongs to a family of four epidermal growth factor receptors (HER1–4) and has a proven role in malignancy when overexpressed or amplified [88]. When activated by dimerization, HER2 triggers phosphorylation of certain tyrosine kinases that promote cell growth/proliferation and malignant transformation through a series of downstream signaling pathways [88]. HER2 overexpression/amplification is seen in 3–20% of BTCs and is less common in iCCA (compared to eCCA and GBC) [89,90]. HER2 inhibitors are commonly used to treat HER2-positive breast, esophageal, and gastric cancers [91,92]. They are now being studied to treat aBTCs.
Trastuzumab, a humanized monoclonal antibody (hmab) directed against the extracellular domain (IV) of HER2, suppresses signaling pathways and degrades HER2 [93]. Pertuzumab, another hmab, alternatively prevents the dimerization of HER2 receptor by attaching to another extracellular domain (II) and inhibiting its activation [94]. Zanidatamab (ZW25) is a bi-specific antibody that binds to both domains II and IV [95]. Neratinib is an irreversible TKI that binds to the intracellular TK-domain and inhibits signaling pathways [96].
The results of the BTC expansion cohort of the phase I trial of zanidatamab (ZW25) were reported [52]. A total of 20 refractory BTC (including 5 patients who received prior-trastuzumab) were given 20 mg/Q2 weeks. No serious AEs were reported, while 70% had G1/2 AE. A BTC cohort that had 25 patients (including AC) reported for a SUMMIT trial, where refractory solid tumors were treated with neratinib alone (24 mg/day). [49]. The ORR (primary end-point) was appreciated in just 12%. Despite receiving loperamide prophylactically, 56% had diarrhea, including G3 in 24%, but the drug was not discontinued in any patient with diarrhea. GC with varlitinib (reversible pan-HER inhibitor) was well tolerated in treatment-naïve Asian patients [97]. A phase Ib trial determined the maximum tolerated dose of varlitinib and safety of the combination. Dose-limiting toxicity (DLT) was reported in 2/11 and 1/12 patients in 200 mg and 300 mg cohorts, respectively. The 300 mg cohort had a higher rate of ≥grade 3 AEs (67% vs. 36%). The ORR was 35% (PR in 35% and SD in 35%) during the study period.
Ongoing phase II trials with HER2 inhibitors in refractory BTC are: (a) Trastuzumab plus mFOLFOX in aBTC including AC; (b) Trastuzumab plus pertuzumab (part of a basket trial including My Pathway trial); (c) Zanidatamab monotherapy in aBTC; (d) Trastuzumab deruxtecan in a BTCs (DESTINY-PanTumor02, NCT04482309); (e) Trastuzumab emtansine (NCT02999672) [98,99,100,101].
3.6. Other Targeted Therapy Options
The ROAR and NCI-Match trials showed the ORR ranging from 20–38% with dabrafenib plus trametinib combination in BTCs with BRAFV600E mutation and may be an option if they progress on first-line GC [102,103]. Other BRAF inhibitor combinations under investigation are dabrafenib plus JSI-1187 (ERK-inhibitor) in NCT03272464 and ABM-1310 (novel BRAF-inhibitor) plus cobimetinib (MEK-inhibitor) in NCT04190628 (discussed in Table 2). The effect of encorafenib plus bimimetinib (MEK-inhibitor) on non-V600E BRAF mutations is being examined in a phase II trial (NCT03839342). GC in combination with selumetinib (MEK inhibitor) had acceptable toxicity (1/12 had DLT of chest pain) in an ABC-04, phase I trial [104]. Three patients had PR and 5 had SD. A phase II clinical trial comparing this combination (GC plus selumetinib) to GC in aBTC (NCT02151084) is now underway.
Protein kinase CK2 is a phosphorylating enzyme that is essentially active in the normal eukaryotic cell that helps in cell differentiation and immune regulation [105,106]. It has a role in benign diseases such as diabetes, neurological disorders such as Parkinson’s disease and Huntington’s disease, intestinal inflammation, and some autoimmune diseases [105,107,108]. Its role in malignant transformation, distant metastasis, and drug resistance is well established [109]. Silmitasertib (CX-4945) is a selective inhibitor of CK2 with antiproliferative/antiangiogenic capability that showed good efficacy in preclinical studies [110]. In a multicenter, open-label, phase Ib/II study, it was administered along with GC in unresectable CCA (1000 mg bid, 10 days of 21 days GC cycle) [39]. The mPFS (primary end-point) and OS are 11.1 (95% CI 7.6–14.7) and 17.4 (95% CI 13.4–25.7), respectively. Severe AEs reported included diarrhea, neutropenia, nausea, anemia, and thrombocytopenia.
Pralsetinib is a RET inhibitor approved after results from the ARROW study showed the safety and efficacy of this agent in metastatic non-small cell lung cancer and advanced or metastatic thyroid cancer [111]. This study sought to determine efficacy through the ORR of this agent for other cancers with RET fusions, including pancreatic, colon, CCA, and unknown primary. The ORR was 53% (CI 29–76) with 11% CR and 42% PR. This study had 3 CCA patients and 2/3 had a clinical response. The benefit of drugs targeting the DNA damage repair genes, including AT-rich interaction domain 1A (ARID1A), protein poly-bromo1 (PBRM1), and BRCA1-associated protein 1 (BAP1) in aBTCs, is under investigation (NCT03207347; NCT04042831). Ceralasertib (AZD6738), a selective ATR inhibitor that is expected to accentuate DNA damage when used with a PARP inhibitor (olaparib) and ICI (durvalumab), is being studied to treat aBTCs in the second line [112]. Adding DKN-01, a humanized mAb targeting Dickkopf-1 (DKK1), a Wnt pathway GC in the first line for aBTCs was tolerable with no dose-limiting toxicity and ORR exceeding 20% [40]. It is being studied with Nivo in a phase II study (NCT04057365). Novel agents targeting key pathways promoting carcinogenesis in BTCs, such as JAK/STAT (BBI503), Wnt/β-catenin (NCT03507998), and NOTCH (brontictuzumab), have shown promising preclinical and early-trial evidence and are expected to expand the arsenal of targeted therapies in coming years [113]
4. Immunotherapy in Biliary Tract Cancers
In the current clinical practice, immunotherapy can be broadly divided into ICIs and less explored adoptive cell therapy (chimeric antigen receptor T cell therapy or CAR-T) and vaccines. Reported results and ongoing trials with immunotherapy are summarized in Table 1 (above) and Table 3 (below).

Table 3.
Ongoing trials with immunotherapy in biliary tract cancer.
4.1. Immune Checkpoint Inhibitors
The current ICIs can be broadly divided into three classes: (i) Cytotoxic T-Lymphocyte Antigen 4 (CTLA4) inhibitors such as ipilimumab and tremelimumab; (ii) Programmed cell death-1 (PD-1) inhibitors such as pembrolizumab and nivolumab; (iii) Programmed death-ligand 1 (PD-L1) inhibitors such as durvalumab, avelumab, and atezolizumab. PD-1 and PD-L1 inhibitors were studied alone or in combination with chemotherapy or targeted therapy, while CTLA4 inhibitors were combined with PD-1 or PD-L1 inhibitors. Pembrolizumab is recommended in patients with mismatch repair deficient (d-MMR) or microsatellite instability—high (MSI-H) and higher TMB (>10) aBTCs in the first line [114,115,116]. In one of the first reports published in 2017, 86 MSI-H/ d-MMR advanced cancer patients (12 different cancer types, including 40 colorectal cancers) were treated with pembrolizumab [114]. The ORR of the entire group was 56% (21% CR, 33% PR, and 23% SD). About 5% (4/86) of the enrolled cancers were CCA and the ORR among them was 25% (1 CR) with 100% DCR (3 SD + 1 CR). In KEYNOTE-158, MSI-H/dMMR refractory non-colorectal advanced tumors (27 types including CCAs) were treated with pembrolizumab [115]. It had 22/233 (9.3%) CCA patients and the ORR among them was 41% (2-CR and 7-PR). The PFS and OS were 4.2 months and 24.3 months, respectively. The median DOR of this cohort had not been reached at the time of publication
Nivolumab has a category 2B recommendation (per national comprehensive cancer network or NCCN guidelines) in the second line and is typically offered to patients who do not have targetable mutations and may not tolerate chemotherapy [117]. This approval was based on a trial published in 2020 where 54 refractory aBTC (>1 and ≤3 lines) patients were treated with nivolumab. Tumor samples were available for 42 patients and 18 of them (43%) expressed PD-L1. The ORR was 22% (11/46, 0-CR, 10-PR, and 1 unconfirmed PR), with 37% (17/46) having an SD based on RECIST criteria and investigator-review. The ORR and SD were 11% and 39%, respectively, on central review in this study. Interestingly, 50% (9/18) and 28% (5/18) of the patients expressing PD-L1 had an evaluable response in the investigator review and central review, respectively.
