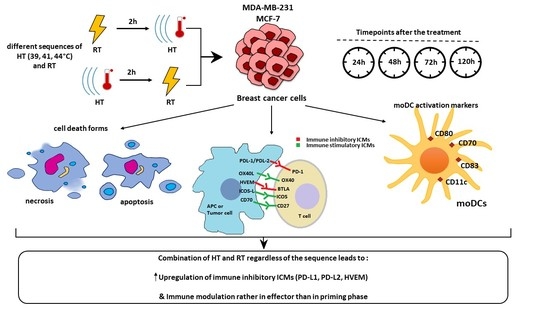
The Effect of Hyperthermia and Radiotherapy Sequence on Cancer Cell Death and the Immune Phenotype of Breast Cancer Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
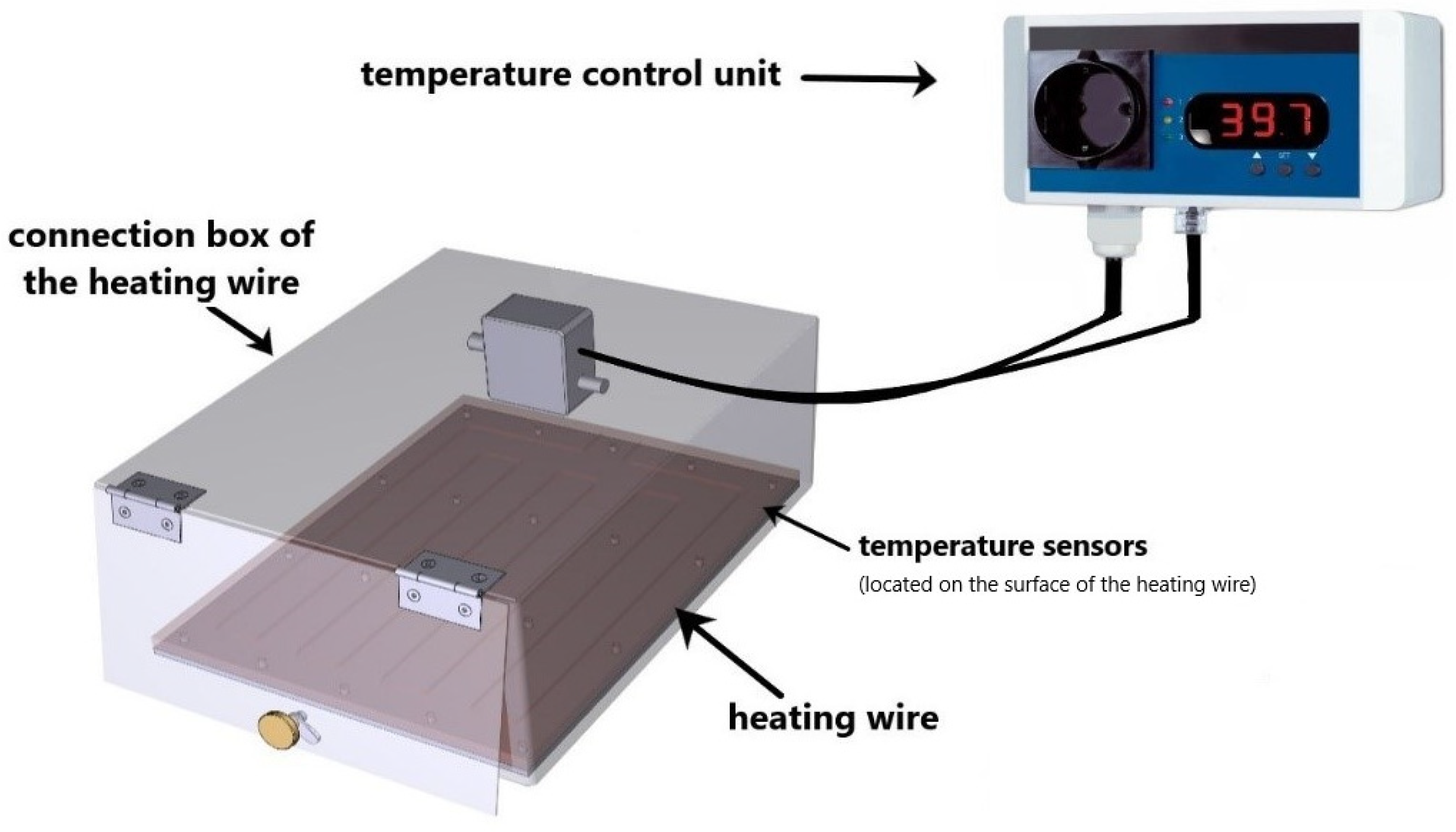
2.1. Ex Vivo Heating System for Hyperthermia Treatment of Tumor Cells
2.2. Cell Lines and Cell Culture
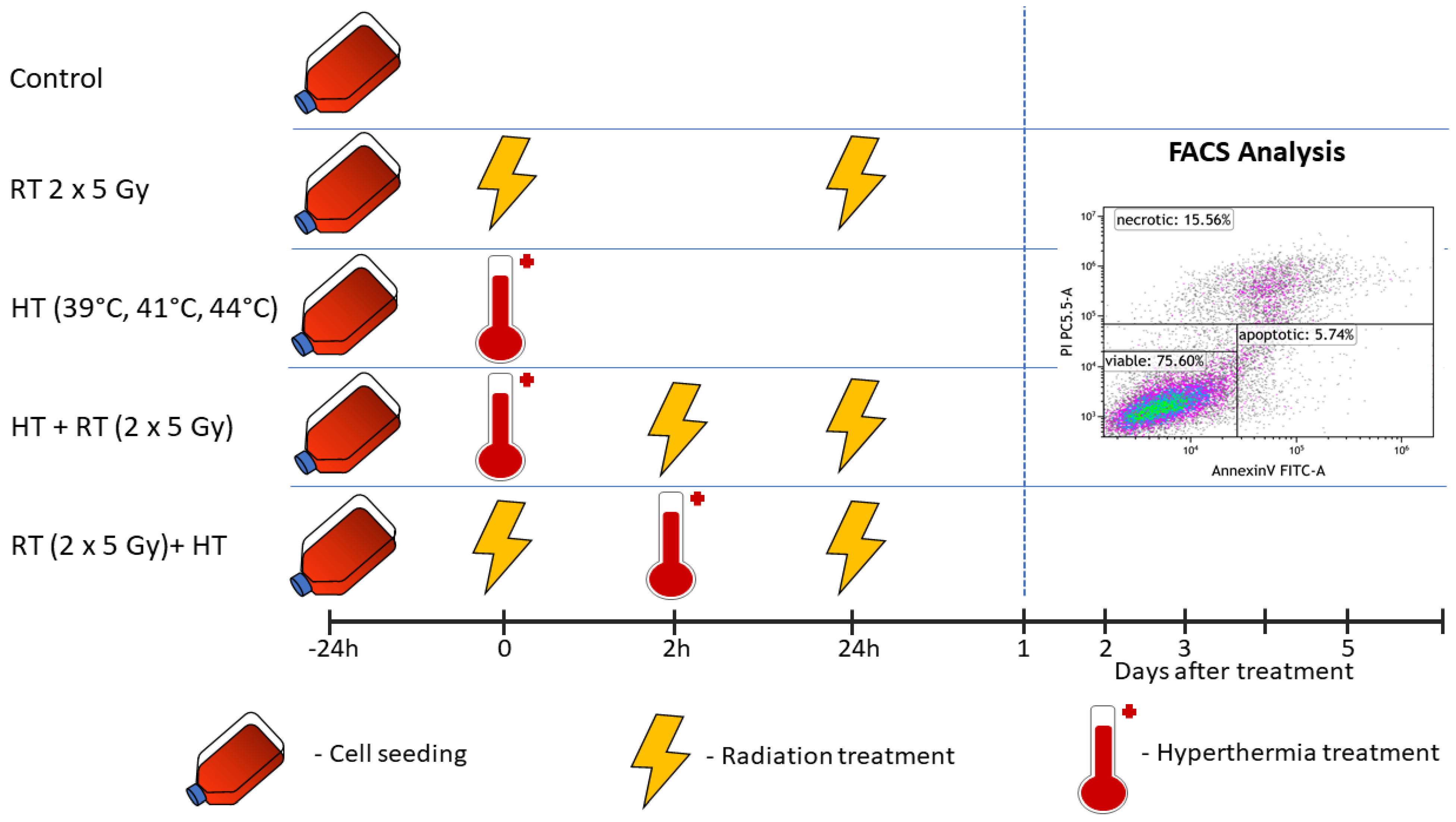
2.3. Treatments and Sampling
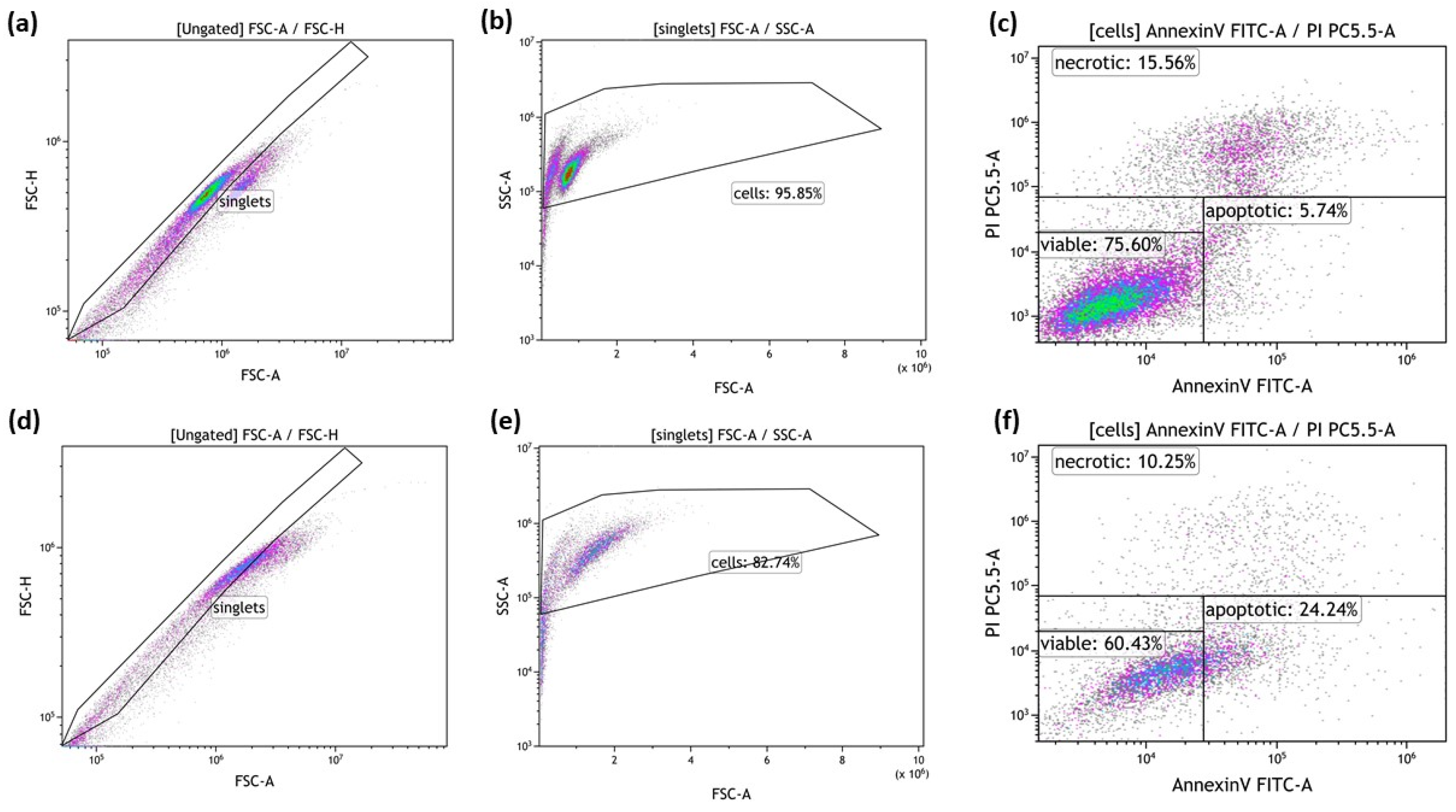
2.4. Detection of Tumor Cell Death Forms by Annexin V/PI Staining
2.5. Detection of Immune Checkpoint Molecules by Multicolor Flow Cytometry
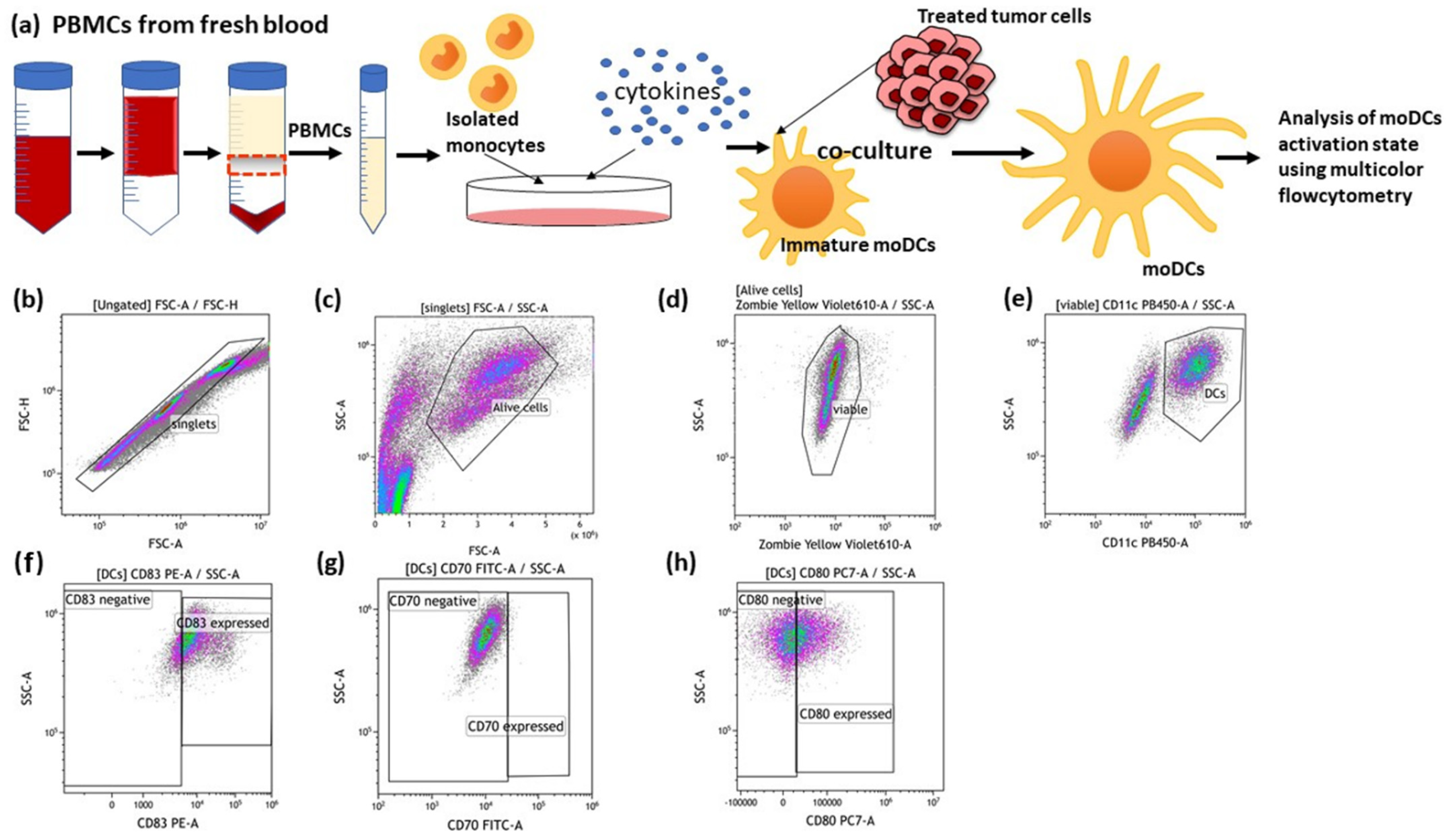
2.6. Isolation of Human Peripheral Blood Mononuclear Cells (PBMCs), Monocyte Enrichment and Differentiation into Immature Dendritic Cells (imm. moDCs)
2.7. Co-Culture of moDCs with Treated and Untreated MCF-7 Cancer Cells and Detection of DC Activation Markers on the Surface of moDCs
2.8. Statistical Analysis
3. Results
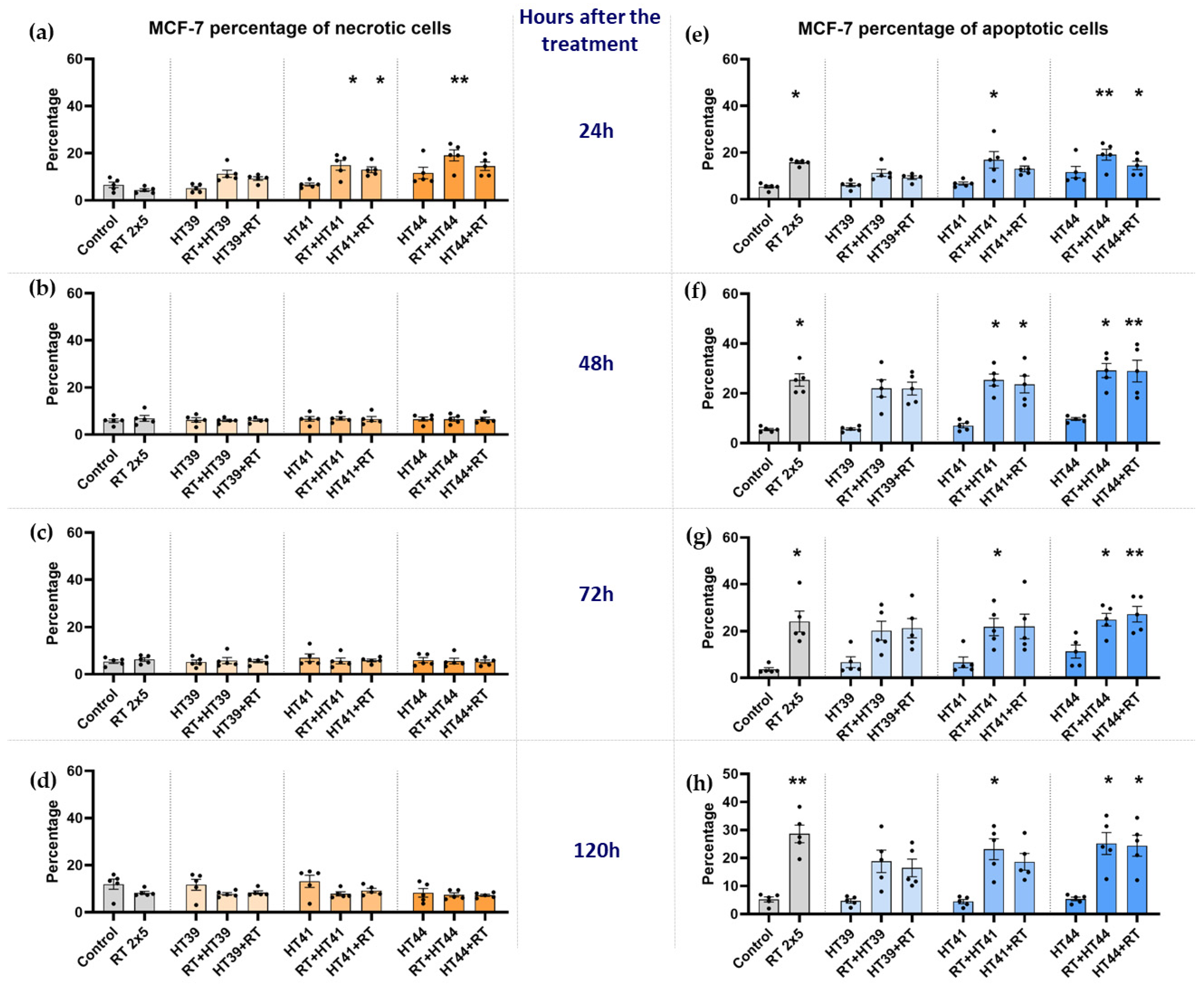
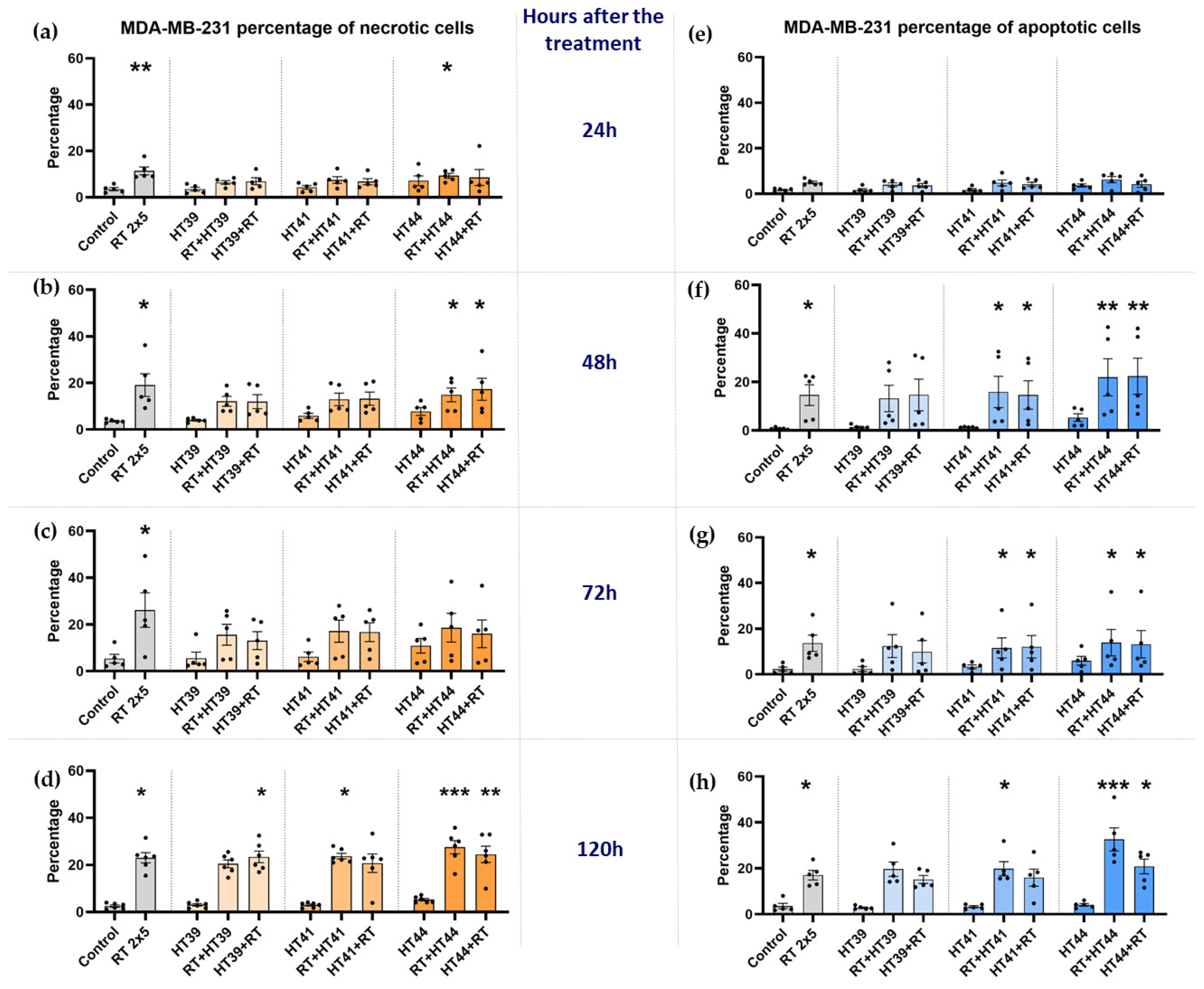
3.1. Radiotherapy in Combination with Hyperthermia Significantly Induces Apoptosis in MCF-7 Cells Andboth Apoptosis and Necrosis in MDA-MB-231 Breast Cancer Cells
3.1.1. Radiotherapy in Combination with Hyperthermia Regardless of the Treatment Sequence Significantly Induces Apoptosis in MCF-7 Breast Cancer Cells
3.1.2. Radiotherapy in Combination with Hyperthermia Regardless of the Treatment Sequence Significantly Induce Apoptosis and Necrosis in MDA-MB-231 Breast Cancer Cells
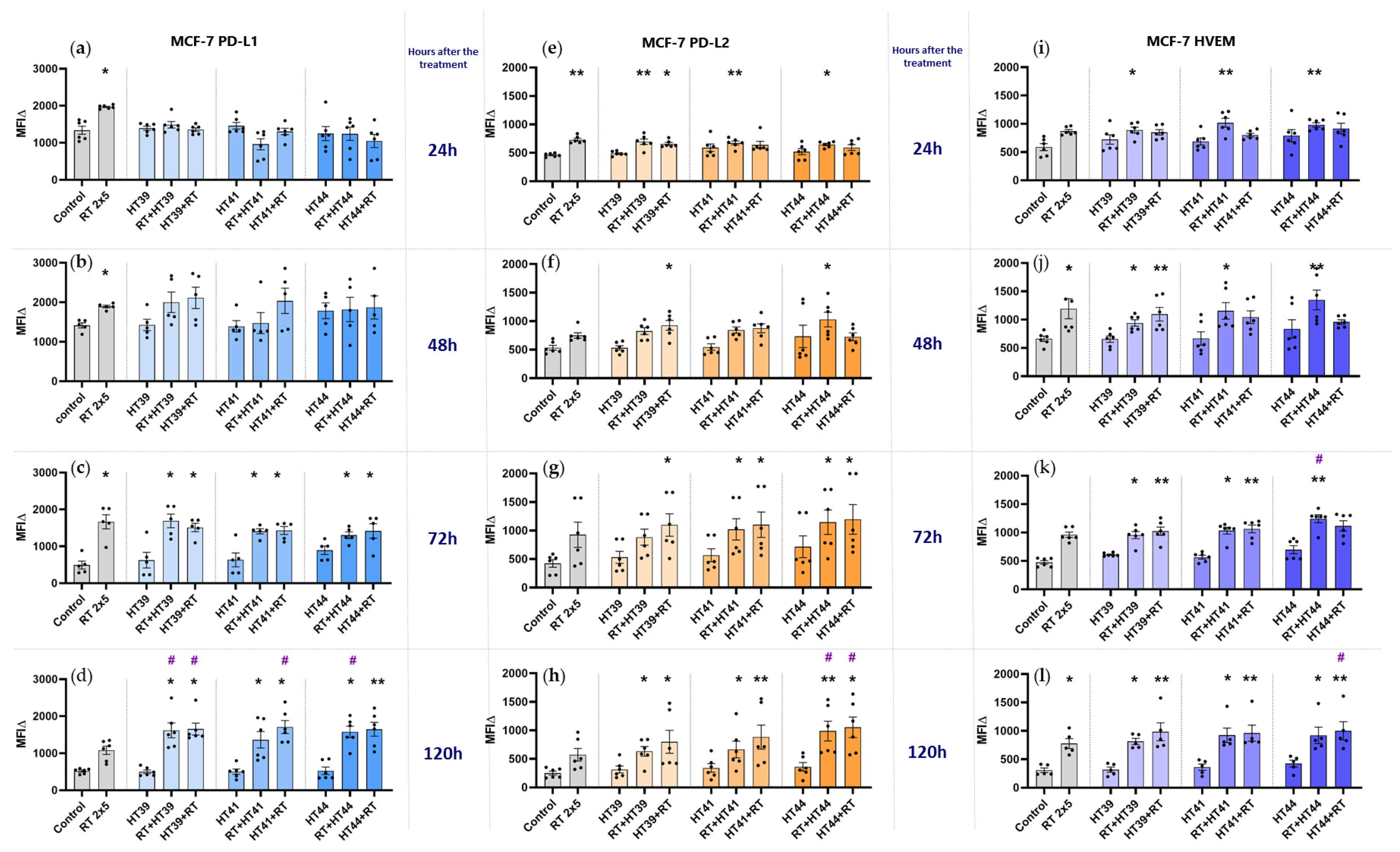
3.2. Hyperthermia in Combination with Radiotherapy Affects the Expression of Immune Checkpoint Molecules on Breast Cancer Cells
3.2.1. Hyperthermia in Combination with Radiotherapy Upregulates the Expression of Several Inhibitory Immune Checkpoint Molecules on MCF-7 Breast Cancer Cells
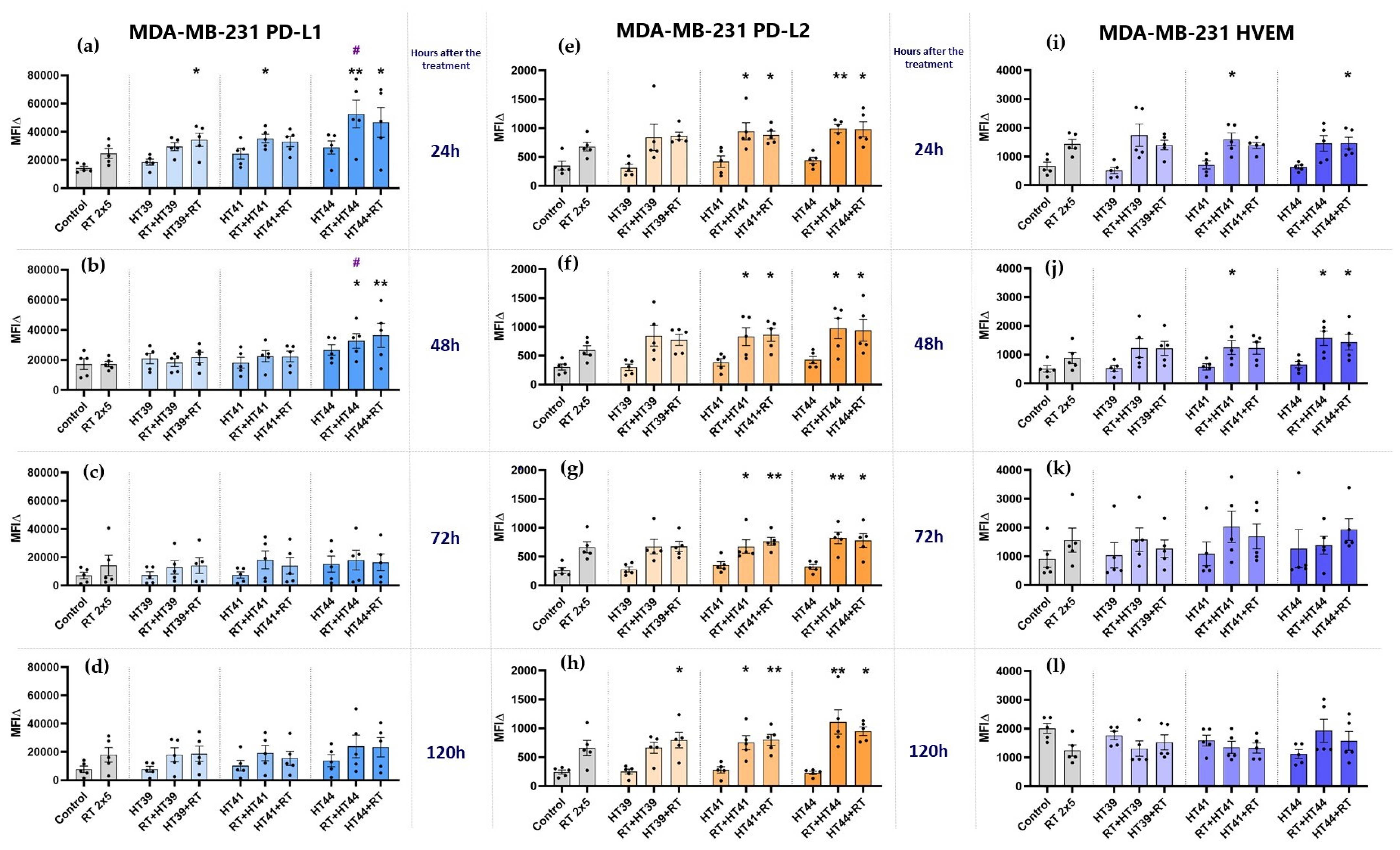
3.2.2. Hyperthermia in Combination with Radiotherapy Upregulates the Expression of Several Inhibitory Immune Checkpoint Molecules on MDA-MB-231 Breast Cancer Cells
