Changing Landscape of Systemic Therapy in Biliary Tract Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
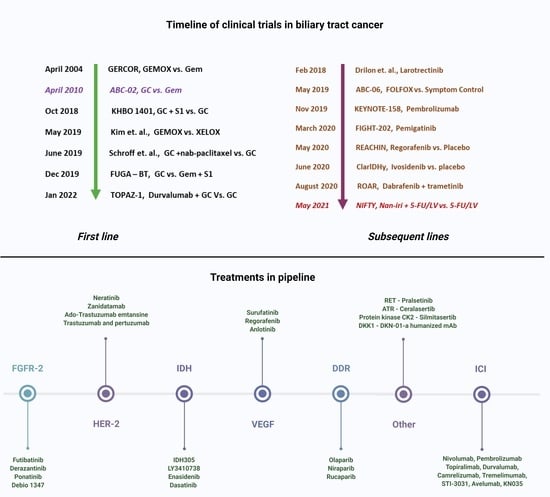
2. Chemotherapy in Biliary Tract Cancers
2.1. Chemotherapy in the First Line
2.2. Chemotherapy in the Second Line
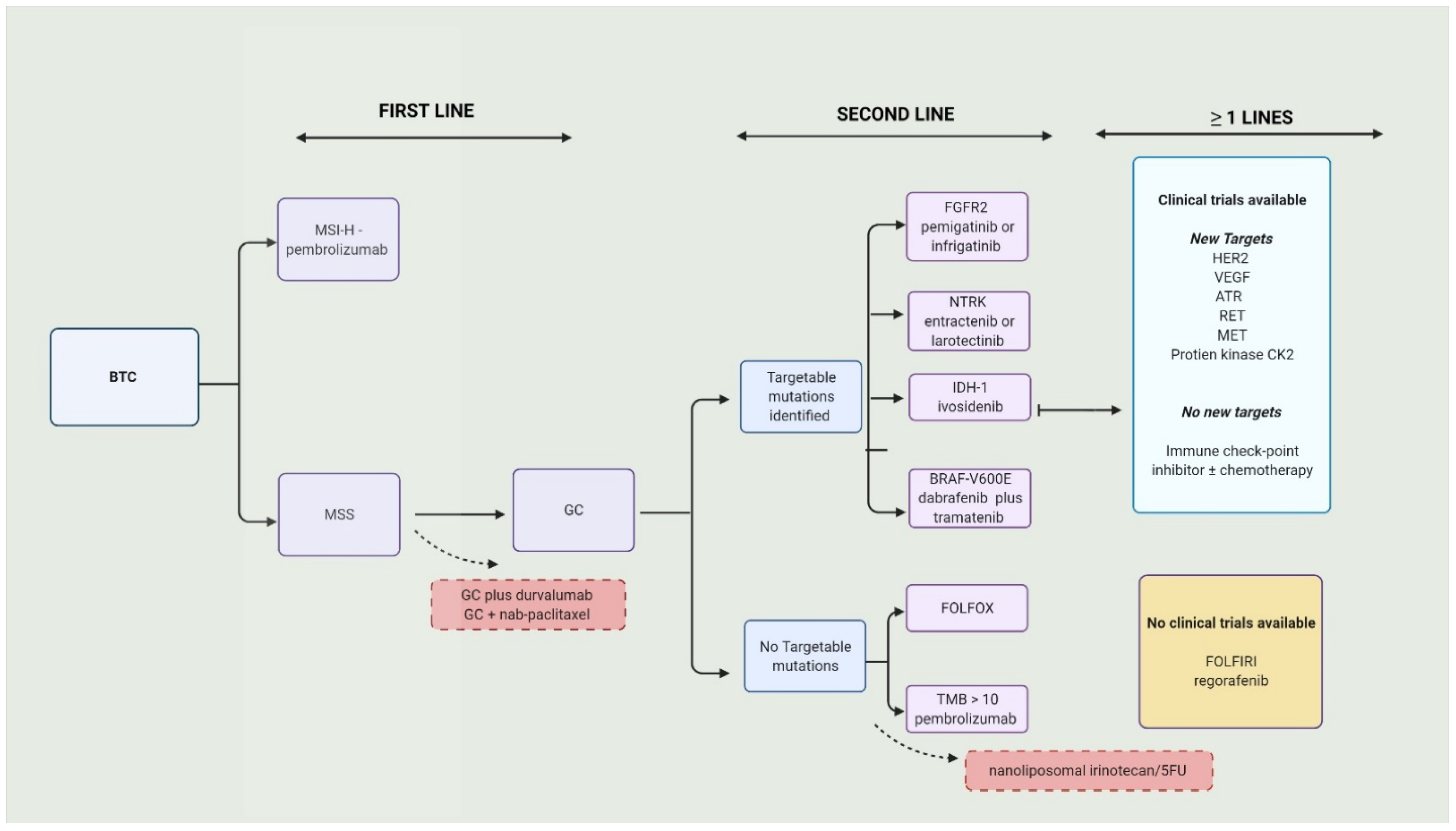
3. Targeted Therapy in Biliary Tract Cancers
| Line | Phase (N) | Clinical Trial Identifier | Treated Cancer Group | Experimental Arm | Target of the Drug (If Applicable) | Comparative Arm | Primary Outcome Studied in the Trial | Top 3 Treatment-Related Adverse Events | Notes |
|---|---|---|---|---|---|---|---|---|---|
| First line | III | NCT03875235 [27] | BTC | Durvalumab (D) + GC | PD-1 | GC + placebo (Pbo) | OS—12.8 m vs. 11.5 m (D vs. Pbo, HR = 0.80; 95% CI, 0.66–0.97; p = 0.021) | Anemia Low neutrophil count Low platelet count | PFS-7.2 m vs. 5.7 m (D vs. Pbo, HR, 0.75; 95% CI, 0.64–0.89; p = 0.001); ORR—26.7% vs. 18.7% (D vs. Pbo); Grade 3/4—62.7% vs. 64.9% (D vs. Pbo) |
| II | NCT03796429 [36] | BTC | Toripalimab + GC | PD-1 | Single arm | PFS—6.7 m OS—NR | Leukopenia Anemia Rash | ORR—21 DCR—85% G3/4, non-hematological in 20% and hematological—69% | |
| II | NCT03951597 [37] | iCCA | Toripalimab + lenvatinib + GemOx + | PD-1 + TKI | Single arm | ORR—80% (1CR and three patients obtained enough control to allow for resection) | Jaundice Rash Proteinuria | DCR—93.3%, PFS—10 m OS—NR DOR—9.8 m | |
| II | NCT04361331 [38] | iCCA | Lenvatinib + GemOx | TKI | Single arm | ORR—30% 1/30 was down staged to have resection | Fatigue Jaundice Vomiting | PFS and OS—NR DCRc—87% No G5, ≥G3 in 40% | |
| Ib II | NCT02992340 | BTC | Varlitinib + GC | Pan-HER 2 | Single arm | DLT—1/11 (200 mg); 1/12 (300 mg) | blood and lymphatic system disorders | PR = 8/23; SD = 12/23 ORR—35%, DCR—87%, DoR—4 m, PFS—6.8 m | |
| Ib II | NCT02128282 [39] | CCA | Silmitasertib (CX-4945) + GC | Casein kinase 2 (CK2) | Single arm | PFS 11 m | Diarrhea Neutropenia Nausea | Compared to GC—Better PFS Lesser neutropenia | |
| I | NCT02375880 [40] | BTC | DKN-01 + GC | Dickkopf-1 (DKK1) | Single arm | Safety—no DLT | Neutropenia Thrombocytopenia Leukopenia | ORR—21.3% PFS—8.7 m | |
| Subsequent lines | III | NCT02989857 (ClarIDHy) [41] | CCA | Ivosidenib (IVO) | IDH-1 | IVO alone vs. placebo | PFS—2.7 m vs. 1.4 m (HR = 0.37; 95% CI 0.25–0.54; p < 0.0001). | Ascites Fatigue Anemia | OS in updated analysis 10.3 m IVO vs. 7.5 m (HR = 0.79; 95% CI 0.56–1.12; p = 0.093) |
| II | NCT02966821 [42] | BTC | Surufatinib | VEGF | Single arm | PFS rate at 16 wks—46.33% (95%, 24.38–65.73) | Elevated bilirubin Hypertension Proteinuria | PFS—3.7 m OS—6.9 m | |
| II | ChiCTR1900022003 [43]. | BTC | Anlotinib + sintlimab | TKI + PD-1 | Single arm | OS—NR | Hypertension ** Diarrhea Hypothyroidism | PFS—6.5 m ORR—40% DCR—87% | |
| II | NCT02052778 [44]. | iCCA # | Futibatinib | FGFR2 | Single arm | ORR 37% | Hyperphosphatemia Diarrhea * Dry mouth * | DoR—8.3 m and DCR = 82% | |
| II | NCT03230318 [45] | iCCA | Derazantinib | FGFR2—mutations and amplifications | Single arm | 3-month PFS rate—76% | Not specified | DCR = 80% PFS = 7.3 m 6-month PFS rate = 50% | |
| II | NCT03797326 [46] | BTC # | Pembrolizumab + lenvatinib | PD-1 + TKI | Single arm | ORR—10% Safety—TRAE in 97% (>G354%) | Hypertension Dysphonia Diarrhea | DCR—68% PFS—6.1 m OS—8.6 m | |
| II | NCT02265341 [47] | BTC | Ponatinib | FGFR2 | Single arm | ORR—9% | Lymphopenia, Rash Fatigue (50%) | CR = 0, PR—8%, SD = 36%. PFS—2.4 m and OS—15.7 m | |
| II | NCT03834220 [48] | CCA among Solid tumors | Debio 1347 | FGFR Fusion | Single arm | ORR—2/5 (40%) of CCA | Fatigue Hyperphosphatemia Anemia | DoR and PFS were 16.1 weeks and 18.3 weeks (in all patients), respectively. | |
| II | NCT01953926 [49] | BTC + AC # | Neratinib | HER2 or EGFR Exon 18 | Single arm | ORR—12% | Diarrhea * Vomiting * | PSS—2.8 m OS—5.4 m | |
| I/ II | NCT01752920 [50] | iCCA | Derazantinib | FGFR2—fusions | Single arm | Safety—all-grade TRAE in 93% | Fatigue Eye-toxicity Hyperphospatemia | ≥3 Grade TRAE in 28% ORR—27% DCR—83% | |
| I | NCT02699515 [51] | BTC # | Bintrafusp alfa, | TGF-β and PD-L1 | Single arm | Safety—emergent and all adverse events | Rash Fever Increased lipase | 63% had TRAE 37% ≥ G3 | |
| I | NCT02892123 [52] | BTC # | ZW25 (Zanidatamab) | bispecific HER2 | Single arm | Safety/tolerability—only G1–G2 reported in 70% | Fatigue ** Diarrhea Infusion reaction | ORR—47 DCR—65% DoR—6.6 m | |
| Ib | NCT03996408 [53] | BTC | Anlotinib TQB2450 | TKI + PDL1 | Single arm | DLT/ MTD in first 3 weeks (one cycle)—none RP2D—25 mg ORR—42% | * Hypertension Leukopenia Increased total bilirubin Neutropenia | PFS—240 days DCR—75% |
3.1. Fibroblastic Growth Factors Receptor Inhibitors (FGFRis)
3.2. Isocitrate Dehydrogenase Inhibitors
3.3. Neurotrophic Tyrosine Receptor Kinase Fusion Inhibitors
3.4. Vascular Endothelial Growth Factor Inhibitors
3.5. Human Epidermal Growth Factor Receptor 2 Inhibitors
3.6. Other Targeted Therapy Options
4. Immunotherapy in Biliary Tract Cancers
4.1. Immune Checkpoint Inhibitors
4.2. Chimeric Antigen Receptor T Cell Therapy and Vaccines in Biliary Tract Cancers
5. Systemic Therapy in Early-Stage Biliary Tract Cancers
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef]
- Razumilava, N.; Gores, G.J. Classification, diagnosis, and management of cholangiocarcinoma. Clin. Gastroenterol. Hepatol. 2013, 11, 13–21.e11. [Google Scholar] [CrossRef] [Green Version]
- DeOliveira, M.L.; Cunningham, S.C.; Cameron, J.L.; Kamangar, F.; Winter, J.M.; Lillemoe, K.D.; Choti, M.A.; Yeo, C.J.; Schulick, R.D. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Ann. Surg. 2007, 245, 755–762. [Google Scholar] [CrossRef]
- Patel, T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001, 33, 1353–1357. [Google Scholar] [CrossRef]
- Ouyang, G.; Liu, Q.; Wu, Y.; Liu, Z.; Lu, W.; Li, S.; Pan, G.; Chen, X. The global, regional, and national burden of gallbladder and biliary tract cancer and its attributable risk factors in 195 countries and territories, 1990 to 2017: A systematic analysis for the Global Burden of Disease Study 2017. Cancer 2021, 127, 2238–2250. [Google Scholar] [CrossRef] [PubMed]
- Zatonski, W.A.; Lowenfels, A.B.; Boyle, P.; Maisonneuve, P.; Bueno de Mesquita, H.B.; Ghadirian, P.; Jain, M.; Przewozniak, K.; Baghurst, P.; Moerman, C.J.; et al. Epidemiologic aspects of gallbladder cancer: A case-control study of the SEARCH Program of the International Agency for Research on Cancer. J. Natl. Cancer Inst. 1997, 89, 1132–1138. [Google Scholar] [CrossRef] [Green Version]
