The Effect of Intratumoral Interrelation among FOXP3+ Regulatory T Cells on Treatment Response and Survival in Triple-Negative Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Patients and Tumor Samples
2.3. Pathological Assessment and Evaluation
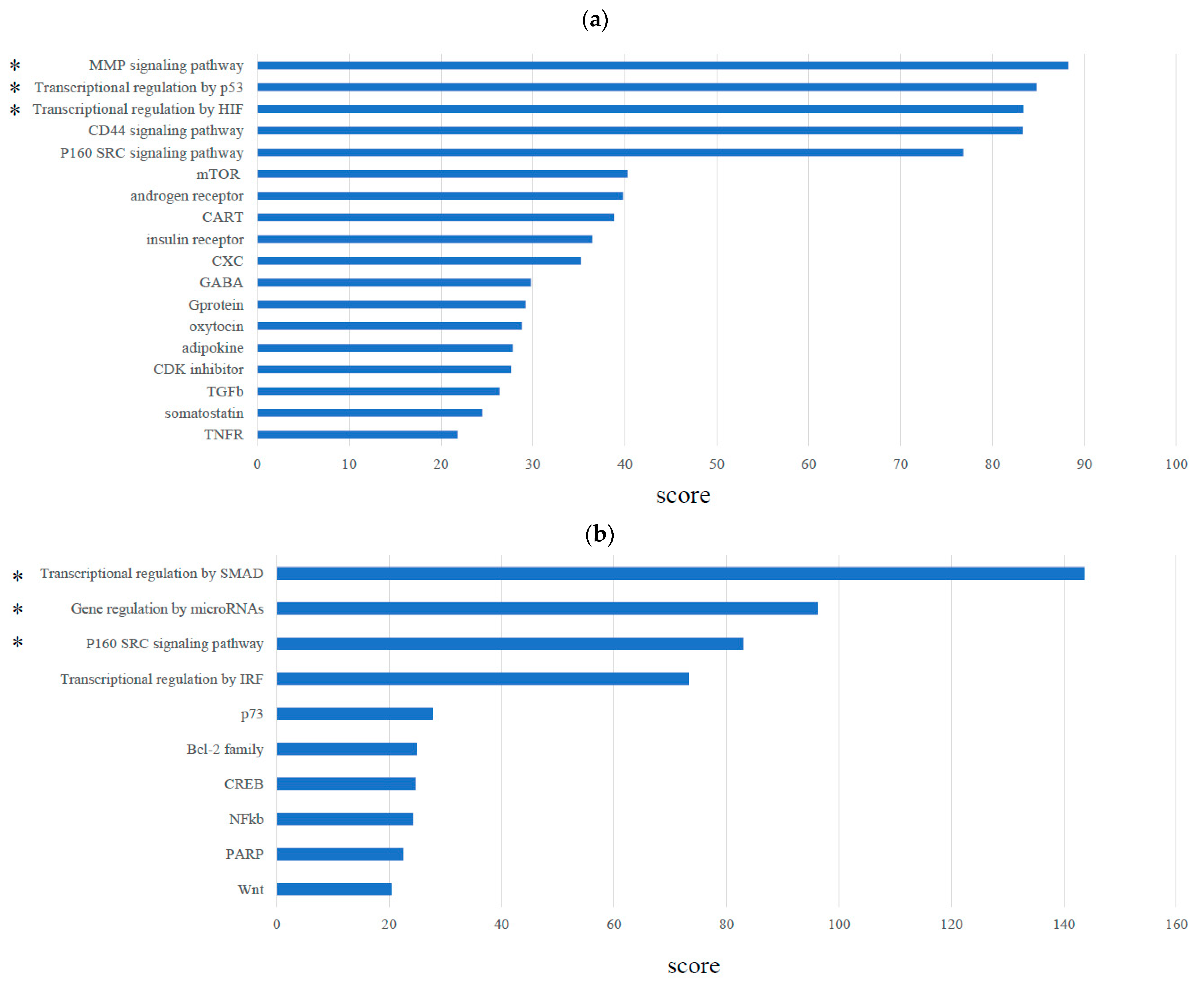
2.4. Molecular Network and Pathway Analysis
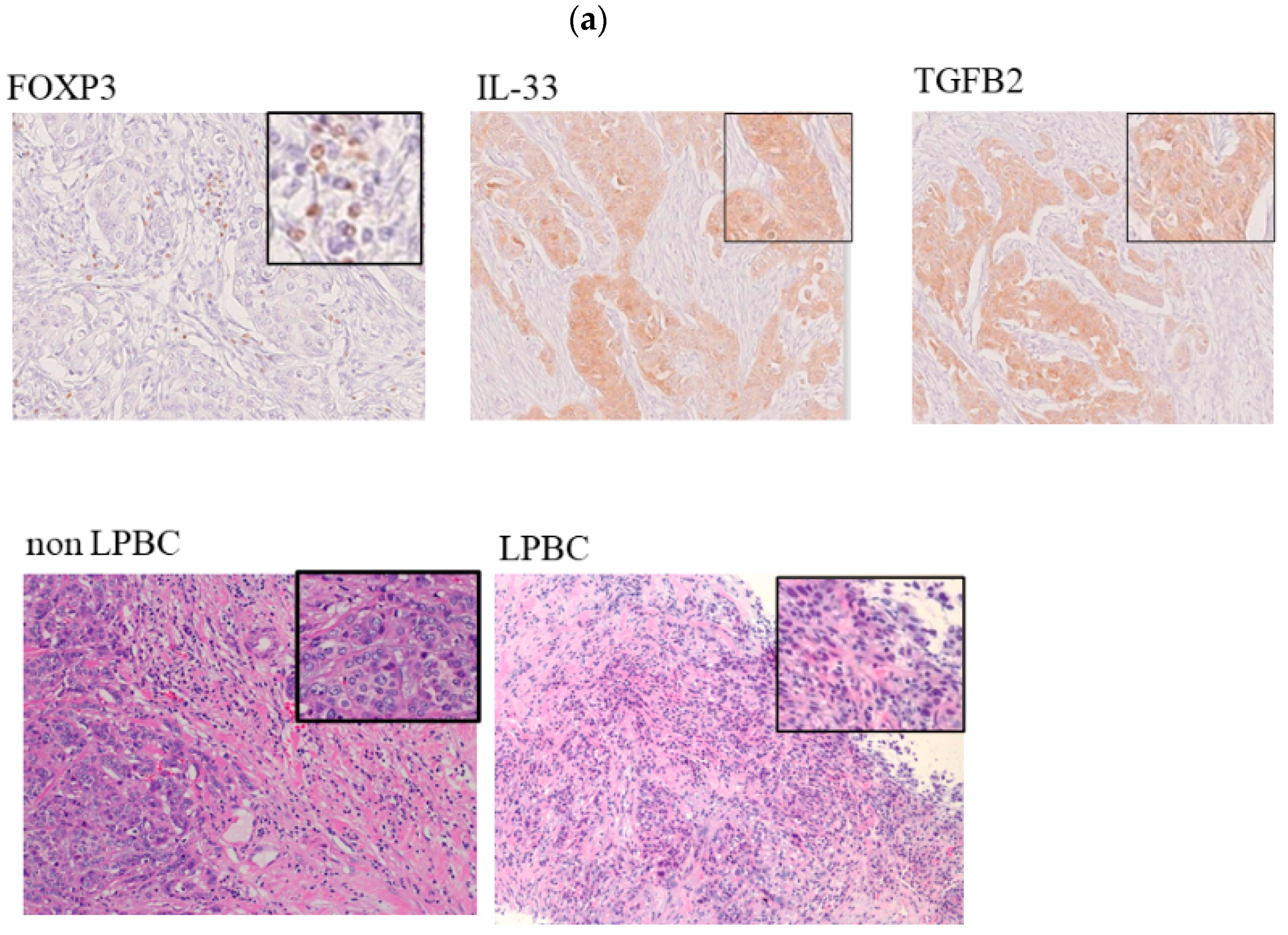
2.5. Immunohistochemistry
2.6. External Gene Expression Data Analysis
2.7. Statistical Analyses
3. Results
3.1. Molecular Network Analysis of Treg Infiltration of TNBC
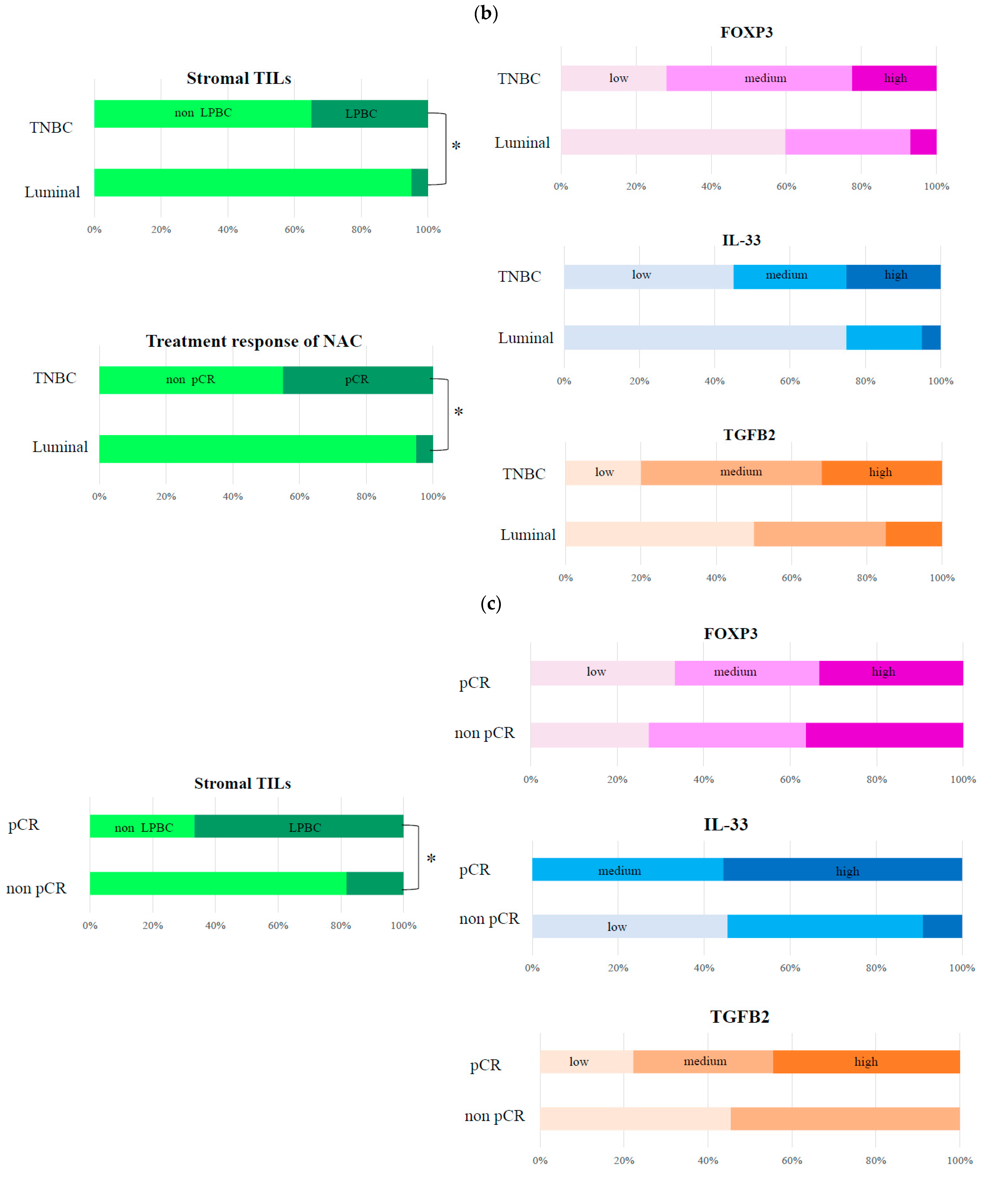
3.2. Histopathological Evaluation of Markers Involved in FOXP3 in TNBC
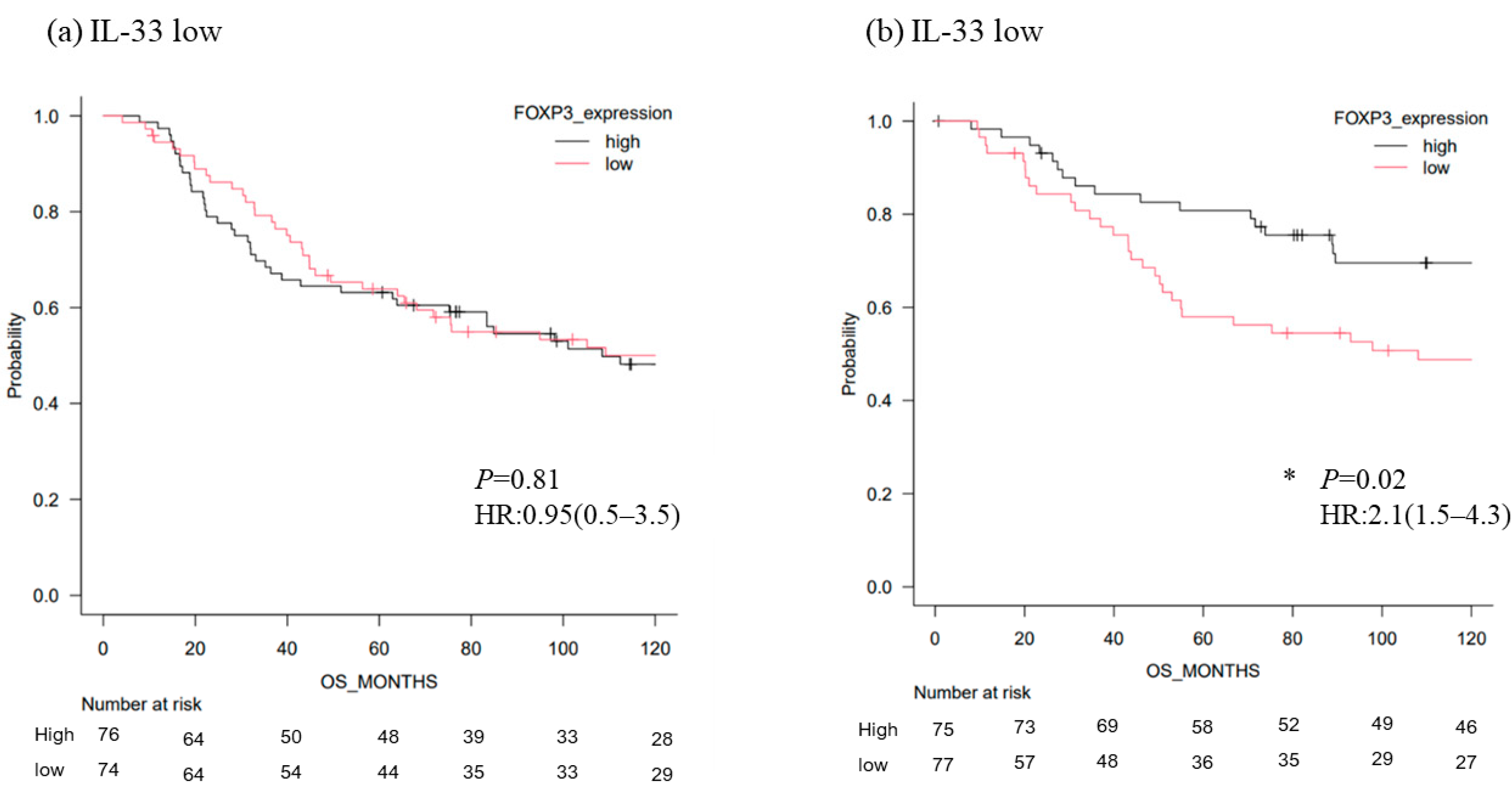
3.3. Survival Analysis of TNBC According to the Level of FOXP3 mRNA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-Negative Breast Cancer Molecular Subtyping and Treatment Progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Karantza, V.; Aktan, G.; Lala, M. Current Treatment Landscape for Patients with Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer: A Systematic Literature Review. Breast Cancer Res. 2019, 21, 143. [Google Scholar] [CrossRef] [Green Version]
- Masuda, N.; Lee, S.J.; Ohtani, S.; Im, Y.H.; Lee, E.S.; Yokota, I.; Kuroi, K.; Im, S.A.; Park, B.W.; Kim, S.B.; et al. Adjuvant Capecitabine for Breast Cancer After Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Savas, P.; Salgado, R.; Denkert, C.; Sotiriou, C.; Darcy, P.K.; Smyth, M.J.; Loi, S. Clinical Relevance of Host Immunity in Breast Cancer: From TILs to the Clinic. Nat. Rev. Clin. Oncol. 2016, 13, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.L.; Bono, P.; Kataja, V.; Desmedt, C.; et al. Tumor-Infiltrating Lymphocytes Are Prognostic in Triple Negative Breast Cancer and Predictive for Trastuzumab Benefit in Early Breast Cancer: Results from the FinHER Trial. Ann. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Stanton, S.E.; Disis, M.L. Clinical Significance of Tumor-Infiltrating Lymphocytes in Breast Cancer. J. Immunother. Cancer 2016, 4, 59. [Google Scholar] [CrossRef] [Green Version]
- Adams, S.; Gray, R.J.; Demaria, S.; Goldstein, L.; Perez, E.A.; Shulman, L.N.; Martino, S.; Wang, M.; Jones, V.E.; Saphner, T.J.; et al. Prognostic Value of Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancers from Two phase III Randomized Adjuvant Breast Cancer Trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 2014, 32, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Baretta, Z.; Mocellin, S.; Goldin, E.; Olopade, O.I.; Huo, D. Effect of BRCA Germline Mutations on Breast Cancer Prognosis: A Systematic Review and Meta-Analysis. Medicine 2016, 95, e4975. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.E.; Tung, N.; Conte, P.; Im, S.A.; Senkus, E.; Xu, B.; Masuda, N.; Delaloge, S.; Li, W.; Armstrong, A.; et al. OlympiAD Final Overall Survival and Tolerability Results: Olaparib Versus Chemotherapy Treatment of Physician’s Choice in Patients with a Germline BRCA Mutation and HER2-Negative Metastatic Breast Cancer. Ann. Oncol. 2019, 30, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, J.; Peng, L.; Sahin, A.A.; Huo, L.; Ward, K.C.; O’Regan, R.; Torres, M.A.; Meisel, J.L. Triple-Negative Breast Cancer Has Worse Overall Survival and Cause-Specific Survival than Non-Triple-Negative Breast Cancer. Breast Cancer Res. Treat. 2017, 161, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Miyara, M.; Costantino, C.M.; Hafler, D.A. FOXP3+ Regulatory T Cells in the Human Immune System. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Sakaguchi, S. Regulatory T Cells in Cancer Immunotherapy. Curr. Opin. Immunol. 2014, 27, 1–7. [Google Scholar] [CrossRef] [Green Version]