Combining ICI with chemotherapy and TKI is being studied in the first line. The TOPAZ-1 trial results (discussed above) are very encouraging and may open doors for many such combinations going forward [27]. In KEYNOTE-966 (phase III trial), the efficacy and safety of pembrolizumab and GC combination are being studied (vs. GC + placebo) [118]. Interim results of phase II trials with pembrolizumab and olaparib showed acceptable safety [119]. The combination of nivolumab and Nan-Iri/5FU did not provide the expected results in the BiT-03 trial [120]. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, had promising success in the phase I trial reported in 2020 [51]. It was studied with second-line (N = 30) Asian patients with BTC (including one ampullary cancer). It was well tolerated with ≥G3 events in 37% (11/30) and G5 events in 10% (3/30). The ORR was 20% with 18 m of DOR. It is currently being studied in the first line combined with GC (GC + bintrafusp alfa vs. GC + placebo) [121].
The LEAP-005 study that evaluated the safety and efficacy of lenvatinib and pembrolizumab as second-line therapy for advanced solid tumors had 31 for BTC patients [46]. The ORR was 10% (95% CI 2–26) with DCR 68% (95% CI 49–83.) There were treatment-related AEs for 97% of patients, including 48% having grade 3–4 AEs. It was concluded that lenvatinib and pembrolizumab have some efficacy as second-line agents with tolerable side effects in patients with BTC. JS001-ZS-BC001 trial, an open-label, phase II clinical study, evaluated the efficacy and safety of toripalimab or Tor (PD-1 inhibitor) plus Gem/S-1 in the first line [36]. A total of 39 patients received this combination, with the response rate being 20.6% and DCR 85.3%. The PFS was 6.7 months, with grade 3/4 non-hematologic AEs seen in 20.5% of patients, while grade 3/4 hematologic AEs were seen in 69.2%. The study showed promising results in line with the TOPAZ trial. When Tor was combined with gemcitabine/oxaliplatin (D1 and D8, Q3W for six cycles) and lenvatinib (8 mg) in a phase II trial with locally advanced iCCA (N = 30), the ORR was 80% (1CR and 3 patients obtained enough control to allow for resection), DCR was 93.3%, PFS was 10 m, and DOR was 9.8 m. ORR was related to PD-L1 expression and DNA damage repair mutations in the tumors [122]. On the other hand, ORR and DCR were 30% and 87%, respectively, when lenvatinib plus the GemOX arm of a phase II trial reported in gemcitabine/oxaliplatin combination was presented last year [38].
In the CTEP 10139 trial, atezolizumab (Atezo) alone was compared with the combination of MEK inhibitor combimetinib (Cobi) [123]. Although the PFS was higher in the combination group (3.6 months vs. 1.9 months; p = 0.027), the OS and the ORR were similar in both groups. The combination of Atezo with varlilumab, a CD 27 agonist (NCT04941287), is now being studied with and without Cobi. Arginase inhibition by a novel agent (INCB001158) was well tolerated in the first line when given with GC and is being explored as another option in this area [124].
Immune-related adverse events (irAE) are organ-specific inflammatory responses invoked by ICIs similar to autoimmune diseases [16]. They can affect any organ (such as colitis, dermatitis, hepatitis, pneumonitis, hypophysitis, myocarditis, myositis, and thyroiditis) and can be life-threatening [125,126]. In the nivolumab trial, only 17% of the study population had grade 3 or grade 4 irAE [117]. In the bintrafusp alfa trial, only 37% had ≥ grade 3 irAEs [51]. The typical management for low-grade irAEs is holding the therapy and restarting it after resolution [127]. High-dose steroids and immunosuppressants such as infliximab are used in severe irAEs.
4.2. Chimeric Antigen Receptor T Cell Therapy and Vaccines in Biliary Tract Cancers
CAR T-cell therapy involves creating a chimeric antigen receptor (CAR) that targets an antigen in the cancer cells, allowing the host T-cells to identify the tumor cells and destroy them [128]. This approach is approved for use in various hematologic malignancies, but its use in solid tumors remains experimental [129]. The key to successful development involves determining the correct antigen to target, which is expressed in large numbers on tumor cells but is found in small numbers on healthy cells. More specific and reliable biomarkers are being studied to target CCA more effectively with CAR-T [130]. Studies with several other CAR targets, including CD133, EGFR, Integrin αvβ6, and Anti-MUC1, have shown positive results [130,131,132,133]. Of 19 patients enrolled in a study evaluating CART-EGFR, and with 17 evaluable, 1 patient saw CR for 22 months and 10 saw SD ranging from 2.5 to 15 months, with a median PFS of 4 months [134]. Another study using anti-MUC1 CAR T-cells showed significantly decreased fluorescence of MUC1 expressing cholangiocarcinoma cells after 3 and 5 days of exposure [133]. While there have been positive results in these trials, more data and larger studies are needed to further assess the safety and efficacy of CAR-T therapy in CCA.
Additionally, vaccines have been developed from various peptides such as MUC1, WT1, and other combinations to mount an immune response against the patient’s cancer. A three-peptide vaccine consisting of cell division cycle associated 1 (CDCA1), cadherin 3 (CDH3), and kinesin family member 20A (KIF20A) showed response in 5 of 9 patients enrolled, with a median PFS of 3.4 months and OS of 9.7 months [135]. A study using MUC1 peptide showed a response in only 1 of 8 patients enrolled but had a tolerable side effect profile [136]. Another study used lymphocyte antigen 6 complex locus K, TTK protein kinase, insulin-like growth factor-II mRNA-binding protein 3, and DEP domain containing 1, with 7 of 9 patients showing response and producing a median PFS of 5.2 months and OS of 12.7 months [137].
5. Systemic Therapy in Early-Stage Biliary Tract Cancers
Capecitabine is the preferred agent for AT in BTCs based on the BILCAP trial [138]. On the other hand, BCAT and PRODIGE 12 trials could not show the clinical benefit of gemcitabine or gemcitabine/oxaliplatin combination over observation [139,140,141]. A recently presented pooled analysis of these two trials further proved this point [142]. A total of 419 patients were included in the two studies, which showed no difference in PFS (2.9 years in gem-based vs. 2.1 years in observation; HR = 0.91; p = 0.45) or OS (5.1 years vs. 5 years; HR = 1.03; p = 0.83). Radiation alone (XRT) or chemoradiation (CRT) in the adjuvant setting is not a popular approach in managing BTC. CRT is offered to eCCA and GBC patients with positive margins or lymph nodes [143,144,145]. Retrospective studies showed benefits with chemotherapy only in resected BTCs, but it is difficult to compare the AT strategies as CRT or XRT is offered to BTCs with high-risk factors (positive margins/lymph nodes) [146].
Neoadjuvant (NAT) systemic therapy is not a standard approach in resectable BTCs. Some case reports and retrospective studies show the benefit of NAT downstaging the locally advanced or unresectable BTCs enough to have resection [147,148,149]. The addition of pre-operative radiation can increase the probability of R0 resection in these tumors [150,151]. On the other hand, NAT did not result in any survival advantage in managing resectable BTCs in the reported studies [152]. Multiple trials investigating the role of neoadjuvant therapy in resectable (GC-D in NCT04308174 or DEBATE; GC in NCT03673072; GC/NP in NCT03579771) and unresectable/locally advanced BTCs (FOLOXIRI in NCT03603834; toripalimab + GEMOX + lenvatinib in NCT0450628) are underway that may give us a definite answer in the coming years. In the current practice, systemic options typically for NAT are similar to those used for treating aBTCs (such as GC).