3.2.3. Hyperthermia in Combination with Radiotherapy Only Slightly Affects the Expression of Stimulatory Immune Checkpoint Molecules on MCF-7 and MDA-MB-231 Breast Cancer Cells
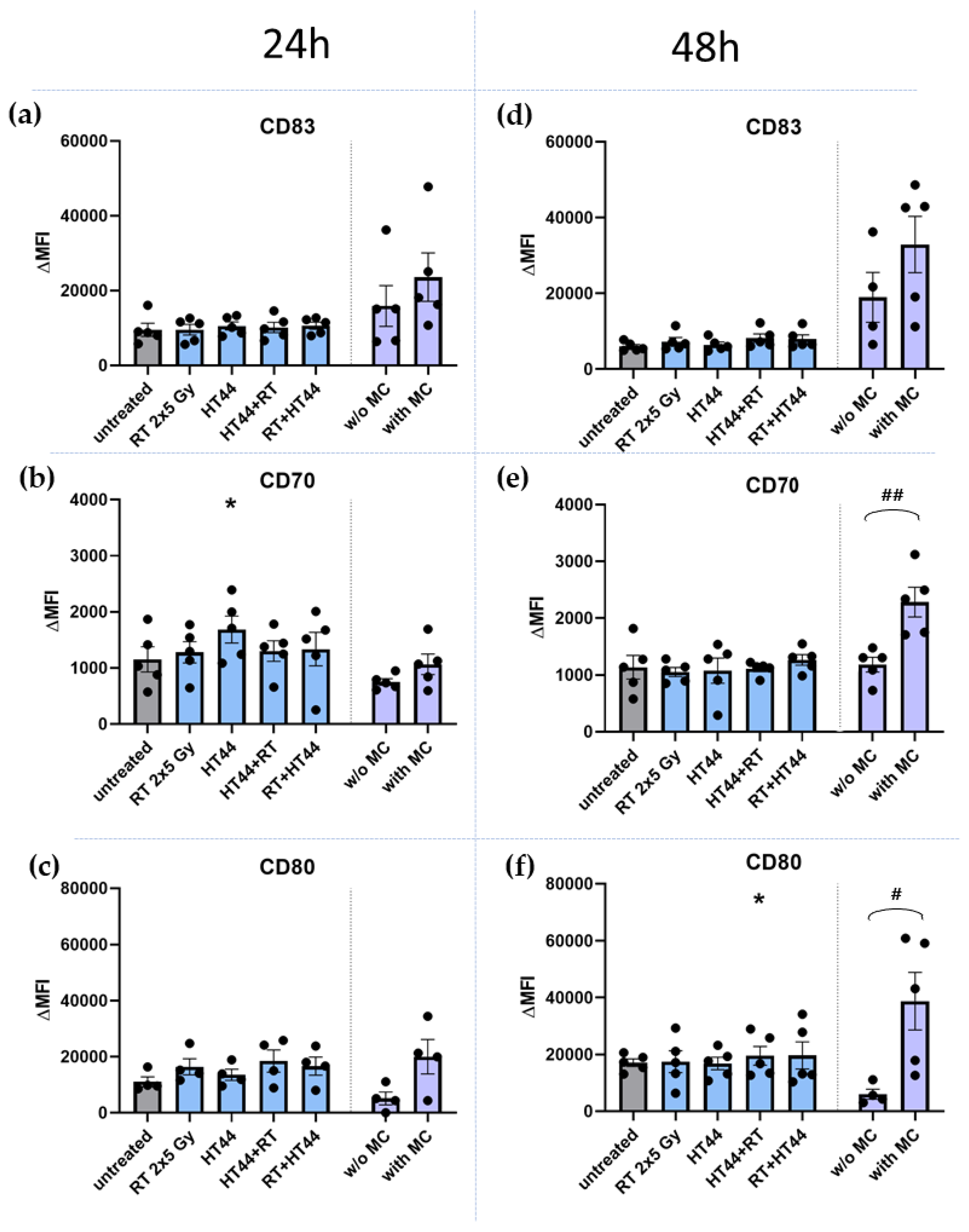
3.3. The Impact of HT- and RT-Treated Breast Cancer Cells on the Activation State of moDCs
4. Discussion
4.1. In Human Breast Cancer Cells, Radiotherapy Rather Than Moderate Hyperthermia Is the Key Trigger for Cell Death Induction
4.2. The Combination of Hyperthermia and Radiotherapy Affects Particularly the Expression of Immune Suppressive Immune Checkpoint Molecules of Breast Cancer Cells, but Independently of the Treatment Sequence
4.3. The Co-Incubation of RT- and HT-Treated Breast Cancer Cells Does Not Affect the Activation State of Dendritic Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- O’Shaughnessy, J. Extending Survival with Chemotherapy in Metastatic Breast Cancer. Oncologist 2005, 10, 20–29. [Google Scholar] [CrossRef]
- Villacampa, G.; Tolosa, P.; Salvador, F.; Sánchez-Bayona, R.; Villanueva, L.; Dienstmann, R.; Ciruelos, E.; Pascual, T. Addition of immune checkpoint inhibitors to chemotherapy versus chemotherapy alone in first-line metastatic triple-negative breast cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 2022, 104, 102352. [Google Scholar] [CrossRef]
- Frey, B.; Rückert, M.; Deloch, L.; Rühle, P.F.; Derer, A.; Fietkau, R.; Gaipl, U.S. Immunomodulation by ionizing radiation-impact for design of radio-immunotherapies and for treatment of inflammatory diseases. Immunol. Rev. 2017, 280, 231–248. [Google Scholar] [CrossRef]
- Issels, R.; Kampmann, E.; Kanaar, R.; Lindner, L. Hallmarks of hyperthermia in driving the future of clinical hyperthermia as targeted therapy: Translation into clinical application. Int. J. Hyperth. 2016, 32, 89–95. [Google Scholar] [CrossRef]
- Hurwitz, M.; Stauffer, P. Hyperthermia, Radiation and Chemotherapy: The Role of Heat in Multidisciplinary Cancer Care. Semin. Oncol. 2014, 41, 714–729. [Google Scholar] [CrossRef]