- de Kruijf, E.M.; van Nes, J.G.; Sajet, A.; Tummers, Q.R.J.G.; Putter, H.; Osanto, S.; Speetjens, F.M.; Smit, V.T.; Liefers, G.J.; van de Velde, C.J.; et al. The Predictive Value of HLA Class I Tumor Cell Expression and Presence of Intratumoral Tregs for Chemotherapy in Patients with Early Breast Cancer. Clin. Cancer Res. 2010, 16, 1272–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladoire, S.; Arnould, L.; Apetoh, L.; Coudert, B.; Martin, F.; Chauffert, B.; Fumoleau, P.; Ghiringhelli, F. Pathologic Complete Response to Neoadjuvant Chemotherapy of Breast Carcinoma Is Associated with the Disappearance of Tumor-Infiltrating foxp3+ Regulatory T Cells. Clin. Cancer Res. 2008, 14, 2413–2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, G.J.; Fox, S.B.; Han, C.; Leek, R.D.; Garcia, J.F.; Harris, A.L.; Banham, A.H. Quantification of Regulatory T Cells Enables the Identification of High-Risk Breast Cancer Patients and Those At Risk of Late Relapse. J. Clin. Oncol. 2006, 24, 5373–5380. [Google Scholar] [CrossRef] [PubMed]
- Yeong, J.; Thike, A.A.; Lim, J.C.; Lee, B.; Li, H.; Wong, S.C.; Hue, S.S.; Tan, P.H.; Iqbal, J. Higher Densities of Foxp3+ Regulatory T Cells Are Associated with Better Prognosis in Triple-Negative Breast Cancer. Breast Cancer Res. Treat. 2017, 163, 21–35. [Google Scholar] [CrossRef] [PubMed]
- deLeeuw, R.J.; Kost, S.E.; Kakal, J.A.; Nelson, B.H. The Prognostic Value of FoxP3+ Tumor-Infiltrating Lymphocytes in Cancer: A Critical Review of the Literature. Clin. Cancer Res. 2012, 18, 3022–3029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, H.; Ishida, S.; Toda, K.; Matsuda, R.; Hayashi, Y.; Shigetaka, M.; Fukuda, M.; Wakamatsu, Y.; Itai, A. New Approaches to Mechanism Analysis for Drug Discovery Using DNA Microarray Data Combined with KeyMolnet. Curr. Drug Discov. Technol. 