Locoregional therapy (LRT) with high-dose XRT (58–67.5 Gy in 15 fractions) and SBRT (30–50 Gy in 3 to 5 fractions) improves local control and OS in unresectable iCCA, and can be an option for suitable patients [153,154]. Other LRTs such as transcatheter arterial chemoembolization (TACE) and transarterial radioembolization (TARE) are not typically employed in treating BTCs. SBRT plus capecitabine combination increased local control rates (≈80%) with minimal toxicity (no ≥ grade 3 toxicity) in unresectable perihilar CCA [155]. Other trials intended to see the benefit of SBRT and chemotherapy combinations were closed due to low accrual (NCT01151761 and NCT00983541). ICI with TACE or SBRT, or TARE trials, are underway (NCT03898895, NCT04866836, NCT03937830, NCT02821754, NCT04238637, and NCT04708067), which may open up more options in the near future.
6. Conclusions
GC has been the standard of care for first-line treatment of BTCs for more than a decade. It took considerable time to compile the present arsenal of therapeutic options to treat this lethal cancer (illustrated in the graphical abstract). Currently, the benefit of adding NP to it or replacing gemcitabine with NUC-1031 is being studied. Second-line systemic therapy for patients ineligible for targeted therapy is limited. Although the NIFTY trial suggested the benefit of Nan-Iri/5FU, FOLFOX is typically used. The success of FGFR2, IDH, NTRK, and BRAF (V600E) inhibitors in the second line is remarkable, but their use is restricted by the low prevalence of the respective targets in BTCs. Therapy directed at new targets such as VEGF, HER2, and RET are being studied and may open doors for new options. Pembrolizumab is preferred in MSI-H patients, with little evidence for the use of nivolumab in second-line MSS patients. The TOPAZ-1 trial results may be a game-changer and can bring ICI into the first line.
The landscape of BTC management has started to change in recent years. The oncology practice is moving away from traditional chemotherapy to personalized medicine. The accessibility to tumor mutation profiling and circulating tumor DNA or ctDNA genomic profiling contributed to the success of targeted therapy and paved the way for many new agents. By studying the ongoing trials, it is clear that the focus is also on expanding the use of ICIs with chemotherapy (GC or GEMOX) or selected targeted therapy (rucaparib, DKN-01, entinostat). Such combinations will cater to broader BTC populations as the prevalence of mutations does not restrict eligibility. Other investigational therapies such as CAR-T therapy and vaccines are driving the advancement in treatment options and patient outcomes. With the continued success of clinical trials, these agents could be seen in the near future to join the fight against BTC and provide a much-needed breath of fresh air to the treatment options that we can offer our patients.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers14092137/s1. Table S1: Comparing the trial data of three prominent trials with chemotherapy in the first line (this is not a head–head comparison).
Author Contributions
Conceptualization, A.M.; writing—original draft preparation, E.W. and A.M.; writing—review and editing, D.L. and B.K.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef]
- Razumilava, N.; Gores, G.J. Classification, diagnosis, and management of cholangiocarcinoma. Clin. Gastroenterol. Hepatol. 2013, 11, 13–21.e11. [Google Scholar] [CrossRef] [Green Version]
- DeOliveira, M.L.; Cunningham, S.C.; Cameron, J.L.; Kamangar, F.; Winter, J.M.; Lillemoe, K.D.; Choti, M.A.; Yeo, C.J.; Schulick, R.D. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Ann. Surg. 2007, 245, 755–762. [Google Scholar] [CrossRef]
- Patel, T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001, 33, 1353–1357. [Google Scholar] [CrossRef]
- Ouyang, G.; Liu, Q.; Wu, Y.; Liu, Z.; Lu, W.; Li, S.; Pan, G.; Chen, X. The global, regional, and national burden of gallbladder and biliary tract cancer and its attributable risk factors in 195 countries and territories, 1990 to 2017: A systematic analysis for the Global Burden of Disease Study 2017. Cancer 2021, 127, 2238–2250. [Google Scholar] [CrossRef] [PubMed]
- Zatonski, W.A.; Lowenfels, A.B.; Boyle, P.; Maisonneuve, P.; Bueno de Mesquita, H.B.; Ghadirian, P.; Jain, M.; Przewozniak, K.; Baghurst, P.; Moerman, C.J.; et al. Epidemiologic aspects of gallbladder cancer: A case-control study of the SEARCH Program of the International Agency for Research on Cancer. J. Natl. Cancer Inst. 1997, 89, 1132–1138. [Google Scholar] [CrossRef] [Green Version]
- Massarweh, N.N.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control. 2017, 24, 1073274817729245. [Google Scholar] [CrossRef]
- Sithithaworn, P.; Yongvanit, P.; Duenngai, K.; Kiatsopit, N.; Pairojkul, C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2014, 21, 301–308. [Google Scholar] [CrossRef]
- Strom, B.L.; Soloway, R.D.; Rios-Dalenz, J.L.; Rodriguez-Martinez, H.A.; West, S.L.; Kinman, J.L.; Polansky, M.; Berlin, J.A. Risk factors for gallbladder cancer. An international collaborative case-control study. Cancer 1995, 76, 1747–1756. [Google Scholar] [CrossRef]
- Florio, A.A.; Ferlay, J.; Znaor, A.; Ruggieri, D.; Alvarez, C.S.; Laversanne, M.; Bray, F.; Mcglynn, K.A.; Petrick, J.L. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer 2020, 126, 2666–2678. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Zhu, A.X.; Fuchs, C.S.; Brooks, G.A. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016, 21, 594–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaib, Y.H.; Davila, J.A.; McGlynn, K.; El-Serag, H.B. Rising incidence of intrahepatic cholangiocarcinoma in the United States: A true increase? J. Hepatol. 2004, 40, 472–477. [Google Scholar] [CrossRef]
- Forner, A.; Vidili, G.; Rengo, M.; Bujanda, L.; Ponz-Sarvisé, M.; Lamarca, A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019, 39, 98–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, A.; Saborowski, A. Current and Future Systemic Therapies in Biliary Tract Cancer. Visc. Med. 2021, 37, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Neumann, U.P.; Schmeding, M. Role of surgery in cholangiocarcinoma: From resection to transplantation. Best Pract Res. Clin. Gastroenterol. 2015, 29, 295–308. [Google Scholar] [CrossRef]