- Issels, R.D. Hyperthermia adds to chemotherapy. Eur. J. Cancer 2008, 44, 2546–2554. [Google Scholar] [CrossRef]
- De-Colle, C.; Weidner, N.; Heinrich, V.; Brucker, S.; Hahn, M.; Macmillan, K.; Lamprecht, U.; Gaupp, S.; Voigt, O.; Zips, D. Hyperthermic chest wall re-irradiation in recurrent breast cancer: A prospective observational study. Strahlenther. Onkol. 2019, 195, 318–326. [Google Scholar] [CrossRef]
- Oldenborg, S.; Rasch, C.R.N.; Van Os, R.; Kusumanto, Y.H.; Oei, B.S.; Venselaar, J.L.; Heymans, M.; Vörding, P.J.Z.V.S.; Crezee, J.; Van Tienhoven, G. Reirradiation + hyperthermia for recurrent breast cancer en cuirasse. Strahlenther. Onkol. 2017, 194, 206–214. [Google Scholar] [CrossRef]
- Song, C.W.; Shakil, A.; Osborn, J.L.; Iwata, K. Tumour oxygenation is increased by hyperthermia at mild temperatures. Int. J. Hyperth. 2009, 12, 367–373. [Google Scholar] [CrossRef]
- Horsman, M.R.; Overgaard, J. Hyperthermia: A Potent Enhancer of Radiotherapy. Clin. Oncol. 2007, 19, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Tsang, Y.-W.; Huang, C.-C.; Yang, K.-L.; Chi, M.-S.; Chiang, H.-C.; Wang, Y.-S.; Andocs, G.; Szasz, A.; Li, W.-T.; Chi, K.-H. Improving immunological tumor microenvironment using electro-hyperthermia followed by dendritic cell immunotherapy. BMC Cancer 2015, 15, 708. [Google Scholar] [CrossRef][Green Version]
- Rao, W.; Deng, Z.-S.; Liu, J. A Review of Hyperthermia Combined With Radiotherapy/Chemotherapy on Malignant Tumors. Crit. Rev. Biomed. Eng. 2010, 38, 101–116. [Google Scholar] [CrossRef]
- Chen, T.; Guo, J.; Han, C.; Yang, M.; Cao, X. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J. Immunol. 2009, 182, 1449–1459. [Google Scholar] [CrossRef]
- Datta, N.R.; Gómez Ordóñez, S.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef]
- Schildkopf, P.; Frey, B.; Mantel, F.; Ott, O.J.; Weiss, E.-M.; Sieber, R.; Janko, C.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Application of hyperthermia in addition to ionizing irradiation fosters necrotic cell death and HMGB1 release of colorectal tumor cells. Biochem. Biophys. Res. Commun. 2010, 391, 1014–1020. [Google Scholar] [CrossRef]