2005, 2, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Colaprico, A.; Silva, T.C.; Olsen, C.; Garofano, L.; Cava, C.; Garolini, D.; Sabedot, T.S.; Malta, T.M.; Pagnotta, S.M.; Castiglioni, I.; et al. TCGAbiolinks: An R/Bioconductor Package for Integrative Analysis of TCGA Data. Nucleic Acids Res. 2016, 44, e71. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Shah, S.P.; Chin, S.F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The Genomic and Transcriptomic Architecture of 2,000 Breast Tumours Reveals Novel Subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Hothorn, T.; Lausen, B. On the Exact Distribution of Maximally Selected Rank Statistics. Comput. Stat. Data Anal. 2003, 43, 121–137. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.I.; Ding, J.; Sun, Q.; Zhang, S. The Predictive and Prognostic Value of Foxp3+/CD25+ Regulatory T Cells and PD-L1 Expression in Triple Negative Breast Cancer. Ann. Diagn. Pathol. 2019, 40, 143–151. [Google Scholar] [CrossRef]
- Liu, F.; Lang, R.; Zhao, J.; Zhang, X.; Pringle, G.A.; Fan, Y.; Yin, D.; Gu, F.; Yao, Z.; Fu, L. CD8⁺ Cytotoxic T Cell and FOXP3⁺ Regulatory T Cell Infiltration in Relation to Breast Cancer Survival and Molecular Subtypes. Breast Cancer Res. Treat. 2011, 130, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Sasano, H.; Tamaki, K.; Chan, M.; Hirakawa, H.; Suzuki, A.; Tada, H.; Watanabe, G.; Nemoto, N.; Nakagawa, S.; et al. Tumor-Infiltrating CD8+ and FOXP3+ Lymphocytes in Triple-Negative Breast Cancer: Its Correlation with Pathological Complete Response to Neoadjuvant Chemotherapy. Breast Cancer Res. Treat. 