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients with Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014, 149, 565–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapisochín, G. Liver transplantation for cholangiocarcinoma: Current status and new insights. World J. Hepatol. 2015, 7, 2396. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valle, J.W.; Wasan, H.; Johnson, P.; Jones, E.; Dixon, L.; Swindell, R.; Baka, S.; Maraveyas, A.; Corrie, P.; Falk, S.; et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: A multicentre randomised phase II study—The UK ABC-01 Study. Br. J. Cancer 2009, 101, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Phelip, J.M.; Desrame, J.; Edeline, J.; Barbier, E.; Terrebonne, E.; Michel, P.; Perrier, H.; Dahan, L.; Bourgeois, V.; Akouz, F.K.; et al. Modified FOLFIRINOX Versus CISGEM Chemotherapy for Patients with Advanced Biliary Tract Cancer (PRODIGE 38 AMEBICA): A Randomized Phase II Study. J. Clin. Oncol. 2022, 40, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.J.; McNamara, M.G.; Goyal, L.; Cosgrove, D.; Springfeld, C.; Sjoquist, K.M.; Park, J.O.; Verdaguer, H.; Braconi, C.; Ross, P.J.; et al. Phase III study of NUC-1031 + cisplatin versus gemcitabine + cisplatin for first-line treatment of patients with advanced biliary tract cancer (NuTide:121). J. Clin. Oncol. 2021, 39, TPS4164. [Google Scholar] [CrossRef]
- Kapacee, Z.A.; Knox, J.J.; Palmer, D.; Blagden, S.P.; Lamarca, A.; Valle, J.W.; Mcnamara, M.G. NUC-1031, use of ProTide technology to circumvent gemcitabine resistance: Current status in clinical trials. Med. Oncol. 2020, 37, 61. [Google Scholar] [CrossRef]
- Assenat, E.; Blanc, J.F.; Bouattour, M.; Gauthier, L.; Touchefeu, Y.; Portales, F.; Borg, C.; Fares, N.; Mineur, L.; Bleuse, J.-P.; et al. 48P (BREGO) Regorafenib combined with modified m-GEMOX in patients with advanced biliary tract cancer (BTC): A phase II randomized trial. Ann. Oncol. 2021, 32, S376–S377. [Google Scholar] [CrossRef]
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.S.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers. JAMA Oncol. 2019, 5, 824. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.; Lee, C.-K.; Sang, Y.B.; Choi, H.J.; Kim, M.H.; Ji, J.H.; Ko, K.H.; Kwon, C.-I.; Kim, D.J.; Choi, S.H.; et al. Real-world efficacy and safety of nab-paclitaxel plus gemcitabine-cisplatin in patients with advanced biliary tract cancers: A multicenter retrospective analysis. Ther. Adv. Med. Oncol. 2021, 13, 175883592110359. [Google Scholar] [CrossRef]
- Sakai, D.; Kanai, M.; Kobayashi, S.; Eguchi, H.; Baba, H.; Seo, S.; Taketomi, A.; Takayama, T.; Yamaue, H.; Ishioka, C.; et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 (GCS) versus gemcitabine, cisplatin (GC) for advanced biliary tract cancer (KHBO1401-MITSUBA). Ann. Oncol. 2018, 29, viii205. [Google Scholar] [CrossRef]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J. Clin. Oncol. 2022, 40, 378. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Rizzo, A.; Salati, M.; Frega, G.; Merz, V.; Caputo, F.; Ricci, A.D.; Palloni, A.; Messina, C.; Spallanzani, A.; Saccoccio, G.; et al. Second-line chemotherapy (2L) in elderly patients with advanced biliary tract cancer (ABC): A multicenter real-world study. J. Clin. Oncol. 2021, 39, 322. [Google Scholar] [CrossRef]
- Yoo, C.; Kim, K.-P.; Jeong, J.H.; Kim, I.; Kang, M.J.; Cheon, J.; Kang, B.W.; Ryu, H.; Lee, J.S.; Kim, K.W.; et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): A multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021, 22, 1560–1572. [Google Scholar] [CrossRef]
- Belkouz, A.; de Vos-Geelen, J.; Mathôt, R.A.A.; Eskens, F.A.L.M.; van Gulik, T.M.; van Oijen, M.G.H.; Punt, C.J.A.; Wilmink, J.W.; Klümpen, H.J. Efficacy and safety of FOLFIRINOX as salvage treatment in advanced biliary tract cancer: An open-label, single arm, phase 2 trial. Br. J. Cancer 2020, 122, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Makawita, S.; Abou-Alfa, G.K.; Roychowdhury, S.; Sadeghi, S.; Borbath, I.; Goyal, L.; Cohn, A.; Lamarca, A.; Oh, D.Y.; Macarulla, T.; et al. Infigratinib in patients with advanced cholangiocarcinoma with. Future Oncol. 2020, 16, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Liu, T.; Li, W.; Yu, Y.; Guo, X.; Xu, X.; Wang, Y.; Li, Q.; Wang, Y.; Cui, Y.; Liu, H.; et al. 53P Toripalimab with chemotherapy as first-line treatment for advanced biliary tract tumors: A preliminary analysis of safety and efficacy of an open-label phase II clinical study. Ann. Oncol. 2020, 31, S261. [Google Scholar] [CrossRef]
- Zhou, J.; Fan, J.; Shi, G.; Huang, X.; Wu, D.; Yang, G.; Ge, N.; Hou, Y.; Sun, H.; Huang, X.; et al. 56P Anti-PD1 antibody toripalimab, lenvatinib and gemox chemotherapy as first-line treatment of advanced and unresectable intrahepatic cholangiocarcinoma: A phase II clinical trial. Ann. Oncol. 2020, 31, S262–S263. [Google Scholar] [CrossRef]
- Shi, G.-M.; Jian, Z.; Fan, J.; Huang, X.-Y.; Wu, D.; Liang, F.; Lu, J.-C.; Yang, G.-H.; Chen, Y.; Ge, N.-L.; et al. Phase II study of lenvatinib in combination with GEMOX chemotherapy for advanced intrahepatic cholangiocarcinoma. J. Clin. Oncol. 2021, 39, e16163. [Google Scholar] [CrossRef]
- Borad, M.J.; Bai, L.-Y.; Chen, M.-H.; Hubbard, J.M.; Mody, K.; Rha, S.Y.; Richards, D.A.; Davis, S.L.; Soong, J.; Huang, C.-E.C.-E.; et al. Silmitasertib (CX-4945) in combination with gemcitabine and cisplatin as first-line treatment for patients with locally advanced or metastatic cholangiocarcinoma: A phase Ib/II study. J. Clin. Oncol. 2021, 39, 312. [Google Scholar] [CrossRef]
- Goyal, L.; Sirard, C.; Schrag, M.; Kagey, M.H.; Eads, J.R.; Stein, S.; El-Khoueiry, A.B.; Manji, G.A.; Abrams, T.A.; Khorana, A.A.; et al. Phase I and Biomarker Study of the Wnt Pathway Modulator DKN-01 in Combination with Gemcitabine/Cisplatin in Advanced Biliary Tract Cancer. Clin. Cancer Res. 2020, 26, 6158–6167. [Google Scholar] [CrossRef]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final results from ClarIDHy, a global, phase III, randomized, double-blind study of ivosidenib (IVO) versus placebo (PBO) in patients (pts) with previously treated cholangiocarcinoma (CCA) and an isocitrate dehydrogenase 1 (IDH1) mutation. J. Clin. Oncol. 2021, 39, 266. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, J.; Sun, H.; Bai, C.; Jia, R.; Li, Y.; Zhang, W.; Liu, L.; Huang, C.; Guan, M.; et al. A single-arm, multicenter, open-label phase 2 trial of surufatinib in patients with unresectable or metastatic biliary tract cancer. J. Clin. Oncol. 2021, 39, e16123. [Google Scholar] [CrossRef]
- Zong, H.; Zhong, Q.; Zhao, R.; Jin, S.; Zhou, C.; Zhang, X.; Shi, J.; Qiao, S.; Han, J.; Jiang, M. Phase II study of anlotinib plus sintlimab as second-line treatment for patients with advanced biliary tract cancers. J. Clin. Oncol. 2021, 39, 307. [Google Scholar] [CrossRef]
- Bridgewater, J.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.; Abrams, T.; Furuse, J.; Kelley, R.K.; Cassier, P.; et al. 54P Efficacy and safety of futibatinib in intrahepatic cholangiocarcinoma (iCCA) harboring FGFR2 fusions/other rearrangements: Subgroup analyses of a phase II study (FOENIX-CCA2). Ann. Oncol. 2020, 31, S261–S262. [Google Scholar] [CrossRef]