- Massarweh, N.N.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control. 2017, 24, 1073274817729245. [Google Scholar] [CrossRef]
- Sithithaworn, P.; Yongvanit, P.; Duenngai, K.; Kiatsopit, N.; Pairojkul, C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2014, 21, 301–308. [Google Scholar] [CrossRef]
- Strom, B.L.; Soloway, R.D.; Rios-Dalenz, J.L.; Rodriguez-Martinez, H.A.; West, S.L.; Kinman, J.L.; Polansky, M.; Berlin, J.A. Risk factors for gallbladder cancer. An international collaborative case-control study. Cancer 1995, 76, 1747–1756. [Google Scholar] [CrossRef]
- Florio, A.A.; Ferlay, J.; Znaor, A.; Ruggieri, D.; Alvarez, C.S.; Laversanne, M.; Bray, F.; Mcglynn, K.A.; Petrick, J.L. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer 2020, 126, 2666–2678. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Zhu, A.X.; Fuchs, C.S.; Brooks, G.A. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016, 21, 594–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaib, Y.H.; Davila, J.A.; McGlynn, K.; El-Serag, H.B. Rising incidence of intrahepatic cholangiocarcinoma in the United States: A true increase? J. Hepatol. 2004, 40, 472–477. [Google Scholar] [CrossRef]
- Forner, A.; Vidili, G.; Rengo, M.; Bujanda, L.; Ponz-Sarvisé, M.; Lamarca, A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019, 39, 98–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, A.; Saborowski, A. Current and Future Systemic Therapies in Biliary Tract Cancer. Visc. Med. 2021, 37, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Neumann, U.P.; Schmeding, M. Role of surgery in cholangiocarcinoma: From resection to transplantation. Best Pract Res. Clin. Gastroenterol. 2015, 29, 295–308. [Google Scholar] [CrossRef]
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients with Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014, 149, 565–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapisochín, G. Liver transplantation for cholangiocarcinoma: Current status and new insights. World J. Hepatol. 2015, 7, 2396. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valle, J.W.; Wasan, H.; Johnson, P.; Jones, E.; Dixon, L.; Swindell, R.; Baka, S.; Maraveyas, A.; Corrie, P.; Falk, S.; et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: A multicentre randomised phase II study—The UK ABC-01 Study. Br. J. Cancer 2009, 101, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Phelip, J.M.; Desrame, J.; Edeline, J.; Barbier, E.; Terrebonne, E.; Michel, P.; Perrier, H.; Dahan, L.; Bourgeois, V.; Akouz, F.K.; et al. Modified FOLFIRINOX Versus CISGEM Chemotherapy for Patients with Advanced Biliary Tract Cancer (PRODIGE 38 AMEBICA): A Randomized Phase II Study. J. Clin. Oncol. 2022, 40, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.J.; McNamara, M.G.; Goyal, L.; Cosgrove, D.; Springfeld, C.; Sjoquist, K.M.; Park, J.O.; Verdaguer, H.; Braconi, C.; Ross, P.J.; et al. Phase III study of NUC-1031 + cisplatin versus gemcitabine + cisplatin for first-line treatment of patients with advanced biliary tract cancer (NuTide:121). J. Clin. Oncol. 2021, 39, TPS4164. [Google Scholar] [CrossRef]
- Kapacee, Z.A.; Knox, J.J.; Palmer, D.; Blagden, S.P.; Lamarca, A.; Valle, J.W.; Mcnamara, M.G. NUC-1031, use of ProTide technology to circumvent gemcitabine resistance: Current status in clinical trials. Med. Oncol. 2020, 37, 61. [Google Scholar] [CrossRef]
- Assenat, E.; Blanc, J.F.; Bouattour, M.; Gauthier, L.; Touchefeu, Y.; Portales, F.; Borg, C.; Fares, N.; Mineur, L.; Bleuse, J.-P.; et al. 48P (BREGO) Regorafenib combined with modified m-GEMOX in patients with advanced biliary tract cancer (BTC): A phase II randomized trial. Ann. Oncol. 2021, 32, S376–S377. [Google Scholar] [CrossRef]
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.S.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers. JAMA Oncol. 2019, 5, 824. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.; Lee, C.-K.; Sang, Y.B.; Choi, H.J.; Kim, M.H.; Ji, J.H.; Ko, K.H.; Kwon, C.-I.; Kim, D.J.; Choi, S.H.; et al. Real-world efficacy and safety of nab-paclitaxel plus gemcitabine-cisplatin in patients with advanced biliary tract cancers: A multicenter retrospective analysis. Ther. Adv. Med. Oncol. 2021, 13, 175883592110359. [Google Scholar] [CrossRef]
- Sakai, D.; Kanai, M.; Kobayashi, S.; Eguchi, H.; Baba, H.; Seo, S.; Taketomi, A.; Takayama, T.; Yamaue, H.; Ishioka, C.; et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 (GCS) versus gemcitabine, cisplatin (GC) for advanced biliary tract cancer (KHBO1401-MITSUBA). Ann. Oncol. 2018, 29, viii205. [Google Scholar] [CrossRef]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin (GemCis) in patients (pts) with advanced biliary tract cancer (BTC): TOPAZ-1. J. Clin. Oncol. 2022, 40, 378. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Rizzo, A.; Salati, M.; Frega, G.; Merz, V.; Caputo, F.; Ricci, A.D.; Palloni, A.; Messina, C.; Spallanzani, A.; Saccoccio, G.; et al. Second-line chemotherapy (2L) in elderly patients with advanced biliary tract cancer (ABC): A multicenter real-world study. J. Clin. Oncol. 2021, 39, 322. [Google Scholar] [CrossRef]
- Yoo, C.; Kim, K.-P.; Jeong, J.H.; Kim, I.; Kang, M.J.; Cheon, J.; Kang, B.W.; Ryu, H.; Lee, J.S.; Kim, K.W.; et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): A multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021, 22, 1560–1572. [Google Scholar] [CrossRef]
- Belkouz, A.; de Vos-Geelen, J.; Mathôt, R.A.A.; Eskens, F.A.L.M.; van Gulik, T.M.; van Oijen, M.G.H.; Punt, C.J.A.; Wilmink, J.W.; Klümpen, H.J. Efficacy and safety of FOLFIRINOX as salvage treatment in advanced biliary tract cancer: An open-label, single arm, phase 2 trial. Br. J. Cancer 2020, 122, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Makawita, S.; Abou-Alfa, G.K.; Roychowdhury, S.; Sadeghi, S.; Borbath, I.; Goyal, L.; Cohn, A.; Lamarca, A.; Oh, D.Y.; Macarulla, T.; et al. Infigratinib in patients with advanced cholangiocarcinoma with. Future Oncol. 2020, 16, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Liu, T.; Li, W.; Yu, Y.; Guo, X.; Xu, X.; Wang, Y.; Li, Q.; Wang, Y.; Cui, Y.; Liu, H.; et al. 53P Toripalimab with chemotherapy as first-line treatment for advanced biliary tract tumors: A preliminary analysis of safety and efficacy of an open-label phase II clinical study. Ann. Oncol. 2020, 31, S261. [Google Scholar] [CrossRef]