2014, 148, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Sasano, H.; Tamaki, K.; Hirakawa, H.; Takahashi, Y.; Nakagawa, S.; Watanabe, G.; Tada, H.; Suzuki, A.; Ohuchi, N.; et al. Prognostic Significance of Tumor-Infiltrating CD8+ and FOXP3+ Lymphocytes in Residual Tumors and Alterations in These Parameters After Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer: A Retrospective Multicenter Study. Breast Cancer Res. 2015, 17, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asano, Y.; Kashiwagi, S.; Goto, W.; Kurata, K.; Noda, S.; Takashima, T.; Onoda, N.; Tanaka, S.; Ohsawa, M.; Hirakawa, K. Tumour-Infiltrating CD8 to FOXP3 Lymphocyte Ratio in Predicting Treatment Responses to Neoadjuvant Chemotherapy of Aggressive Breast Cancer. Br. J. Surg. 2016, 103, 845–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semeraro, M.; Adam, J.; Stoll, G.; Louvet, E.; Chaba, K.; Poirier-Colame, V.; Sauvat, A.; Senovilla, L.; Vacchelli, E.; Bloy, N.; et al. The Ratio of CD8+/FOXP3 T Lymphocytes Infiltrating Breast Tissues Predicts the Relapse of Ductal Carcinoma In Situ. Oncoimmunology 2016, 5, e1218106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wing, J.B.; Tanaka, A.; Sakaguchi, S. Human FOXP3+ Regulatory T Cell Heterogeneity and Function in Autoimmunity and Cancer. Immunity 2019, 50, 302–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyara, M.; Yoshioka, Y.; Kitoh, A.; Shima, T.; Wing, K.; Niwa, A.; Parizot, C.; Taflin, C.; Heike, T.; Valeyre, D.; et al. Functional Delineation and Differentiation Dynamics of Human CD4+ T Cells Expressing the FoxP3 Transcription Factor. Immunity 2009, 30, 899–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiyama, D.; Nishikawa, H.; Maeda, Y.; Nishioka, M.; Tanemura, A.; Katayama, I.; Ezoe, S.; Kanakura, Y.; Sato, E.; Fukumori, Y.; et al. Anti-CCR4 