- Schildkopf, P.; Frey, B.; Ott, O.J.; Rubner, Y.; Multhoff, G.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Radiation combined with hyperthermia induces HSP70-dependent maturation of dendritic cells and release of pro-inflammatory cytokines by dendritic cells and macrophages. Radiother. Oncol. 2011, 101, 109–115. [Google Scholar] [CrossRef]
- Skitzki, J.J.; A Repasky, E.; Evans, S.S. Hyperthermia as an immunotherapy strategy for cancer. Curr. Opin. Investig. Drugs 2009, 10, 550–558. [Google Scholar]
- Werthmöller, N.; Frey, B.; Rückert, M.; Lotter, M.; Fietkau, R.; Gaipl, U.S. Combination of ionising radiation with hyperthermia increases the immunogenic potential of B16-F10 melanoma cells in vitro and in vivo. Int. J. Hyperth. 2016, 32, 23–30. [Google Scholar] [CrossRef]
- Schmid, T.E.; Multhoff, G. Radiation-induced stress proteins—The role of heat shock proteins (HSP) in anti-tumor responses. Curr. Med. Chem. 2012, 19, 1765–1770. [Google Scholar] [CrossRef]
- Peer, A.J.; Grimm, M.J.; Zynda, E.R.; Repasky, E.A. Diverse immune mechanisms may contribute to the survival benefit seen in cancer patients receiving hyperthermia. Immunol. Res. 2009, 46, 137–154. [Google Scholar] [CrossRef]
- Knippertz, I.; Stein, M.F.; Dörrie, J.; Schaft, N.; Müller, I.; Deinzer, A.; Steinkasserer, A.; Nettelbeck, D.M. Mild hyperthermia enhances human monocyte-derived dendritic cell functions and offers potential for applications in vaccination strategies. Int. J. Hyperth. 2011, 27, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Crezee, J.; Oei, A.L.; Franken, N.A.P.; Stalpers, L.J.A.; Kok, H.P. Response: Commentary: The Impact of the Time Interval between Radiation and Hyperthermia on Clinical Outcome in Patients with Locally Advanced Cervical Cancer. Front. Oncol. 