- Zhou, J.; Fan, J.; Shi, G.; Huang, X.; Wu, D.; Yang, G.; Ge, N.; Hou, Y.; Sun, H.; Huang, X.; et al. 56P Anti-PD1 antibody toripalimab, lenvatinib and gemox chemotherapy as first-line treatment of advanced and unresectable intrahepatic cholangiocarcinoma: A phase II clinical trial. Ann. Oncol. 2020, 31, S262–S263. [Google Scholar] [CrossRef]
- Shi, G.-M.; Jian, Z.; Fan, J.; Huang, X.-Y.; Wu, D.; Liang, F.; Lu, J.-C.; Yang, G.-H.; Chen, Y.; Ge, N.-L.; et al. Phase II study of lenvatinib in combination with GEMOX chemotherapy for advanced intrahepatic cholangiocarcinoma. J. Clin. Oncol. 2021, 39, e16163. [Google Scholar] [CrossRef]
- Borad, M.J.; Bai, L.-Y.; Chen, M.-H.; Hubbard, J.M.; Mody, K.; Rha, S.Y.; Richards, D.A.; Davis, S.L.; Soong, J.; Huang, C.-E.C.-E.; et al. Silmitasertib (CX-4945) in combination with gemcitabine and cisplatin as first-line treatment for patients with locally advanced or metastatic cholangiocarcinoma: A phase Ib/II study. J. Clin. Oncol. 2021, 39, 312. [Google Scholar] [CrossRef]
- Goyal, L.; Sirard, C.; Schrag, M.; Kagey, M.H.; Eads, J.R.; Stein, S.; El-Khoueiry, A.B.; Manji, G.A.; Abrams, T.A.; Khorana, A.A.; et al. Phase I and Biomarker Study of the Wnt Pathway Modulator DKN-01 in Combination with Gemcitabine/Cisplatin in Advanced Biliary Tract Cancer. Clin. Cancer Res. 2020, 26, 6158–6167. [Google Scholar] [CrossRef]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final results from ClarIDHy, a global, phase III, randomized, double-blind study of ivosidenib (IVO) versus placebo (PBO) in patients (pts) with previously treated cholangiocarcinoma (CCA) and an isocitrate dehydrogenase 1 (IDH1) mutation. J. Clin. Oncol. 2021, 39, 266. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, J.; Sun, H.; Bai, C.; Jia, R.; Li, Y.; Zhang, W.; Liu, L.; Huang, C.; Guan, M.; et al. A single-arm, multicenter, open-label phase 2 trial of surufatinib in patients with unresectable or metastatic biliary tract cancer. J. Clin. Oncol. 2021, 39, e16123. [Google Scholar] [CrossRef]
- Zong, H.; Zhong, Q.; Zhao, R.; Jin, S.; Zhou, C.; Zhang, X.; Shi, J.; Qiao, S.; Han, J.; Jiang, M. Phase II study of anlotinib plus sintlimab as second-line treatment for patients with advanced biliary tract cancers. J. Clin. Oncol. 2021, 39, 307. [Google Scholar] [CrossRef]
- Bridgewater, J.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.; Abrams, T.; Furuse, J.; Kelley, R.K.; Cassier, P.; et al. 54P Efficacy and safety of futibatinib in intrahepatic cholangiocarcinoma (iCCA) harboring FGFR2 fusions/other rearrangements: Subgroup analyses of a phase II study (FOENIX-CCA2). Ann. Oncol. 2020, 31, S261–S262. [Google Scholar] [CrossRef]
- Javle, M.M.; Abou-Alfa, G.K.; Macarulla, T.; Personeni, N.; Adeva, J.; Bergamo, F.; Malka, D.; Vogel, A.; Knox, J.J.; Evans, T.R.J.; et al. Efficacy of derazantinib in intrahepatic cholangiocarcinoma patients with FGFR2 mutations or amplifications: Interim results from the phase 2 study FIDES-01. J. Clin. Oncol. 2022, 40, 427. [Google Scholar] [CrossRef]
- Villanueva, L.; Lwin, Z.; Chung, H.C.C.; Gomez-Roca, C.A.; Longo, F.; Yanez, E.; Senellart, H.; Doherty, M.; Garcia-Corbacho, J.; Hendifar, A.E.; et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase 2 LEAP-005 study. J. Clin. Oncol. 2021, 39, 4080. [Google Scholar] [CrossRef]
- Ahn, D.H.; Uson Junior, P.L.S.; Masci, P.; Kosiorek, H.; Halfdanarson, T.R.; Mody, K.; Babiker, H.; DeLeon, T.; Sonbol, M.B.; Gores, G.; et al. A pilot study of Pan-FGFR inhibitor ponatinib in patients with FGFR-altered advanced cholangiocarcinoma. Invest New Drugs. 2022, 40, 134–141. [Google Scholar] [CrossRef]
- Cleary, J.M.; Iyer, G.; Oh, D.-Y.; Mellinghoff, I.K.; Goyal, L.; Ng, M.C.H.; Meric-Bernstam, F.; Matos, I.; Chao, T.-Y.; Sarkouh, R.A.; et al. Final results from the phase I study expansion cohort of the selective FGFR inhibitor Debio 1,347 in patients with solid tumors harboring an FGFR gene fusion. J. Clin. Oncol. 2020, 38, 3603. [Google Scholar] [CrossRef]
- Harding, J.J.; Cleary, J.M.; Quinn, D.I.; Braña, I.; Moreno, V.; Borad, M.J.; Loi, S.; Spanggaard, I.; Park, H.; Ford, J.M.; et al. Targeting HER2 (ERBB2) mutation-positive advanced biliary tract cancers with neratinib: Results from the phase II SUMMIT ‘basket’ trial. J. Clin. Oncol. 2021, 39, 320. [Google Scholar] [CrossRef]
- Mazzaferro, V.; El-Rayes, B.F.; Droz Dit Busset, M.; Cotsoglou, C.; Harris, W.P.; Damjanov, N.; Masi, G.; Rimassa, L.; Personeni, N.; Braiteh, F.; et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br. J. Cancer 2019, 120, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Yoo, C.; Oh, D.-Y.; Choi, H.J.; Kudo, M.; Ueno, M.; Kondo, S.; Chen, L.-T.; Osada, M.; Helwig, C.; Dussault, I.; et al. 73P Long-term follow-up of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. Ann. Oncol. 2020, 31, S268–S269. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Hanna, D.L.; El-Khoueiry, A.B.; Kang, Y.-K.; Oh, D.-Y.; Chaves, J.M.; Rha, S.Y.; Hamilton, E.P.; Pant, S.; Javle, M.M.; et al. Zanidatamab (ZW25) in HER2-positive biliary tract cancers (BTCs): Results from a phase I study. J. Clin. Oncol. 2021, 39, 299. [Google Scholar] [CrossRef]
- Zhou, J.; Gong, J.; Cao, Y.; Peng, Z.; Yuan, J.; Wang, X.; LU, M.; Shen, L. Anlotinib plus TQB2450 in patients with advanced refractory biliary tract cancer (BTC): An open-label, dose-escalating, and dose-expansion cohort of phase Ib trial. J. Clin. Oncol. 2021, 39, 292. [Google Scholar] [CrossRef]
- Beenken, A.; Mohammadi, M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009, 8, 235–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallinan, N.; Finn, S.; Cuffe, S.; Rafee, S.; O’Byrne, K.; Gately, K. Targeting the fibroblast growth factor receptor family in cancer. Cancer Treat. Rev. 2016, 46, 51–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, L.; Kongpetch, S.; Crolley, V.E.; Bridgewater, J. Targeting FGFR inhibition in cholangiocarcinoma. Cancer Treat. Rev. 2021, 95, 102170. [Google Scholar] [CrossRef]
- Presta, M.; Chiodelli, P.; Giacomini, A.; Rusnati, M.; Ronca, R. Fibroblast growth factors (FGFs) in cancer: FGF traps as a new therapeutic approach. Pharmacol. Ther. 2017, 179, 171–187. [Google Scholar] [CrossRef]
- Consortium, A.P.G. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831. [Google Scholar] [CrossRef] [Green Version]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kurzrock, R. The FGFR Landscape in Cancer: Analysis of 4853 Tumors by Next-Generation Sequencing. Clin. Cancer Res. 2016, 22, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Arai, Y.; Totoki, Y.; Hosoda, F.; Shirota, T.; Hama, N.; Nakamura, H.; Ojima, H.; Furuta, K.; Shimada, K.; Okusaka, T.; et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology 2014, 59, 1427–1434. [Google Scholar] [CrossRef]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef] [Green Version]