- Javle, M.M.; Abou-Alfa, G.K.; Macarulla, T.; Personeni, N.; Adeva, J.; Bergamo, F.; Malka, D.; Vogel, A.; Knox, J.J.; Evans, T.R.J.; et al. Efficacy of derazantinib in intrahepatic cholangiocarcinoma patients with FGFR2 mutations or amplifications: Interim results from the phase 2 study FIDES-01. J. Clin. Oncol. 2022, 40, 427. [Google Scholar] [CrossRef]
- Villanueva, L.; Lwin, Z.; Chung, H.C.C.; Gomez-Roca, C.A.; Longo, F.; Yanez, E.; Senellart, H.; Doherty, M.; Garcia-Corbacho, J.; Hendifar, A.E.; et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase 2 LEAP-005 study. J. Clin. Oncol. 2021, 39, 4080. [Google Scholar] [CrossRef]
- Ahn, D.H.; Uson Junior, P.L.S.; Masci, P.; Kosiorek, H.; Halfdanarson, T.R.; Mody, K.; Babiker, H.; DeLeon, T.; Sonbol, M.B.; Gores, G.; et al. A pilot study of Pan-FGFR inhibitor ponatinib in patients with FGFR-altered advanced cholangiocarcinoma. Invest New Drugs. 2022, 40, 134–141. [Google Scholar] [CrossRef]
- Cleary, J.M.; Iyer, G.; Oh, D.-Y.; Mellinghoff, I.K.; Goyal, L.; Ng, M.C.H.; Meric-Bernstam, F.; Matos, I.; Chao, T.-Y.; Sarkouh, R.A.; et al. Final results from the phase I study expansion cohort of the selective FGFR inhibitor Debio 1,347 in patients with solid tumors harboring an FGFR gene fusion. J. Clin. Oncol. 2020, 38, 3603. [Google Scholar] [CrossRef]
- Harding, J.J.; Cleary, J.M.; Quinn, D.I.; Braña, I.; Moreno, V.; Borad, M.J.; Loi, S.; Spanggaard, I.; Park, H.; Ford, J.M.; et al. Targeting HER2 (ERBB2) mutation-positive advanced biliary tract cancers with neratinib: Results from the phase II SUMMIT ‘basket’ trial. J. Clin. Oncol. 2021, 39, 320. [Google Scholar] [CrossRef]
- Mazzaferro, V.; El-Rayes, B.F.; Droz Dit Busset, M.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 2019, 120, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Yoo, C.; Oh, D.-Y.; Choi, H.J.; Kudo, M.; Ueno, M.; Kondo, S.; Chen, L.-T.; Osada, M.; Helwig, C.; Dussault, I.; et al. 73P Long-term follow-up of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. Ann. Oncol. 2020, 31, S268–S269. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Hanna, D.L.; El-Khoueiry, A.B.; Kang, Y.-K.; Oh, D.-Y.; Chaves, J.M.; Rha, S.Y.; Hamilton, E.P.; Pant, S.; Javle, M.M.; et al. Zanidatamab (ZW25) in HER2-positive biliary tract cancers (BTCs): Results from a phase I study. J. Clin. Oncol. 2021, 39, 299. [Google Scholar] [CrossRef]
- Zhou, J.; Gong, J.; Cao, Y.; Peng, Z.; Yuan, J.; Wang, X.; LU, M.; Shen, L. Anlotinib plus TQB2450 in patients with advanced refractory biliary tract cancer (BTC): An open-label, dose-escalating, and dose-expansion cohort of phase Ib trial. J. Clin. Oncol. 2021, 39, 292. [Google Scholar] [CrossRef]
- Beenken, A.; Mohammadi, M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009, 8, 235–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallinan, N.; Finn, S.; Cuffe, S.; Rafee, S.; O’Byrne, K.; Gately, K. Targeting the fibroblast growth factor receptor family in cancer. Cancer Treat. Rev. 2016, 46, 51–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, L.; Kongpetch, S.; Crolley, V.E.; Bridgewater, J. Targeting FGFR inhibition in cholangiocarcinoma. Cancer Treat. Rev. 2021, 95, 102170. [Google Scholar] [CrossRef]
- Presta, M.; Chiodelli, P.; Giacomini, A.; Rusnati, M.; Ronca, R. Fibroblast growth factors (FGFs) in cancer: FGF traps as a new therapeutic approach. Pharmacol. Ther. 2017, 179, 171–187. [Google Scholar] [CrossRef]
- Consortium, A.P.G. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831. [Google Scholar] [CrossRef] [Green Version]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kurzrock, R. The FGFR Landscape in Cancer: Analysis of 4853 Tumors by Next-Generation Sequencing. Clin. Cancer Res. 2016, 22, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Arai, Y.; Totoki, Y.; Hosoda, F.; Shirota, T.; Hama, N.; Nakamura, H.; Ojima, H.; Furuta, K.; Shimada, K.; Okusaka, T.; et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology 2014, 59, 1427–1434. [Google Scholar] [CrossRef]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef] [Green Version]
- Abou-Alfa, G.K.; Bibeau, K.; Schultz, N.; Yaqubie, A.; Millang, B.M.; Ren, H.; Féliz, L. Effect of FGFR2 alterations on survival in patients receiving systemic chemotherapy for intrahepatic cholangiocarcinoma. J. Clin. Oncol. 2021, 39, 303. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Javle, M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Weiss, K.H.; Waldschmidt, D.T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.; et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: Mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol. Hepatol. 2021, 6, 803–815. [Google Scholar] [CrossRef]
- Bahleda, R.; Meric-Bernstam, F.; Goyal, L.; Tran, B.; He, Y.; Yamamiya, I.; Benhadji, K.A.; Matos, I.; Arkenau, H.T. Phase I, first-in-human study of futibatinib, a highly selective, irreversible FGFR1-4 inhibitor in patients with advanced solid tumors. Ann. Oncol. 2020, 31, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.H.; Hierro, C.; Heist, R.S.; Cleary, J.M.; Meric-Bernstam, F.; Tabernero, J.; Janku, F.; Gandhi, L.; Iafrate, A.J.; Borger, D.R.; et al. A Phase I, Open-Label, Multicenter, Dose-escalation Study of the Oral Selective FGFR Inhibitor Debio 1347 in Patients with Advanced Solid Tumors Harboring. Clin. Cancer Res. 2019, 25, 2699–2707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.-H.; Su, W.-C.; Oh, D.-Y.; Shen, L.; Kim, K.-P.; Liu, X.; Liao, H.; Qing, M.; Qian, J.; Triantos, S.; et al. Updated analysis with longer follow up of a phase 2a study evaluating erdafitinib in Asian patients (pts) with advanced cholangiocarcinoma (CCA) and fibroblast growth factor receptor (FGFR) alterations. J. Clin. Oncol. 2022, 40, 430. [Google Scholar] [CrossRef]
- Alabduladhem, T.O.; Bordoni, B. Physiology, Krebs Cycle. In StatPearls; StatPearls Publishing© 2021, StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Rakheja, D.; Medeiros, L.J.; Bevan, S.; Chen, W. The emerging role of d-2-hydroxyglutarate as an oncometabolite in hematolymphoid and central nervous system neoplasms. Front. Oncol. 2013, 3, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makawita, S.; Borad, M.J.; Carapeto, F.; Kwong, L.; Bekaii-Saab, T.S.; Murugesan, K.; Ross, J.S.; Danziger, N.; Israel, M.A.; McGregor, K.; et al. IDH1 and IDH2 Driven Intrahepatic Cholangiocarcinoma (IHCC): A comprehensive genomic and immune profiling study. J. Clin. Oncol. 2021, 39, 4009. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.; Wu, F.; Ni, C.; Wang, Y.; Chen, S.; Bai, Y. 76P The distribution of tumor mutational burden in IDH-mutant solid tumors. Ann. Oncol. 2020, 31, S270. [Google Scholar] [CrossRef]
- Manne, A.; Woods, E.; Tsung, A.; Mittra, A. Biliary Tract Cancers: Treatment Updates and Future Directions in the Era of Precision Medicine and Immuno-Oncology. Front. Oncol. 2021, 11, 768009. [Google Scholar] [CrossRef]