2020, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.; Van Der Zee, J.; Van Tienhoven, G.; Kok, H.P.; Rasch, C.R.N.; Crezee, H. Temperature and thermal dose during radiotherapy and hyperthermia for recurrent breast cancer are related to clinical outcome and thermal toxicity: A systematic review. Int. J. Hyperth. 2019, 36, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Crezee, H.; Kok, H.P.; Oei, A.L.; Franken, N.A.P.; Stalpers, L.J.A. The Impact of the Time Interval between Radiation and Hyperthermia on Clinical Outcome in Patients with Locally Advanced Cervical Cancer. Front. Oncol. 2019, 9, 412. [Google Scholar] [CrossRef]
- Dobšíček Trefná, H.; Schmidt, M.; van Rhoon, G.C.; Kok, H.P.; Gordeyev, S.S.; Lamprecht, U.; Marder, D.; Nadobny, J.; Ghadjar, P.; Abdel-Rahman, S.; et al. Quality assurance guidelines for interstitial hyperthermia. Int. J. Hyperth. 2019, 36, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Lagendijk, J.J.W.; Van Rhoon, G.C.; Hornsleth, S.N.; Wust, P.; De Leeuw, A.C.C.; Schneider, C.J.; Van Ddk, J.D.P.; Van Der Zee, J.; Van Heek-Romanowski, R.; Rahman, S.A.; et al. Esho Quality Assurance Guidelines for Regional Hyperthermia. Int. J. Hyperth. 2009, 14, 125–133. [Google Scholar] [CrossRef]
- Elming, P.B.; Sørensen, B.S.; Oei, A.L.; Franken, N.A.P.; Crezee, J.; Overgaard, J.; Horsman, M.R. Hyperthermia: The Optimal Treatment to Overcome Radiation Resistant Hypoxia. Cancers 2019, 11, 60. [Google Scholar] [CrossRef]
- Vernon, C.C.; Hand, J.W.; Field, S.B.; Machin, B.; Whaley, J.B.; van der Zee, J.; van Putten, W.L.; van Rhoon, G.C.; van Dijk, J.D.; González González, D.; et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. Int. J. Radiat. Oncol. Biol. Phys. 1996, 35, 731–744. [Google Scholar]
- Overgaard, J.; Bentzen, S.M.; Overgaard, J.; Gonzalez, D.G.; Hulshof, M.C.C.M.; Arcangeli, G.; Dahl, O.; Mella, O. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. Lancet 1995, 345, 540–543. [Google Scholar] [CrossRef]
- Van Der Zee, J.; González, D.G. The Dutch Deep Hyperthermia Trial: Results in cervical cancer. Int. J. Hyperth. 2009, 18, 1–12. [Google Scholar] [CrossRef]