- Abou-Alfa, G.K.; Bibeau, K.; Schultz, N.; Yaqubie, A.; Millang, B.M.; Ren, H.; Féliz, L. Effect of FGFR2 alterations on survival in patients receiving systemic chemotherapy for intrahepatic cholangiocarcinoma. J. Clin. Oncol. 2021, 39, 303. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Javle, M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Weiss, K.H.; Waldschmidt, D.T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.; et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: Mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol. Hepatol. 2021, 6, 803–815. [Google Scholar] [CrossRef]
- Bahleda, R.; Meric-Bernstam, F.; Goyal, L.; Tran, B.; He, Y.; Yamamiya, I.; Benhadji, K.A.; Matos, I.; Arkenau, H.T. Phase I, first-in-human study of futibatinib, a highly selective, irreversible FGFR1-4 inhibitor in patients with advanced solid tumors. Ann. Oncol. 2020, 31, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.H.; Hierro, C.; Heist, R.S.; Cleary, J.M.; Meric-Bernstam, F.; Tabernero, J.; Janku, F.; Gandhi, L.; Iafrate, A.J.; Borger, D.R.; et al. A Phase I, Open-Label, Multicenter, Dose-escalation Study of the Oral Selective FGFR Inhibitor Debio 1347 in Patients with Advanced Solid Tumors Harboring. Clin. Cancer Res. 2019, 25, 2699–2707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.-H.; Su, W.-C.; Oh, D.-Y.; Shen, L.; Kim, K.-P.; Liu, X.; Liao, H.; Qing, M.; Qian, J.; Triantos, S.; et al. Updated analysis with longer follow up of a phase 2a study evaluating erdafitinib in Asian patients (pts) with advanced cholangiocarcinoma (CCA) and fibroblast growth factor receptor (FGFR) alterations. J. Clin. Oncol. 2022, 40, 430. [Google Scholar] [CrossRef]
- Alabduladhem, T.O.; Bordoni, B. Physiology, Krebs Cycle. In StatPearls; StatPearls Publishing© 2021, StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Rakheja, D.; Medeiros, L.J.; Bevan, S.; Chen, W. The emerging role of d-2-hydroxyglutarate as an oncometabolite in hematolymphoid and central nervous system neoplasms. Front. Oncol. 2013, 3, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makawita, S.; Borad, M.J.; Carapeto, F.; Kwong, L.; Bekaii-Saab, T.S.; Murugesan, K.; Ross, J.S.; Danziger, N.; Israel, M.A.; McGregor, K.; et al. IDH1 and IDH2 Driven Intrahepatic Cholangiocarcinoma (IHCC): A comprehensive genomic and immune profiling study. J. Clin. Oncol. 2021, 39, 4009. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.; Wu, F.; Ni, C.; Wang, Y.; Chen, S.; Bai, Y. 76P The distribution of tumor mutational burden in IDH-mutant solid tumors. Ann. Oncol. 2020, 31, S270. [Google Scholar] [CrossRef]
- Manne, A.; Woods, E.; Tsung, A.; Mittra, A. Biliary Tract Cancers: Treatment Updates and Future Directions in the Era of Precision Medicine and Immuno-Oncology. Front. Oncol. 2021, 11, 768009. [Google Scholar] [CrossRef]
- Salama, V.; Brooks, N.; Skwarska, A.; Kays, L.; Milligan, P.; Newell, K.; Roth, K.; Geeganage, S.; Gilmour, R.; Chan, S.M.; et al. Abstract 6417: LY3410738, a novel inhibitor of mutant IDH1 is more effective than Ivosidenib and potentiates antileukemic activity of standard chemotherapy in preclinical models of acute myeloid leukemia (AML). Cancer Res. 2020, 80, 6417. [Google Scholar] [CrossRef]
- Pauff, J.M.; Papadopoulos, K.P.; Janku, F.; Turk, A.A.; Goyal, L.; Shroff, R.T.; Shimizu, T.; Ikeda, M.; Azad, N.S.; Cleary, J.M.; et al. A phase I study of LY3410738, a first-in-class covalent inhibitor of mutant IDH1 in cholangiocarcinoma and other advanced solid tumors. J. Clin. Oncol. 2021, 39, TPS350. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef]
- Demols, A.; Perez-Casanova, L.; Rocq, L.; Charry, M.; De Nève, N.; Verrellen, A.; Ramadhan, A.; Van Campenhout, C.; De Clercq, S.; Maris, C.; et al. 71P NTRK gene fusions in bilio-pancreatic cancers. Ann. Oncol. 2020, 31, S268. [Google Scholar] [CrossRef]
- Yoshikawa, D.; Ojima, H.; Iwasaki, M.; Hiraoka, N.; Kosuge, T.; Kasai, S.; Hirohashi, S.; Shibata, T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br. J. Cancer 2008, 98, 418–425. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Koukourakis, M.I.; Simopoulos, C.; Polychronidis, A.; Sivridis, E. Vascular endothelial growth factor (VEGF) expression in operable gallbladder carcinomas. Eur. J. Surg. Oncol. 2003, 29, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shroff, R.T.; Makawita, S.; Xiao, L.; Danner De Armas, A.; Bhosale, P.; Reddy, K.; Shalaby, A.; Raghav, K.; Pant, S.; et al. Phase II Study of Ramucirumab in Advanced Biliary Tract Cancer Previously Treated by Gemcitabine-based Chemotherapy. Clin. Cancer Res. 2022. [Google Scholar] [CrossRef]
- Rao, J.-h.; Wu, C.; Zhang, H.; Wang, X.; Lu, L.; Cheng, F.; Chen, D. Efficacy and biomarker analysis of neoadjuvant carrizumab plus apatinib in patients with local advanced biliary tract cancers. J. Clin. Oncol. 2021, 39, e16126. [Google Scholar] [CrossRef]
- Wang, D.; Yang, X.; Long, J.; Lin, J.; Mao, J.; Xie, F.; Wang, Y.; Wang, Y.; Xun, Z.; Bai, Y.; et al. The Efficacy and Safety of Apatinib Plus Camrelizumab in Patients With Previously Treated Advanced Biliary Tract Cancer: A Prospective Clinical Study. Front. Oncol. 2021, 11, 646979. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, P.; Shi, R. Anlotinib as a molecular targeted therapy for tumors (Review). Oncol. Lett. 2020, 20, 1001–1014. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, J.; Fang, W.; Lu, P.; Fan, Q.; Shu, Y.; Feng, J.F.; Zhang, S.; Ba, Y.; Liu, Y.; et al. Anlotinib in chemotherapy-refractory metastatic esophageal squamous cell carcinoma (ESCC): A randomized, double-blind, multicenter phase II trial. J. Clin. Oncol. 2019, 37, 95. [Google Scholar] [CrossRef]
- Han, B.; Li, K.; Wang, Q.; Zhang, L.; Shi, J.; Wang, Z.; Cheng, Y.; He, J.; Shi, Y.; Zhao, Y.; et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non–Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 1569. [Google Scholar] [CrossRef]