mAb Selectively Depletes Effector-Type FoxP3+CD4+ Regulatory T Cells, Evoking Antitumor Immune Responses in Humans. Proc. Natl. Acad. Sci. USA 2013, 110, 17945–17950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, T.; Nishikawa, H.; Wada, H.; Nagano, Y.; Sugiyama, D.; Atarashi, K.; Maeda, Y.; Hamaguchi, M.; Ohkura, N.; Sato, E.; et al. Two FOXP3(+)CD4(+) T Cell Subpopulations Distinctly Control the Prognosis of Colorectal Cancers. Nat. Med. 2016, 22, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Schiering, C.; Krausgruber, T.; Chomka, A.; Fröhlich, A.; Adelmann, K.; Wohlfert, E.A.; Pott, J.; Griseri, T.; Bollrath, J.; Hegazy, A.N.; et al. The Alarmin IL-33 Promotes Regulatory T-Cell Function in the Intestine. Nature 2014, 513, 564–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faustino, L.D.; Griffith, J.W.; Rahimi, R.A.; Nepal, K.; Hamilos, D.L.; Cho, J.L.; Medoff, B.D.; Moon, J.J.; Vignali, D.A.A.; Luster, A.D. Interleukin-33 Activates Regulatory T Cells to Suppress Innate γδ T Cell Responses in the Lung. Nat. Immunol. 2020, 21, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Worthington, J.J.; Kelly, A.; Smedley, C.; Bauché, D.; Campbell, S.; Marie, J.C.; Travis, M.A. Integrin αvβ8-Mediated TGF-β Activation by Effector Regulatory T Cells Is Essential for Suppression of T-Cell-Mediated Inflammation. Immunity 2015, 42, 903–915. [Google Scholar] [CrossRef] [Green Version]
- Bhola, N.E.; Balko, J.M.; Dugger, T.C.; Kuba, M.G.; Sánchez, V.; Sanders, M.; Stanford, J.; Cook, R.S.; Arteaga, C.L. TGF-β Inhibition Enhances Chemotherapy Action Against Triple-Negative Breast Cancer. J. Clin. Investig. 2013, 123, 1348–1358. [Google Scholar] [CrossRef]