- van der Zee, J.; González, D.; van Rhoon, G.C.; van Dijk, J.D.; van Putten, W.L.; Hart, A.A. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Lancet 2000, 355, 1119–1125. [Google Scholar] [CrossRef]
- Ott, O.J.; Schmidt, M.; Semrau, S.; Strnad, V.; Matzel, K.E.; Schneider, I.; Raptis, D.; Uter, W.; Grützmann, R.; Fietkau, R. Chemoradiotherapy with and without deep regional hyperthermia for squamous cell carcinoma of the anus. Strahlenther. Onkol. 2018, 195, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Issels, R.D.; Noessner, E.; Lindner, L.H.; Schmidt, M.; Albertsmeier, M.; Blay, J.-Y.; Stutz, E.; Xu, Y.; Buecklein, V.; Altendorf-Hofmann, A.; et al. Immune infiltrates in patients with localised high-risk soft tissue sarcoma treated with neoadjuvant chemotherapy without or with regional hyperthermia: A translational research program of the EORTC 62961-ESHO 95 randomised clinical trial. Eur. J. Cancer 2021, 158, 123–132. [Google Scholar] [CrossRef]
- Schouten, D.; van Os, R.; Westermann, A.M.; Crezee, H.; van Tienhoven, G.; Kolff, M.W.; Bins, A.D. A randomized phase-II study of reirradiation and hyperthermia versus reirradiation and hyperthermia plus chemotherapy for locally recurrent breast cancer in previously irradiated area. Acta Oncol. 2022, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Notter, M.; Stutz, E.; Thomsen, A.R.; Vaupel, P. Radiation-Associated Angiosarcoma of the Breast and Chest Wall Treated with Thermography-Controlled, Contactless wIRA-Hyperthermia and Hypofractionated Re-Irradiation. Cancers 2021, 13, 3911. [Google Scholar] [CrossRef]
- Murray Brunt, A.; Haviland, J.S.; Wheatley, D.A.; Sydenham, M.A.; Alhasso, A.; Bloomfield, D.J.; Chan, C.; Churn, M.; Cleator, S.; Coles, C.E.; et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020, 395, 1613–1626. [Google Scholar] [CrossRef]