- Zhou, A.P.; Bai, Y.; Song, Y.; Luo, H.; Ren, X.B.; Wang, X.; Shi, B.; Fu, C.; Cheng, Y.; Liu, J.; et al. Anlotinib Versus Sunitinib as First-Line Treatment for Metastatic Renal Cell Carcinoma: A Randomized Phase II Clinical Trial. Oncologist 2019, 24, e702–e708. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Du, F.; Gao, M.; Ji, Q.; Li, Z.; Zhang, Y.; Guo, Z.; Wang, J.; Chen, X.; Wang, J.; et al. Anlotinib for the Treatment of Patients with Locally Advanced or Metastatic Medullary Thyroid Cancer. Thyroid 2018, 28, 1455–1461. [Google Scholar] [CrossRef]
- Browne, B.C.; O’Brien, N.; Duffy, M.J.; Crown, J.; O’Donovan, N. HER-2 signaling and inhibition in breast cancer. Curr. Cancer Drug Targets 2009, 9, 419–438. [Google Scholar] [CrossRef]
- Galdy, S.; Lamarca, A.; Mcnamara, M.G.; Hubner, R.A.; Cella, C.A.; Fazio, N.; Valle, J.W. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: A potential therapeutic target? Cancer Metastasis Rev. 2017, 36, 141–157. [Google Scholar] [CrossRef] [Green Version]
- Mondaca, S.; Razavi, P.; Xu, C.; Offin, M.; Myers, M.; Scaltriti, M.; Hechtman, J.F.; Bradley, M.; O’Reilly, E.M.; Berger, M.F.; et al. Genomic Characterization of ERBB2-Driven Biliary Cancer and a Case of Response to Ado-Trastuzumab Emtansine. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.-B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef] [Green Version]
- Ter Veer, E.; Creemers, A.; De Waal, L.; Van Oijen, M.G.H.; Van Laarhoven, H.W.M. Comparing cytotoxic backbones for first-line trastuzumab-containing regimens in human epidermal growth factor receptor 2-positive advanced oesophagogastric cancer: A meta-analysis. Int. J. Cancer 2018, 143, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Valabrega, G.; Montemurro, F.; Aglietta, M. Trastuzumab: Mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann. Oncol. 2007, 18, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Beckmann, M.W.; Rody, A.; Schneeweiss, A.; Müller, V.; Fehm, T.; Marschner, N.; Gluz, O.; Schrader, I.; Heinrich, G.; et al. HER2 Dimerization Inhibitor Pertuzumab—Mode of Action and Clinical Data in Breast Cancer. Breast Care 2013, 8, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Dixit, S.; Abraham, L.; Weiser, N.; Gold, M.R. Abstract 1032: Super-resolution imaging studies of zanidatamab: Providing insights into its bispecific mode of action. Cancer Res. 2021, 81, 1032. [Google Scholar] [CrossRef]
- Kong, A.; Feldinger, K. Profile of neratinib and its potential in the treatment of breast cancer. Breast Cancer Targets Ther. 2015, 7, 147. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.-Y.; Yong, W.-P.; Chen, L.-T.; Kim, J.-W.; Park, J.H.; Hsu, K.; Lindmark, B.; McIntyre, N.; Collins, B.; Firth, C. Varlitinib in combination with gemcitabine and cisplatin for treatment-naïve advanced biliary tract cancer. J. Clin. Oncol. 2022, 40, 439. [Google Scholar] [CrossRef]
- Kudo, R.; Kubo, T.; Mori, Y.; Harada, Y.; Shirota, H.; Hayashi, H.; Kano, M.; Shimizu, Y.; Ishibashi, E.; Akita, H.; et al. A phase 2 basket trial of combination therapy with trastuzumab and pertuzumab in patients with solid cancers harboring HER2 amplification (JUPITER trial). J. Clin. Oncol. 2021, 39, TPS3141. [Google Scholar] [CrossRef]
- Lee, C.-k.; Cheon, J.; Chon, H.J.; Kim, M.H.; Kim, J.W.; Lee, M.A.; Park, H.S.; Kang, M.J.; Jang, J.-S.; Choi, H.J. A phase II trial of trastuzumab plus modified-FOLFOX for gemcitabine/cisplatin refractory HER2-positive biliary tract cancer (BTC): Multi-institutional study of the Korean Cancer Study Group (KCSG-HB19-14). J. Clin. Oncol. 2021, 39, TPS4161. [Google Scholar] [CrossRef]
- Pant, S.; Ducreux, M.; Harding, J.J.; Javle, M.M.; Oh, D.-Y.; Wasan, H.S.; Fortenberry, A.; Josephson, N.C.; Mwatha, A.; Wang, K.; et al. A phase IIb, open-label, single-arm study of zanidatamab (ZW25) monotherapy in subjects with advanced or metastatic HER2-amplified biliary tract cancers. J. Clin. Oncol. 2021, 39, TPS352. [Google Scholar] [CrossRef]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef]
- Salama, A.K.S.; Li, S.; Macrae, E.R.; Park, J.-I.; Mitchell, E.P.; Zwiebel, J.A.; Chen, H.X.; Gray, R.J.; Mcshane, L.M.; Rubinstein, L.V.; et al. Dabrafenib and Trametinib in Patients with Tumors With BRAFV600E Mutations: Results of the NCI-MATCH Trial Subprotocol, H. J. Clin. Oncol. 2020, 38, 3895–3904. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- Bridgewater, J.; Lopes, A.; Beare, S.; Duggan, M.; Lee, D.; Ricamara, M.; Mcentee, D.; Sukumaran, A.; Wasan, H.; Valle, J.W. A phase 1b study of Selumetinib in combination with Cisplatin and Gemcitabine in advanced or metastatic biliary tract cancer: The ABC-04 study. BMC Cancer 2016, 16, 153. [Google Scholar] [CrossRef]
- Allende, J.E.; Allende, C.C. Protein kinase CK2: An enzyme with multiple substrates and a puzzling regulation. FASEB J. 1995, 9, 313–323. [Google Scholar] [CrossRef]
- Jang, S.W.; Hwang, S.S.; Kim, H.S.; Lee, K.O.; Kim, M.K.; Lee, W.; Kim, K.; Lee, G.R. Casein kinase 2 is a critical determinant of the balance of Th17 and Treg cell differentiation. Exp. Mol. Med. 2017, 49, e375. [Google Scholar] [CrossRef] [Green Version]
- Ampofo, E.; Nalbach, L.; Menger, M.D.; Montenarh, M.; Götz, C. Protein Kinase CK2—A Putative Target for the Therapy of Diabetes Mellitus? Int. J. Mol. Sci. 2019, 20, 4398. [Google Scholar] [CrossRef] [Green Version]
- Castello, J.; Ragnauth, A.; Friedman, E.; Rebholz, H. CK2—An Emerging Target for Neurological and Psychiatric Disorders. Pharmaceuticals 2017, 10, 7. [Google Scholar] [CrossRef] [Green Version]
- Silva-Pavez, E.; Tapia, J.C. Protein Kinase CK2 in Cancer Energetics. Front. Oncol. 2020, 10, 893. [Google Scholar] [CrossRef]
- Siddiqui-Jain, A.; Drygin, D.; Streiner, N.; Chua, P.; Pierre, F.; O’Brien, S.E.; Bliesath, J.; Omori, M.; Huser, N.; Ho, C.; et al. CX-4945, an Orally Bioavailable Selective Inhibitor of Protein Kinase CK2, Inhibits Prosurvival and Angiogenic Signaling and Exhibits Antitumor Efficacy. Cancer Res. 2010, 70, 10288–10298. [Google Scholar] [CrossRef] [Green Version]
- Subbiah, V.; Cassier, P.A.; Siena, S.; Alonso, G.; Paz-Ares, L.G.; Garrido, P.; Nadal, E.; Curigliano, G.; Vuky, J.; Lopes, G.; et al. Clinical activity and safety of the RET inhibitor pralsetinib in patients with RET fusion-positive solid tumors: Update from the ARROW trial. J. Clin. Oncol. 2021, 39, 3079. [Google Scholar] [CrossRef]