- Salama, V.; Brooks, N.; Skwarska, A.; Kays, L.; Milligan, P.; Newell, K.; Roth, K.; Geeganage, S.; Gilmour, R.; Chan, S.M.; et al. Abstract 6417: LY3410738, a novel inhibitor of mutant IDH1 is more effective than Ivosidenib and potentiates antileukemic activity of standard chemotherapy in preclinical models of acute myeloid leukemia (AML). Cancer Res. 2020, 80, 6417. [Google Scholar] [CrossRef]
- Pauff, J.M.; Papadopoulos, K.P.; Janku, F.; Turk, A.A.; Goyal, L.; Shroff, R.T.; Shimizu, T.; Ikeda, M.; Azad, N.S.; Cleary, J.M.; et al. A phase I study of LY3410738, a first-in-class covalent inhibitor of mutant IDH1 in cholangiocarcinoma and other advanced solid tumors. J. Clin. Oncol. 2021, 39, TPS350. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
- Demols, A.; Perez-Casanova, L.; Rocq, L.; Charry, M.; De Nève, N.; Verrellen, A.; Ramadhan, A.; Van Campenhout, C.; De Clercq, S.; Maris, C.; et al. 71P NTRK gene fusions in bilio-pancreatic cancers. Ann. Oncol. 2020, 31, S268. [Google Scholar] [CrossRef]
- Yoshikawa, D.; Ojima, H.; Iwasaki, M.; Hiraoka, N.; Kosuge, T.; Kasai, S.; Hirohashi, S.; Shibata, T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br. J. Cancer 2008, 98, 418–425. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Koukourakis, M.I.; Simopoulos, C.; Polychronidis, A.; Sivridis, E. Vascular endothelial growth factor (VEGF) expression in operable gallbladder carcinomas. Eur. J. Surg. Oncol. 2003, 29, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shroff, R.T.; Makawita, S.; Xiao, L.; Danner De Armas, A.; Bhosale, P.; Reddy, K.; Shalaby, A.; Raghav, K.; Pant, S.; et al. Phase II Study of Ramucirumab in Advanced Biliary Tract Cancer Previously Treated by Gemcitabine-based Chemotherapy. Clin. Cancer Res. 2022. [Google Scholar] [CrossRef]
- Rao, J.-h.; Wu, C.; Zhang, H.; Wang, X.; Lu, L.; Cheng, F.; Chen, D. Efficacy and biomarker analysis of neoadjuvant carrizumab plus apatinib in patients with local advanced biliary tract cancers. J. Clin. Oncol. 2021, 39, e16126. [Google Scholar] [CrossRef]
- Wang, D.; Yang, X.; Long, J.; Lin, J.; Mao, J.; Xie, F.; Wang, Y.; Wang, Y.; Xun, Z.; Bai, Y.; et al. The Efficacy and Safety of Apatinib Plus Camrelizumab in Patients With Previously Treated Advanced Biliary Tract Cancer: A Prospective Clinical Study. Front. Oncol. 2021, 11, 646979. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, P.; Shi, R. Anlotinib as a molecular targeted therapy for tumors (Review). Oncol. Lett. 2020, 20, 1001–1014. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, J.; Fang, W.; Lu, P.; Fan, Q.; Shu, Y.; Feng, J.F.; Zhang, S.; Ba, Y.; Liu, Y.; et al. Anlotinib in chemotherapy-refractory metastatic esophageal squamous cell carcinoma (ESCC): A randomized, double-blind, multicenter phase II trial. J. Clin. Oncol. 2019, 37, 95. [Google Scholar] [CrossRef]
- Han, B.; Li, K.; Wang, Q.; Zhang, L.; Shi, J.; Wang, Z.; Cheng, Y.; He, J.; Shi, Y.; Zhao, Y.; et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non–Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 1569. [Google Scholar] [CrossRef]
- Zhou, A.P.; Bai, Y.; Song, Y.; Luo, H.; Ren, X.B.; Wang, X.; Shi, B.; Fu, C.; Cheng, Y.; Liu, J.; et al. Anlotinib Versus Sunitinib as First-Line Treatment for Metastatic Renal Cell Carcinoma: A Randomized Phase II Clinical Trial. Oncologist 2019, 24, e702–e708. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Du, F.; Gao, M.; Ji, Q.; Li, Z.; Zhang, Y.; Guo, Z.; Wang, J.; Chen, X.; Wang, J.; et al. Anlotinib for the Treatment of Patients with Locally Advanced or Metastatic Medullary Thyroid Cancer. Thyroid 2018, 28, 1455–1461. [Google Scholar] [CrossRef]
- Browne, B.C.; O’Brien, N.; Duffy, M.J.; Crown, J.; O’Donovan, N. HER-2 signaling and inhibition in breast cancer. Curr. Cancer Drug Targets 2009, 9, 419–438. [Google Scholar] [CrossRef]
- Galdy, S.; Lamarca, A.; Mcnamara, M.G.; Hubner, R.A.; Cella, C.A.; Fazio, N.; Valle, J.W. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: A potential therapeutic target? Cancer Metastasis Rev. 2017, 36, 141–157. [Google Scholar] [CrossRef] [Green Version]
- Mondaca, S.; Razavi, P.; Xu, C.; Offin, M.; Myers, M.; Scaltriti, M.; Hechtman, J.F.; Bradley, M.; O’Reilly, E.M.; Berger, M.F.; et al. Genomic Characterization of ERBB2-Driven Biliary Cancer and a Case of Response to Ado-Trastuzumab Emtansine. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.-B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef] [Green Version]
- Ter Veer, E.; Creemers, A.; De Waal, L.; Van Oijen, M.G.H.; Van Laarhoven, H.W.M. Comparing cytotoxic backbones for first-line trastuzumab-containing regimens in human epidermal growth factor receptor 2-positive advanced oesophagogastric cancer: A meta-analysis. Int. J. Cancer 2018, 143, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Valabrega, G.; Montemurro, F.; Aglietta, M. Trastuzumab: Mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann. Oncol. 2007, 18, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Beckmann, M.W.; Rody, A.; Schneeweiss, A.; Müller, V.; Fehm, T.; Marschner, N.; Gluz, O.; Schrader, I.; Heinrich, G.; et al. HER2 Dimerization Inhibitor Pertuzumab—Mode of Action and Clinical Data in Breast Cancer. Breast Care 2013, 8, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Dixit, S.; Abraham, L.; Weiser, N.; Gold, M.R. Abstract 1032: Super-resolution imaging studies of zanidatamab: Providing insights into its bispecific mode of action. Cancer Res. 2021, 81, 1032. [Google Scholar] [CrossRef]
- Kong, A.; Feldinger, K. Profile of neratinib and its potential in the treatment of breast cancer. Breast Cancer Targets Ther. 2015, 7, 147. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.-Y.; Yong, W.-P.; Chen, L.-T.; Kim, J.-W.; Park, J.H.; Hsu, K.; Lindmark, B.; McIntyre, N.; Collins, B.; Firth, C. Varlitinib in combination with gemcitabine and cisplatin for treatment-naïve advanced biliary tract cancer. J. Clin. Oncol. 2022, 40, 439. [Google Scholar] [CrossRef]
- Kudo, R.; Kubo, T.; Mori, Y.; Harada, Y.; Shirota, H.; Hayashi, H.; Kano, M.; Shimizu, Y.; Ishibashi, E.; Akita, H.; et al. A phase 2 basket trial of combination therapy with trastuzumab and pertuzumab in patients with solid cancers harboring HER2 amplification (JUPITER trial). J. Clin. Oncol. 2021, 39, TPS3141. [Google Scholar] [CrossRef]
- Lee, C.-k.; Cheon, J.; Chon, H.J.; Kim, M.H.; Kim, J.W.; Lee, M.A.; Park, H.S.; Kang, M.J.; Jang, J.-S.; Choi, H.J. A phase II trial of trastuzumab plus modified-FOLFOX for gemcitabine/cisplatin refractory HER2-positive biliary tract cancer (BTC): Multi-institutional study of the Korean Cancer Study Group (KCSG-HB19-14). J. Clin. Oncol. 2021, 39, TPS4161. [Google Scholar] [CrossRef]