- Panagi, M.; Voutouri, C.; Mpekris, F.; Papageorgis, P.; Martin, M.R.; Martin, J.D.; Demetriou, P.; Pierides, C.; Polydorou, C.; Stylianou, A.; et al. TGF-β Inhibition Combined with Cytotoxic Nanomedicine Normalizes Triple Negative Breast Cancer Microenvironment Towards Anti-Tumor Immunity. Theranostics 2020, 10, 1910–1922. [Google Scholar] [CrossRef]




| Variate | TNBC (n = 20) Number (%) | Luminal (n = 20) Number (%) |
|---|---|---|
| Age (year), median (range) | 57 (38–65) | 59 (40–66) |
| Histological type | ||
| Infiltrating duct carcinoma | 16 (80) | 15 (75) |
| Lobular carcinoma | 0 (0) | 4 (20) |
| Other | 4 (20) | 1 (5) |
| T status | ||
| T1 | 3 (15) | 0 (0) |
| T2 | 7 (35) | 12 (60) |
| T3 | 6 (30) | 5 (25) |
| T4 | 4 (20) | 3 (15) |
| Nodal metastasis | ||
| Negative | 9 (45) | 3 (15) |
| Positive | 11 (55) | 17 (85) |
| Histological grade | ||
| 1 | 1 (5) | 2 (10) |
| 2 | 8 (40) | 9 (45) |
| 3 | 11 (55) | 9 (45) |
| LVI | ||
| Negative | 5 (25) | 12 (60) |
| Positive | 15 (75) | 8 (40) |
| Ki-67 labeling index | ||
| <20% | 2 (10) | 6 (30) |
| ≥20% | 18 (90) | 14 (70) |
| Stromal TILs | ||
| Non-LPBC (stromal TIL <50%) | 13 (65) | 19 (95) |
| LPBC (stromal TILs ≥50%) | 7 (35) | 1 (5) |
| Neoadjuvant chemotherapy | ||
| Non-pCR | 11 (55) | 19 (95) |
| pCR | 9 (45) | 1 (5) |
| Variate | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Ki-67 labeling index (≥20% vs. <20%) | 2.34 (0.48–9.79) | 0.158 | 1.89 (0.33–8.97) | 0.264 |
| TILs (LPBC vs. non LPBC) | 2.13 (1.88–5.648) | 0.002 | 5.45 (1.08–13.8) | 0.002 |
| Histological grade (0–1 vs. 2–3) | 1.89 (0.22–5.98) | 0.685 | 2.17 (0.45–6.58) | 0.475 |
| LVI (positive vs. negative) | 5.12 (0.68–6.85) | 0.445 | 4.98 (0.59–1.57) | 0.555 |
| IL33 (high vs. low) | 3.54 (1.65–6.948) | 0.001 | 6.32 (2.65–12.5) | 0.003 |
| TGFb2 (high vs. low) | 4.12 (0.95–2.32) | 0.542 | 3.85 (0.54–3.98) | 0.335 |