- Yoon, J.S.; Kim, J.W.; Kim, J.-W.; Kim, T.-Y.; Nam, A.-R.; Bang, J.-H.; Seo, H.-R.; Kim, J.-M.; Oh, K.S.; Mortimer, P.G.; et al. DNA-damage response-umbrella study of the combination of ceralasertib and olaparib, or ceralasertib and durvalumab in advanced biliary tract cancer: A phase 2 trial-in-progress. J. Clin. Oncol. 2021, 39, TPS4166. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Eckhardt, G.; Patnaik, A.; Lorusso, P.; Faoro, L.; Heymach, J.V.; Kapoun, A.M.; Xu, L.; Munster, P. A phase I dose-escalation and dose-expansion study of brontictuzumab in subjects with selected solid tumors. Ann. Oncol. 2018, 29, 1561–1568. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [Green Version]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Merino, D.M.; Mcshane, L.M.; Fabrizio, D.; Funari, V.; Chen, S.-J.; White, J.R.; Wenz, P.; Baden, J.; Barrett, J.C.; Chaudhary, R.; et al. Establishing guidelines to harmonize tumor mutational burden (TMB): In silico assessment of variation in TMB quantification across diagnostic platforms: Phase I of the Friends of Cancer Research TMB Harmonization Project. J. ImmunoTherapy Cancer 2020, 8, e000147. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.-M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients with Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888. [Google Scholar] [CrossRef]
- Valle, J.W.; Kelley, R.K.; Furuse, J.; Edeline, J.; Finn, R.S.; Ren, Z.; Su, S.-C.; Malhotra, U.; Siegel, A.B.; Vogel, A. 78TiP KEYNOTE-966 trial in progress: Pembrolizumab plus gemcitabine and cisplatin for advanced biliary tract cancer. Ann. Oncol. 2020, 31, S270–S271. [Google Scholar] [CrossRef]
- Yin, C.; Armstrong, S.A.; Agarwal, S.; Wang, H.; Noel, M.S.; Weinberg, B.A.; Marshall, J.; He, A.R. Phase II study of combination pembrolizumab and olaparib in patients with advanced cholangiocarcinoma: Interim results. J. Clin. Oncol. 2022, 40, 452. [Google Scholar] [CrossRef]
- Sahai, V.; Griffith, K.A.; Lin, B.S.-L.; Soares, H.P.; Chandana, S.R.; Crysler, O.V.; Enzler, T.; Zalupski, M. A multicenter phase Ib/II study of liposomal-irinotecan, 5-fluorouracil (5-FU), and leucovorin (LV) with nivolumab as second-line therapy for patients with advanced biliary tract cancer (BilT-03). J. Clin. Oncol. 2022, 40, 438. [Google Scholar] [CrossRef]
- Oh, D.-Y.; De Braud, F.; Bridgewater, J.; Furuse, J.; Hsu, C.-H.; Ikeda, M.; Javle, M.; Moehler, M.; Park, J.O.; Shen, L.; et al. 79TiP A phase II/III, randomized, placebo-controlled study of bintrafusp alfa with gemcitabine plus cisplatin as first-line treatment of biliary tract cancer. Ann. Oncol. 2020, 31, S271–S272. [Google Scholar] [CrossRef]
- Jian, Z.; Fan, J.; Shi, G.-M.; Huang, X.-Y.; Wu, D.; Yang, G.-H.; Ji, Y.; Chen, Y.; Liang, F.; Lu, J.-C.; et al. Gemox chemotherapy in combination with anti-PD1 antibody toripalimab and lenvatinib as first-line treatment for advanced intrahepatic cholangiocarcinoma: A phase 2 clinical trial. J. Clin. Oncol. 2021, 39, 4094. [Google Scholar] [CrossRef]
- Yarchoan, M.; Cope, L.; Ruggieri, A.N.; Anders, R.A.; Noonan, A.M.; Goff, L.W.; Goyal, L.; Lacy, J.; Li, D.; Patel, A.K.; et al. Multicenter randomized phase II trial of atezolizumab with or without cobimetinib in biliary tract cancers. J. Clin. Investig. 2021, 131, e152670. [Google Scholar] [CrossRef]
- Javle, M.M.; Bridgewater, J.A.; Gbolahan, O.B.; Jungels, C.; Cho, M.T.; Papadopoulos, K.P.; Thistlethwaite, F.C.; Canon, J.-L.R.; Cheng, L.; Ioannidis, S.; et al. A phase I/II study of safety and efficacy of the arginase inhibitor INCB001158 plus chemotherapy in patients (Pts) with advanced biliary tract cancers. J. Clin. Oncol. 2021, 39, 311. [Google Scholar] [CrossRef]
- Khoja, L.; Day, D.; Wei-Wu Chen, T.; Siu, L.L.; Hansen, A.R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann. Oncol. 2017, 28, 2377–2385. [Google Scholar] [CrossRef]
- Kartolo, A.; Sattar, J.; Sahai, V.; Baetz, T.; Lakoff, J.M. Predictors of immunotherapy-induced immune-related adverse events. Curr. Oncol. 2018, 25, 403–410. [Google Scholar] [CrossRef] [Green Version]
- NCCN. Management of Immunotherapy—Related Toxicities. Available online: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf (accessed on 7 October 2021).
- Sadelain, M.; Rivière, I.; Brentjens, R. Targeting tumours with genetically enhanced T lymphocytes. Nat. Rev. Cancer 2003, 3, 35–45. [Google Scholar] [CrossRef]
- Gill, S.; Brudno, J.N. CAR T-Cell Therapy in Hematologic Malignancies: Clinical Role, Toxicity, and Unanswered Questions. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e246–e265. [Google Scholar] [CrossRef]
- Phanthaphol, N.; Somboonpatarakun, C.; Suwanchiwasiri, K.; Chieochansin, T.; Sujjitjoon, J.; Wongkham, S.; Maher, J.; Junking, M.; Yenchitsomanus, P.T. Chimeric Antigen Receptor T Cells Targeting Integrin αvβ6 Expressed on Cholangiocarcinoma Cells. Front. Oncol. 2021, 11, 657868. [Google Scholar] [CrossRef]
- Sangsuwannukul, T.; Supimon, K.; Sujjitjoon, J.; Phanthaphol, N.; Chieochansin, T.; Poungvarin, N.; Wongkham, S.; Junking, M.; Yenchitsomanus, P.T. Anti-tumour effect of the fourth-generation chimeric antigen receptor T cells targeting CD133 against cholangiocarcinoma cells. Int. Immunopharmacol. 2020, 89, 107069. [Google Scholar] [CrossRef]
- Feng, K.C.; Guo, Y.L.; Liu, Y.; Dai, H.R.; Wang, Y.; Lv, H.Y.; Huang, J.H.; Yang, Q.M.; Han, W.D. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J. Hematol. Oncol. 2017, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Supimon, K.; Sangsuwannukul, T.; Sujjitjoon, J.; Phanthaphol, N.; Chieochansin, T.; Poungvarin, N.; Wongkham, S.; Junking, M.; Yenchitsomanus, P.T. Anti-mucin 1 chimeric antigen receptor T cells for adoptive T cell therapy of cholangiocarcinoma. Sci. Rep. 2021, 11, 6276. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, K.; Liu, Y.; Wu, Z.; Dai, H.; Yang, Q.; Wang, Y.; Jia, H.; Han, W. Phase I Study of Chimeric Antigen Receptor-Modified T Cells in Patients with EGFR-Positive Advanced Biliary Tract Cancers. Clin. Cancer Res. 2018, 24, 1277–1286. [Google Scholar] [CrossRef] [Green Version]
- Aruga, A.; Takeshita, N.; Kotera, Y.; Okuyama, R.; Matsushita, N.; Ohta, T.; Takeda, K.; Yamamoto, M. Phase I clinical trial of multiple-peptide vaccination for patients with advanced biliary tract cancer. J. Transl. Med. 2014, 12, 61. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Ueno, T.; Kawaoka, T.; Hazama, S.; Fukui, M.; Suehiro, Y.; Hamanaka, Y.; Ikematsu, Y.; Imai, K.; Oka, M.; et al. MUC1 peptide vaccination in patients with advanced pancreas or biliary tract cancer. Anticancer Res. 2005, 25, 3575–3579. [Google Scholar]