- Pant, S.; Ducreux, M.; Harding, J.J.; Javle, M.M.; Oh, D.-Y.; Wasan, H.S.; Fortenberry, A.; Josephson, N.C.; Mwatha, A.; Wang, K.; et al. A phase IIb, open-label, single-arm study of zanidatamab (ZW25) monotherapy in subjects with advanced or metastatic HER2-amplified biliary tract cancers. J. Clin. Oncol. 2021, 39, TPS352. [Google Scholar] [CrossRef]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Salama, A.K.S.; Li, S.; Macrae, E.R.; Park, J.-I.; Mitchell, E.P.; Zwiebel, J.A.; Chen, H.X.; Gray, R.J.; Mcshane, L.M.; Rubinstein, L.V.; et al. Dabrafenib and Trametinib in Patients with Tumors With BRAFV600E Mutations: Results of the NCI-MATCH Trial Subprotocol, H. J. Clin. Oncol. 2020, 38, 3895–3904. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- Bridgewater, J.; Lopes, A.; Beare, S.; Duggan, M.; Lee, D.; Ricamara, M.; Mcentee, D.; Sukumaran, A.; Wasan, H.; Valle, J.W. A phase 1b study of Selumetinib in combination with Cisplatin and Gemcitabine in advanced or metastatic biliary tract cancer: The ABC-04 study. BMC Cancer 2016, 16, 153. [Google Scholar] [CrossRef]
- Allende, J.E.; Allende, C.C. Protein kinase CK2: An enzyme with multiple substrates and a puzzling regulation. FASEB J. 1995, 9, 313–323. [Google Scholar] [CrossRef]
- Jang, S.W.; Hwang, S.S.; Kim, H.S.; Lee, K.O.; Kim, M.K.; Lee, W.; Kim, K.; Lee, G.R. Casein kinase 2 is a critical determinant of the balance of Th17 and Treg cell differentiation. Exp. Mol. Med. 2017, 49, e375. [Google Scholar] [CrossRef] [Green Version]
- Ampofo, E.; Nalbach, L.; Menger, M.D.; Montenarh, M.; Götz, C. Protein Kinase CK2—A Putative Target for the Therapy of Diabetes Mellitus? Int. J. Mol. Sci. 2019, 20, 4398. [Google Scholar] [CrossRef] [Green Version]
- Castello, J.; Ragnauth, A.; Friedman, E.; Rebholz, H. CK2—An Emerging Target for Neurological and Psychiatric Disorders. Pharmaceuticals 2017, 10, 7. [Google Scholar] [CrossRef] [Green Version]
- Silva-Pavez, E.; Tapia, J.C. Protein Kinase CK2 in Cancer Energetics. Front. Oncol. 2020, 10, 893. [Google Scholar] [CrossRef]
- Siddiqui-Jain, A.; Drygin, D.; Streiner, N.; Chua, P.; Pierre, F.; O’Brien, S.E.; Bliesath, J.; Omori, M.; Huser, N.; Ho, C.; et al. CX-4945, an Orally Bioavailable Selective Inhibitor of Protein Kinase CK2, Inhibits Prosurvival and Angiogenic Signaling and Exhibits Antitumor Efficacy. Cancer Res. 2010, 70, 10288–10298. [Google Scholar] [CrossRef] [Green Version]
- Subbiah, V.; Cassier, P.A.; Siena, S.; Alonso, G.; Paz-Ares, L.G.; Garrido, P.; Nadal, E.; Curigliano, G.; Vuky, J.; Lopes, G.; et al. Clinical activity and safety of the RET inhibitor pralsetinib in patients with RET fusion-positive solid tumors: Update from the ARROW trial. J. Clin. Oncol. 2021, 39, 3079. [Google Scholar] [CrossRef]
- Yoon, J.S.; Kim, J.W.; Kim, J.-W.; Kim, T.-Y.; Nam, A.-R.; Bang, J.-H.; Seo, H.-R.; Kim, J.-M.; Oh, K.S.; Mortimer, P.G.; et al. DNA-damage response-umbrella study of the combination of ceralasertib and olaparib, or ceralasertib and durvalumab in advanced biliary tract cancer: A phase 2 trial-in-progress. J. Clin. Oncol. 2021, 39, TPS4166. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Eckhardt, G.; Patnaik, A.; Lorusso, P.; Faoro, L.; Heymach, J.V.; Kapoun, A.M.; Xu, L.; Munster, P. A phase I dose-escalation and dose-expansion study of brontictuzumab in subjects with selected solid tumors. Ann. Oncol. 2018, 29, 1561–1568. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [Green Version]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Merino, D.M.; Mcshane, L.M.; Fabrizio, D.; Funari, V.; Chen, S.-J.; White, J.R.; Wenz, P.; Baden, J.; Barrett, J.C.; Chaudhary, R.; et al. Establishing guidelines to harmonize tumor mutational burden (TMB): In silico assessment of variation in TMB quantification across diagnostic platforms: Phase I of the Friends of Cancer Research TMB Harmonization Project. J. ImmunoTherapy Cancer 2020, 8, e000147. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.-M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients with Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888. [Google Scholar] [CrossRef]
- Valle, J.W.; Kelley, R.K.; Furuse, J.; Edeline, J.; Finn, R.S.; Ren, Z.; Su, S.-C.; Malhotra, U.; Siegel, A.B.; Vogel, A. 78TiP KEYNOTE-966 trial in progress: Pembrolizumab plus gemcitabine and cisplatin for advanced biliary tract cancer. Ann. Oncol. 2020, 31, S270–S271. [Google Scholar] [CrossRef]
- Yin, C.; Armstrong, S.A.; Agarwal, S.; Wang, H.; Noel, M.S.; Weinberg, B.A.; Marshall, J.; He, A.R. Phase II study of combination pembrolizumab and olaparib in patients with advanced cholangiocarcinoma: Interim results. J. Clin. Oncol. 2022, 40, 452. [Google Scholar] [CrossRef]
- Sahai, V.; Griffith, K.A.; Lin, B.S.-L.; Soares, H.P.; Chandana, S.R.; Crysler, O.V.; Enzler, T.; Zalupski, M. A multicenter phase Ib/II study of liposomal-irinotecan, 5-fluorouracil (5-FU), and leucovorin (LV) with nivolumab as second-line therapy for patients with advanced biliary tract cancer (BilT-03). J. Clin. Oncol. 2022, 40, 438. [Google Scholar] [CrossRef]
- Oh, D.-Y.; De Braud, F.; Bridgewater, J.; Furuse, J.; Hsu, C.-H.; Ikeda, M.; Javle, M.; Moehler, M.; Park, J.O.; Shen, L.; et al. 79TiP A phase II/III, randomized, placebo-controlled study of bintrafusp alfa with gemcitabine plus cisplatin as first-line treatment of biliary tract cancer. Ann. Oncol. 2020, 31, S271–S272. [Google Scholar] [CrossRef]
- Jian, Z.; Fan, J.; Shi, G.-M.; Huang, X.-Y.; Wu, D.; Yang, G.-H.; Ji, Y.; Chen, Y.; Liang, F.; Lu, J.-C.; et al. Gemox chemotherapy in combination with anti-PD1 antibody toripalimab and lenvatinib as first-line treatment for advanced intrahepatic cholangiocarcinoma: A phase 2 clinical trial. J. Clin. Oncol. 2021, 39, 4094. [Google Scholar] [CrossRef]
- Yarchoan, M.; Cope, L.; Ruggieri, A.N.; Anders, R.A.; Noonan, A.M.; Goff, L.W.; Goyal, L.; Lacy, J.; Li, D.; Patel, A.K.; et al. Multicenter randomized phase II trial of atezolizumab with or without cobimetinib in biliary tract cancers. J. Clin. Investig. 2021, 131, e152670. [Google Scholar] [CrossRef]
- Javle, M.M.; Bridgewater, J.A.; Gbolahan, O.B.; Jungels, C.; Cho, M.T.; Papadopoulos, K.P.; Thistlethwaite, F.C.; Canon, J.-L.R.; Cheng, L.; Ioannidis, S.; et al. A phase I/II study of safety and efficacy of the arginase inhibitor INCB001158 plus chemotherapy in patients (Pts) with advanced biliary tract cancers. J. Clin. Oncol. 2021, 39, 311. [Google Scholar] [CrossRef]
- Khoja, L.; Day, D.; Wei-Wu Chen, T.; Siu, L.L.; Hansen, A.R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann. Oncol. 2017, 28, 2377–2385. [Google Scholar] [CrossRef]
- Kartolo, A.; Sattar, J.; Sahai, V.; Baetz, T.; Lakoff, J.M. Predictors of immunotherapy-induced immune-related adverse events. Curr. Oncol. 2018, 25, 403–410. [Google Scholar] [CrossRef] [Green Version]
- NCCN. Management of Immunotherapy—Related Toxicities. Available online: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf (accessed on 7 October 2021).