| Variate | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Ki-67 labeling index (≥20% vs. <20%) | 3.25 (0.84–5.23) | 0.658 | 2.45 (0.33–9.65) | 0.678 |
| TILs (LPBC vs. non LPBC) | 3.42 (2.33–8.65) | 0.008 | 6.54 (1.45–21.4) | 0.015 |
| Histological grade (0–1 vs. 2–3) | 0.54 (0.24–5.75) | 0.651 | 0.98 (0.35–5.42) | 0.524 |
| LVI (positive vs. negative) | 2.57 (0.65–6.51) | 0.447 | 5.26 (1.54–15.4) | 0.284 |
| Treatment response of NAC (pCR vs. non pCR) | 3.55 (2.04–9.58) | 0.002 | 8.45 (1.98–14.5) | 0.001 |
| IL33 (high vs. low) | 4.12 (1.65–12.65) | 0.003 | 6.48 (1.59–17.5) | 0.004 |
| TGFb2 (high vs. low) | 2.31 (0.29–5.75) | 0.514 | 1.57 (0.33–9.87) | 0.246 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goda, N.; Nakashima, C.; Nagamine, I.; Otagaki, S. The Effect of Intratumoral Interrelation among FOXP3+ Regulatory T Cells on Treatment Response and Survival in Triple-Negative Breast Cancer. Cancers 2022, 14, 2138. https://doi.org/10.3390/cancers14092138
Goda N, Nakashima C, Nagamine I, Otagaki S. The Effect of Intratumoral Interrelation among FOXP3+ Regulatory T Cells on Treatment Response and Survival in Triple-Negative Breast Cancer. Cancers. 2022; 14(9):2138. https://doi.org/10.3390/cancers14092138
Chicago/Turabian StyleGoda, Noriko, Chika Nakashima, Ichiro Nagamine, and Sunao Otagaki. 2022. "The Effect of Intratumoral Interrelation among FOXP3+ Regulatory T Cells on Treatment Response and Survival in Triple-Negative Breast Cancer" Cancers 14, no. 9: 2138. https://doi.org/10.3390/cancers14092138
APA StyleGoda, N., Nakashima, C., Nagamine, I., & Otagaki, S. (2022). The Effect of Intratumoral Interrelation among FOXP3+ Regulatory T Cells on Treatment Response and Survival in Triple-Negative Breast Cancer. Cancers, 14(9), 2138. https://doi.org/10.3390/cancers14092138