- Aruga, A.; Takeshita, N.; Kotera, Y.; Okuyama, R.; Matsushita, N.; Ohta, T.; Takeda, K.; Yamamoto, M. Long-term Vaccination with Multiple Peptides Derived from Cancer-Testis Antigens Can Maintain a Specific T-cell Response and Achieve Disease Stability in Advanced Biliary Tract Cancer. Clin. Cancer Res. 2013, 19, 2224–2231. [Google Scholar] [CrossRef] [Green Version]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Ebata, T.; Hirano, S.; Konishi, M.; Uesaka, K.; Tsuchiya, Y.; Ohtsuka, M.; Kaneoka, Y.; Yamamoto, M.; Ambo, Y.; Shimizu, Y.; et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br. J. Surg. 2018, 105, 192–202. [Google Scholar] [CrossRef]
- Edeline, J.; Benabdelghani, M.; Bertaut, A.; Watelet, J.; Hammel, P.; Joly, J.-P.; Boudjema, K.; Fartoux, L.; Bouhier-Leporrier, K.; Jouve, J.-L.; et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J. Clin. Oncol. 2019, 37, 658–667. [Google Scholar] [CrossRef]
- Shroff, R.T.; Kennedy, E.B.; Bachini, M.; Bekaii-Saab, T.; Crane, C.; Edeline, J.; El-Khoueiry, A.; Feng, M.; Katz, M.H.G.; Primrose, J.; et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1015–1027. [Google Scholar] [CrossRef] [Green Version]
- Edeline, J.; Hirano, S.; Bertaut, A.; Konishi, M.; Benabdelghani, M.; Uesaka, K.; Watelet, J.; Ohtsuka, M.; Hammel, P.; Kaneoka, Y.; et al. 55P Adjuvant gemcitabine-based chemotherapy for biliary tract cancer: Pooled analysis of the BCAT and PRODIGE-12 studies. Ann. Oncol. 2020, 31, S262. [Google Scholar] [CrossRef]
- Kim, Y.; Amini, N.; Wilson, A.; Margonis, G.A.; Ethun, C.G.; Poultsides, G.; Tran, T.; Idrees, K.; Isom, C.A.; Fields, R.C.; et al. Impact of Chemotherapy and External-Beam Radiation Therapy on Outcomes among Patients with Resected Gallbladder Cancer: A Multi-institutional Analysis. Ann. Surg. Oncol. 2016, 23, 2998–3008. [Google Scholar] [CrossRef]
- Mallick, S.; Benson, R.; Haresh, K.P.; Julka, P.K.; Rath, G.K. Adjuvant radiotherapy in the treatment of gall bladder carcinoma: What is the current evidence. J. Egypt Natl. Canc. Inst. 2016, 28, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.J.; Lemieux, A.; Kalpathy-Cramer, J.; Ord, C.B.; Walker, G.V.; Fuller, C.D.; Kim, J.-S.; Thomas, C.R. Nomogram for Predicting the Benefit of Adjuvant Chemoradiotherapy for Resected Gallbladder Cancer. J. Clin. Oncol. 2011, 29, 4627–4632. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, S.; Alam, M.N.; Rastogi, N.; Saxena, R. 59P Evolution of adjuvant therapy in radically resected carcinoma gallbladder (GBC) over a decade: A real world experience from a regional cancer centre. Ann. Oncol. 2020, 31, S264. [Google Scholar] [CrossRef]
- Rizzo, A.; Brandi, G. Neoadjuvant therapy for cholangiocarcinoma: A comprehensive literature review. Cancer Treat. Res. Commun. 2021, 27, 100354. [Google Scholar] [CrossRef]
- Hashimoto, K.; Tono, T.; Nishida, K.; Nonaka, R.; Tsunashima, R.; Fujie, Y.; Fujita, S.; Fujita, J.; Yoshida, T.; Ohnishi, T.; et al. A case of curatively resected advanced intrahepatic cholangiocellular carcinoma through effective response to neoadjuvant chemotherapy. Gan Kagaku Ryoho 2014, 41, 2083–2085. [Google Scholar]
- Kato, A.; Shimizu, H.; Ohtsuka, M.; Yoshidome, H.; Yoshitomi, H.; Furukawa, K.; Takeuchi, D.; Takayashiki, T.; Kimura, F.; Miyazaki, M. Surgical Resection after Downsizing Chemotherapy for Initially Unresectable Locally Advanced Biliary Tract Cancer: A Retrospective Single-center Study. Ann. Surg. Oncol. 2013, 20, 318–324. [Google Scholar] [CrossRef]
- McMasters, K.M.; Tuttle, T.M.; Leach, S.D.; Rich, T.; Cleary, K.R.; Evans, D.B.; Curley, S.A. Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am. J. Surg. 1997, 174, 605–608; discussion 608–609. [Google Scholar] [CrossRef]
- Nelson, J.W.; Ghafoori, A.P.; Willett, C.G.; Tyler, D.S.; Pappas, T.N.; Clary, B.M.; Hurwitz, H.I.; Bendell, J.C.; Morse, M.A.; Clough, R.W.; et al. Concurrent Chemoradiotherapy in Resected Extrahepatic Cholangiocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Le Roy, B.; Gelli, M.; Pittau, G.; Allard, M.-A.; Pereira, B.; Serji, B.; Vibert, E.; Castaing, D.; Adam, R.; Cherqui, D.; et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br. J. Surg. 2018, 105, 839–847. [Google Scholar] [CrossRef]
- Hong, T.S.; Wo, J.Y.; Yeap, B.Y.; Ben-Josef, E.; Mcdonnell, E.I.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; Goyal, L.; et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients with Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J. Clin. Oncol. 2016, 34, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Tao, R.; Krishnan, S.; Bhosale, P.R.; Javle, M.M.; Aloia, T.A.; Shroff, R.T.; Kaseb, A.O.; Bishop, A.J.; Swanick, C.W.; Koay, E.J.; et al. Ablative Radiotherapy Doses Lead to a Substantial Prolongation of Survival in Patients with Inoperable Intrahepatic Cholangiocarcinoma: A Retrospective Dose Response Analysis. J. Clin. Oncol. 2016, 34, 219–226. [Google Scholar] [CrossRef]
- Polistina, F.A.; Guglielmi, R.; Baiocchi, C.; Francescon, P.; Scalchi, P.; Febbraro, A.; Costantin, G.; Ambrosino, G. Chemoradiation treatment with gemcitabine plus stereotactic body radiotherapy for unresectable, non-metastatic, locally advanced hilar cholangiocarcinoma. Results of a five year experience. Radiother. Oncol. 2011, 99, 120–123. [Google Scholar] [CrossRef]
| Line | Phase | Clinical Trial Identifier | Target of the Drug | Treated Cancer Group | Experimental Arm | Comparative Arm | Primary Outcome | Secondary Outcome (Main) |
|---|---|---|---|---|---|---|---|---|
| First line | III | NCT03773302 | FGFR rearrangement | CCA | Pemigatinib | GC | PFS | OS, OR, DOR, DCR |
| III | NCT03773302 | FGFR2 fusion/translocation | CCA | Infrigatinib | GC | PFS | OS. DCR, DOR, BOR | |
| III | NCT04093362 | iCCA with FGFR2 | iCCA | Futibatinib | GC | PFS | ORR. DCR. OS. Safety/Tolerability | |
| II | NCT03768414 | Not specific | BTC | GC/NP | GC | OS | PFS, ORR, DCR | |
| II | NCT03579771 | High risk * | Resectable IHC | GC/NP | None | SR | RR, R0; OS; PFS | |
| Subsequent lines k | II | NCT04722133 | HER 2 | aBTC | Trastuzumab-pkrb + FOLFOX | None | ORR | PFS, OS, DCR, incidence of TRAE |
| II | jRCT2031180150 | HER 2 | Advanced solid tumors # | Trastuzumab and pertuzumab | None | ORR | PFS, OS, DoR, safety | |
| II | NCT02091141 (My Pathway) | HER 2 | BTC # | Trastuzumab and pertuzumab | None | ORR | DCR, PFS, OS, AE | |
| II | NCT04466891 | HER 2 | BTC | Zanidatamab monotherapy | None | ORR | DoR; DoR > 16 wks; DCR, PFS, OS; incidence of TRAE, PK | |
| II | NCT02999672 | HER 2 | CCA # | Trastuzumab emtansine | None | BOR | PFS, OS, TRAE, SAE, PK | |
| II | NCT04482309 | HER2 | BTC # | Trastuzumab deruxtecan | None | ORR | DOR, DCR, PFF, OS, AEs, PK and immunogenicity | |