- Demaria, S.; Guha, C.; Schoenfeld, J.; Morris, Z.; Monjazeb, A.; Sikora, A.; Crittenden, M.; Shiao, S.; Khleif, S.; Gupta, S.; et al. Radiation dose and fraction in immunotherapy: One-size regimen does not fit all settings, so how does one choose? J. Immunother. Cancer 2021, 9, e002038. [Google Scholar] [CrossRef]
- Hader, M.; Savcigil, D.P.; Rosin, A.; Ponfick, P.; Gekle, S.; Wadepohl, M.; Bekeschus, S.; Fietkau, R.; Frey, B.; Schlücker, E.; et al. Differences of the Immune Phenotype of Breast Cancer Cells after Ex Vivo Hyperthermia by Warm-Water or Microwave Radiation in a Closed-Loop System Alone or in Combination with Radiotherapy. Cancers 2020, 12, 1082. [Google Scholar] [CrossRef]
- Gillette, E.L.; Ensley, B.A. Effect of heating order on radiation response of mouse tumor and skin. Int. J. Radiat. Oncol. 1979, 5, 209–213. [Google Scholar] [CrossRef]
- Hill, S.A.; Denekamp, J. The response of six mouse tumours to combined heat and X rays: Implications for therapy. Br. J. Radiol. 1979, 52, 209–218. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, J.; de Bruijne, M.; van Rhoon, G.C. Thermal medicine, heat shock proteins and cancer. Int. J. Hyperth. 2006, 22, 433–437; author reply 437–447. [Google Scholar]
- Trefná, H.D.; Crezee, H.; Schmidt, M.; Marder, D.; Lamprecht, U.; Ehmann, M.; Hartmann, J.; Nadobny, J.; Gellermann, J.; Van Holthe, N.; et al. Quality assurance guidelines for superficial hyperthermia clinical trials: I. Clinical requirements. Int. J. Hyperth. 2017, 33, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Lühr, J.J.; Alex, N.; Amon, L.; Kräter, M.; Kubánková, M.; Sezgin, E.; Lehmann, C.H.K.; Heger, L.; Heidkamp, G.F.; Smith, A.-S.; et al. Maturation of Monocyte-Derived DCs Leads to Increased Cellular Stiffness, Higher Membrane Fluidity, and Changed Lipid Composition. Front. Immunol. 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Rückert, M.; Deloch, L.; Fietkau, R.; Frey, B.; Hecht, M.; Gaipl, U.S. Immune modulatory effects of radiotherapy as basis for well-reasoned radioimmunotherapies. Strahlenther. Onkol. 2018, 194, 509–519. [Google Scholar] [CrossRef]