- Sadelain, M.; Rivière, I.; Brentjens, R. Targeting tumours with genetically enhanced T lymphocytes. Nat. Rev. Cancer 2003, 3, 35–45. [Google Scholar] [CrossRef]
- Gill, S.; Brudno, J.N. CAR T-Cell Therapy in Hematologic Malignancies: Clinical Role, Toxicity, and Unanswered Questions. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e246–e265. [Google Scholar] [CrossRef]
- Phanthaphol, N.; Somboonpatarakun, C.; Suwanchiwasiri, K.; Chieochansin, T.; Sujjitjoon, J.; Wongkham, S.; Maher, J.; Junking, M.; Yenchitsomanus, P.T. Chimeric Antigen Receptor T Cells Targeting Integrin αvβ6 Expressed on Cholangiocarcinoma Cells. Front. Oncol. 2021, 11, 657868. [Google Scholar] [CrossRef]
- Sangsuwannukul, T.; Supimon, K.; Sujjitjoon, J.; Phanthaphol, N.; Chieochansin, T.; Poungvarin, N.; Wongkham, S.; Junking, M.; Yenchitsomanus, P.T. Anti-tumour effect of the fourth-generation chimeric antigen receptor T cells targeting CD133 against cholangiocarcinoma cells. Int. Immunopharmacol. 2020, 89, 107069. [Google Scholar] [CrossRef]
- Feng, K.C.; Guo, Y.L.; Liu, Y.; Dai, H.R.; Wang, Y.; Lv, H.Y.; Huang, J.H.; Yang, Q.M.; Han, W.D. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J. Hematol. Oncol. 2017, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Supimon, K.; Sangsuwannukul, T.; Sujjitjoon, J.; Phanthaphol, N.; Chieochansin, T.; Poungvarin, N.; Wongkham, S.; Junking, M.; Yenchitsomanus, P.T. Anti-mucin 1 chimeric antigen receptor T cells for adoptive T cell therapy of cholangiocarcinoma. Sci. Rep. 2021, 11, 6276. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, K.; Liu, Y.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Jia, H.; Han, W. Phase I Study of Chimeric Antigen Receptor-Modified T Cells in Patients with EGFR-Positive Advanced Biliary Tract Cancers. Clin. Cancer Res. 2018, 24, 1277–1286. [Google Scholar] [CrossRef] [Green Version]
- Aruga, A.; Takeshita, N.; Kotera, Y.; Okuyama, R.; Matsushita, N.; Ohta, T.; Takeda, K.; Yamamoto, M. Phase I clinical trial of multiple-peptide vaccination for patients with advanced biliary tract cancer. J. Transl. Med. 2014, 12, 61. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Ueno, T.; Kawaoka, T.; Hazama, S.; Fukui, M.; Suehiro, Y.; Hamanaka, Y.; Ikematsu, Y.; Imai, K.; Oka, M.; et al. MUC1 peptide vaccination in patients with advanced pancreas or biliary tract cancer. Anticancer Res. 2005, 25, 3575–3579. [Google Scholar]
- Aruga, A.; Takeshita, N.; Kotera, Y.; Okuyama, R.; Matsushita, N.; Ohta, T.; Takeda, K.; Yamamoto, M. Long-term Vaccination with Multiple Peptides Derived from Cancer-Testis Antigens Can Maintain a Specific T-cell Response and Achieve Disease Stability in Advanced Biliary Tract Cancer. Clin. Cancer Res. 2013, 19, 2224–2231. [Google Scholar] [CrossRef] [Green Version]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Ebata, T.; Hirano, S.; Konishi, M.; Uesaka, K.; Tsuchiya, Y.; Ohtsuka, M.; Kaneoka, Y.; Yamamoto, M.; Ambo, Y.; Shimizu, Y.; et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br. J. Surg. 2018, 105, 192–202. [Google Scholar] [CrossRef]
- Edeline, J.; Benabdelghani, M.; Bertaut, A.; Watelet, J.; Hammel, P.; Joly, J.-P.; Boudjema, K.; Fartoux, L.; Bouhier-Leporrier, K.; Jouve, J.-L.; et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J. Clin. Oncol. 2019, 37, 658–667. [Google Scholar] [CrossRef]
- Shroff, R.T.; Kennedy, E.B.; Bachini, M.; Bekaii-Saab, T.; Crane, C.; Edeline, J.; El-Khoueiry, A.; Feng, M.; Katz, M.H.G.; Primrose, J.; et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1015–1027. [Google Scholar] [CrossRef] [Green Version]
- Edeline, J.; Hirano, S.; Bertaut, A.; Konishi, M.; Benabdelghani, M.; Uesaka, K.; Watelet, J.; Ohtsuka, M.; Hammel, P.; Kaneoka, Y.; et al. 55P Adjuvant gemcitabine-based chemotherapy for biliary tract cancer: Pooled analysis of the BCAT and PRODIGE-12 studies. Ann. Oncol. 2020, 31, S262. [Google Scholar] [CrossRef]
- Kim, Y.; Amini, N.; Wilson, A.; Margonis, G.A.; Ethun, C.G.; Poultsides, G.; Tran, T.; Idrees, K.; Isom, C.A.; Fields, R.C.; et al. Impact of Chemotherapy and External-Beam Radiation Therapy on Outcomes among Patients with Resected Gallbladder Cancer: A Multi-institutional Analysis. Ann. Surg. Oncol. 2016, 23, 2998–3008. [Google Scholar] [CrossRef]
- Mallick, S.; Benson, R.; Haresh, K.P.; Julka, P.K.; Rath, G.K. Adjuvant radiotherapy in the treatment of gall bladder carcinoma: What is the current evidence. J. Egypt Natl. Canc. Inst. 2016, 28, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.J.; Lemieux, A.; Kalpathy-Cramer, J.; Ord, C.B.; Walker, G.V.; Fuller, C.D.; Kim, J.-S.; Thomas, C.R. Nomogram for Predicting the Benefit of Adjuvant Chemoradiotherapy for Resected Gallbladder Cancer. J. Clin. Oncol. 2011, 29, 4627–4632. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, S.; Alam, M.N.; Rastogi, N.; Saxena, R. 59P Evolution of adjuvant therapy in radically resected carcinoma gallbladder (GBC) over a decade: A real world experience from a regional cancer centre. Ann. Oncol. 2020, 31, S264. [Google Scholar] [CrossRef]
- Rizzo, A.; Brandi, G. Neoadjuvant therapy for cholangiocarcinoma: A comprehensive literature review. Cancer Treat. Res. Commun. 2021, 27, 100354. [Google Scholar] [CrossRef]
- Hashimoto, K.; Tono, T.; Nishida, K.; Nonaka, R.; Tsunashima, R.; Fujie, Y.; Fujita, S.; Fujita, J.; Yoshida, T.; Ohnishi, T.; et al. A case of curatively resected advanced intrahepatic cholangiocellular carcinoma through effective response to neoadjuvant chemotherapy. Gan Kagaku Ryoho 2014, 41, 2083–2085. [Google Scholar]
- Kato, A.; Shimizu, H.; Ohtsuka, M.; Yoshidome, H.; Yoshitomi, H.; Furukawa, K.; Takeuchi, D.; Takayashiki, T.; Kimura, F.; Miyazaki, M. Surgical Resection after Downsizing Chemotherapy for Initially Unresectable Locally Advanced Biliary Tract Cancer: A Retrospective Single-center Study. Ann. Surg. Oncol. 2013, 20, 318–324. [Google Scholar] [CrossRef]
- McMasters, K.M.; Tuttle, T.M.; Leach, S.D.; Rich, T.; Cleary, K.R.; Evans, D.B.; Curley, S.A. Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am. J. Surg. 1997, 174, 605–608; discussion 608–609. [Google Scholar] [CrossRef]
- Nelson, J.W.; Ghafoori, A.P.; Willett, C.G.; Tyler, D.S.; Pappas, T.N.; Clary, B.M.; Hurwitz, H.I.; Bendell, J.C.; Morse, M.A.; Clough, R.W.; et al. Concurrent Chemoradiotherapy in Resected Extrahepatic Cholangiocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Le Roy, B.; Gelli, M.; Pittau, G.; Allard, M.-A.; Pereira, B.; Serji, B.; Vibert, E.; Castaing, D.; Adam, R.; Cherqui, D.; et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br. J. Surg. 2018, 105, 839–847. [Google Scholar] [CrossRef]
- Hong, T.S.; Wo, J.Y.; Yeap, B.Y.; Ben-Josef, E.; Mcdonnell, E.I.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; Goyal, L.; et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients with Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J. Clin. Oncol. 2016, 34, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Tao, R.; Krishnan, S.; Bhosale, P.R.; Javle, M.M.; Aloia, T.A.; Shroff, R.T.; Kaseb, A.O.; Bishop, A.J.; Swanick, C.W.; Koay, E.J.; et al. Ablative Radiotherapy Doses Lead to a Substantial Prolongation of Survival in Patients with Inoperable Intrahepatic Cholangiocarcinoma: A Retrospective Dose Response Analysis. J. Clin. Oncol. 2016, 34, 219–226. [Google Scholar] [CrossRef]
- Polistina, F.A.; Guglielmi, R.; Baiocchi, C.; Francescon, P.; Scalchi, P.; Febbraro, A.; Costantin, G.; Ambrosino, G. Chemoradiation treatment with gemcitabine plus stereotactic body radiotherapy for unresectable, non-metastatic, locally advanced hilar cholangiocarcinoma. Results of a five year experience. Radiother. Oncol. 2011, 99, 120–123. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).