| II | NCT03839342. | Non-V600E BRAF mutations | Advanced solid tumors # | Bimimetinib + encorafenib | None | ORR | Safety, DCR, PFS | |
| II | NCT02428855 | IDH1 mutation | iCCA | Dasatinib | None | ORR | PFS, OS, TRAE | |
| II | NCT02675829 | HER2 amplification | Advanced solid tumors # | Ado-Trastuzumab emtansine | None | ORR | None | |
| II | NCT03207347 | BAP1 and other DDR genes | CCA # | Niraparib | None | ORR | PFS, OS, TRAE | |
| II | NCT03212274 | IDH1/2 mutation | CCA | Olaprib | None | ORR | PFS, OS, safety | |
| II | NCT04042831 | DNA repair gene mutation | BTC | Olaparib | None | ORR | OS, PFS, TRAE, DoR | |
| II | NCT03207347 | DNA repair gene mutation | CCA # | Niraparib | None | ORR | OS, PFS, TRAEs | |
| II | NCT02162914 | VEGF mutation | CCA | Regorafenib | None | PFS | RR, OS | |
| II | NCT03339843 | CDK 4/6 mutation | CCA # | Abemaciclib | None | Anti-tumor activity | PFS, OS, toxicity | |
| II | NCT04003896 | CDK 4/6 mutation | BTC | Abemaciclib | None | ORR | PFS, DCR, OS, QoL | |
| II | NCT02232633 | STAT3 inhibitor | CCA | BBI503 | None | DCR | ORR, OS, PFS, PK TRAE | |
| II | NCT03878095 | IDH1/2 mutation | CCA # | Ceralasertib + olaparib | None | ORR | PFS, OS, DoR, Safety | |
| I/II | NCT02273739 | IDH2 mutation | Advanced solid tumors # | Enasidenib Enasidenib | None | DLT, ECOG | Plasma concentration metrics | |
| I | NCT04764084 | HRR mutations | CCA # | Niraparib + anlotinib | None | DLT, MTD | ORR, PFS | |
| I | NCT04521686 | IDH1 R132-mutant advanced solid tumor types or circulating tumor DNA IDH2 R140 or IDH2 R172 mutation (CCA) | CCA # | LY3410738 LY3410738 + GC | Maximum tolerated dose | ORR Safety and tolerability Efficacy PK properties | ||
| I | NCT02381886 | IDH1 mutation | BTC # | IDH305 | None | DLT | TRAE, PK, delta 2-hydroxyglutarate, ORR, SAE | |
| I | NCT03272464 | BRAF-V600E | BTC # | JSI-1187 + dabrafenib | None | TRAE | DOR, OS, PFS, TTP | |
| I | NCT04190628 | BRAF-V600E | BTC # | ABM-1310 + cobimetinib | None | MTD | TRAE, PK, DOR, OS, PFS, TTP | |
| I | NCT02451553 | No specific target | BTC # | Afatinib dimaleate + capecitabine | None | AE, DLT, MTD | DOR, OS, PFS, RR, TTP, biomarker profile | |
| I | NCT03507998 | Wnt/β-catenin signaling inhibitors | BTC # | CGX1321 | None | TRAE | PK |
| Line | Phase | Clinical Trial Identifier | Treated Cancer Group | Experimental Arm | Comparative Arm | Primary Outcome | Secondary Outcome (Main) |
|---|---|---|---|---|---|---|---|
| First line | III | NCT04003636 | BTC | Pembrolizumab + GC | GC + placebo | OS | PFS, ORR, DOR |
| II/III | NCT04066491 | BTC | Bintrafusp alfa | GC + placebo | OS DLT | PFS, DOR, ORR | |
| II | NCT04217954 | BTC | HAIC (oxaliplatin + 5-FU) + toripalimab (T) + bevacizumab | None | PFS, ORR | OS, AE, CA 19-9, DCE-MRI signal change, DWI MRI signal change | |
| II | NCT04172402 | BTC | TS-1 + gemcitabine + nivolumab | None | ORR | None specified | |
| II | NCT03898895 | iCCA | Camrelizumab + radiotherapy | GC | PFS | OS, AE, tumor response | |
| III | NCT03478488 | BTC | KN035 (PD-L1 antibody) + gemcitabine + oxaliplatin | GEMOX | OS | PFS, ORR, DCR, DOR, TTP | |
| II | NCT03796429 | BTC | Gemcitabine/S-1 + toripalimab | None | PFS, OS | ORR, Safety | |
| II | NCT04027764 | BTC | Toripalimab + S1 and albumin paclitaxel | None | ORR | PFS, DCR, OS | |
| II | NCT04191343 | BTC | Toripalimab + GEMOX | None | ORR | None specified | |
| II | NCT04300959 | BTC | Anlotinib hydrochloride + PD1 + gemcitabine + cisplatin | Gemcitabine Cisplatin | OS 1 yr | OS 2 yr, PFS, ORR, AE | |
| Subsequent lines | II | NCT03482102 | HCC, BTC | Tremelimumab + durvalumab + radiation | None | ORR | AE, OS, DCR, PFS, DOR, TTP |
| II | NCT04238637 | BTC | Durvalumab (D) vs. D + T | None | ORR | Safety, DoR, PFS, OS | |
| II | NCT02821754 | HCC, BTC | D + T | D +T + TACE D + T + RFA D + T + Cryo | PFS | Safety | |
| II | NCT02703714 | BTC | Pembrolizumab and sargramostim (GM-CSF) | None | ORR | AE, PD-L1 positivity, PFS, OS, DOR | |
| I/II | NCT03937895 | BTC * | Allogeneic natural killer cells + pembrolizumab | None | Phase I—DLT Phase II—ORR | TTP, toxicity | |
| II | NCT04306367 | BTC | Pembrolizumab and olaparib | mFOLFOX-historical control | ORR | DOR, PFS, OS, safety | |
| II | NCT04295317 | iCCA—adjuvant | PD-1 blocking antibody SHR-1210 + capecitabine | None | PFS | OS, side effects | |
| II | NCT03250273 | BTC, PDA | Entinostat + nivolumab | None | ORR | Toxicity, PFS, OS, DOR | |
| II | NCT02866383 | BTC, PDA | Nivolumab + ipilimumab + radiotherapy | Nivolumab + radiotherapy | CBR | AE, ORR, PFS, OS, QOL | |
| II | NCT04057365 | BTC | DKN-01 + nivolumab | None | ORR | PFS, OS | |
| II | NCT03639935 | BTC | Rucaparib + nivolumab | None | 4-month PFS rate | Response rate, PFS, OS | |
| II | NCT04299581 | iCCA | Camrelizumab + cryo | None | ORR | DOR, PFS, OS, DCR, AE | |
| II | NCT03999658 | BTC # | STI-3031 anti-PD-L1 antibody | None | ORR | DOR, CR, PFS, 1-year PFS rate, correlative studies | |
| II | NCT03801083 | BTC | Tumor infiltrating lymphocytes (TIL) + aldesleukin | None | ORR | CRR, DOR, DCR, PFS, OS, QOL | |
| I/II | NCT03684811 | BTC # | FT-2102 vs. FT-2102 + nivolumab | None | DLT, Dose, ORR | ORR, AE, PFS, TTP, DOR, OS, TT | |
| I/II | NCT03475953 | BTC # | Regorafenib + avelumab | None | I = dose II = antitumor activity | MTD, DLT, toxicity, AE, PK and correlative studies | |
| I/II | NCT03785873 | BTC | Nal-Irinotecan + nivolumab + 5-Fluorouracil + leucovorin | None | I = DLT II = PFS | AE, ORR, OS | |
| I | NCT03849469 | iCCA # | XmAb®22841 and pembrolizumab | XmAb®22841 Monotherapy | Safety and tolerability | None | |
| I | NCT03257761 | BTC, PDA, HCC | Guadecitabine + durvalumab | None | AE, Tumor response | OS, PFS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woods, E.; Le, D.; Jakka, B.K.; Manne, A. Changing Landscape of Systemic Therapy in Biliary Tract Cancer. Cancers 2022, 14, 2137. https://doi.org/10.3390/cancers14092137
Woods E, Le D, Jakka BK, Manne A. Changing Landscape of Systemic Therapy in Biliary Tract Cancer. Cancers. 2022; 14(9):2137. https://doi.org/10.3390/cancers14092137
Chicago/Turabian StyleWoods, Edward, Dat Le, Bharath Kumar Jakka, and Ashish Manne. 2022. "Changing Landscape of Systemic Therapy in Biliary Tract Cancer" Cancers 14, no. 9: 2137. https://doi.org/10.3390/cancers14092137
APA StyleWoods, E., Le, D., Jakka, B. K., & Manne, A. (2022). Changing Landscape of Systemic Therapy in Biliary Tract Cancer. Cancers, 14(9), 2137. https://doi.org/10.3390/cancers14092137