- Hader, M.; Frey, B.; Fietkau, R.; Hecht, M.; Gaipl, U.S. Immune biological rationales for the design of combined radio- and immunotherapies. Cancer Immunol. Immunother. 2020, 69, 293–306. [Google Scholar] [CrossRef]
- Kolberg, H.C.; Hoffmann, O.; Baumann, R. The Abscopal Effect: Could a Phenomenon Described Decades Ago Become Key to Enhancing the Response to Immune Therapies in Breast Cancer? Breast Care 2020, 15, 443–449. [Google Scholar] [CrossRef]
- Zagar, T.M.; Oleson, J.R.; Vujaskovic, Z.; Dewhirst, M.W.; Craciunescu, O.I.; Blackwell, K.L.; Prosnitz, L.R.; Jones, E.L. Hyperthermia combined with radiation therapy for superficial breast cancer and chest wall recurrence: A review of the randomised data. Int. J. Hyperth. 2010, 26, 612–617. [Google Scholar] [CrossRef]
- Arslan, S.A.; Ozdemir, N.; Sendur, M.A.; Eren, T.; Ozturk, H.F.; Aral, I.P.; Soykut, E.D.; Inan, G.A. Hyperthermia and radiotherapy combination for locoregional recurrences of breast cancer: A review. Breast Cancer Manag. 2017, 6, 117–126. [Google Scholar] [CrossRef]
- Ho, A.Y.; Barker, C.A.; Arnold, B.B.; Powell, S.N.; Hu, Z.I.; Gucalp, A.; Lebron-Zapata, L.; Wen, H.Y.; Kallman, C.; D’Agnolo, A.; et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer 2020, 126, 850–860. [Google Scholar] [CrossRef]
- Li, Z.; Deng, J.; Sun, J.; Ma, Y. Hyperthermia Targeting the Tumor Microenvironment Facilitates Immune Checkpoint Inhibitors. Front. Immunol. 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galassi, C.; Zitvogel, L.; Galluzzi, L. Immunogenic cell stress and death. Nat. Immunol. 2022, 23, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Podolska, M.J.; Shan, X.; Janko, C.; Boukherroub, R.; Gaipl, U.S.; Szunerits, S.; Frey, B.; Muñoz, L.E. Graphene-Induced Hyperthermia (GIHT) Combined with Radiotherapy Fosters Immunogenic Cell Death. Front. Oncol. 2021, 11, 664615. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8, e000337. [Google Scholar] [CrossRef]
- Scheidegger, S.; Barba, S.M.; Gaipl, U.S. Theoretical Evaluation of the Impact of Hyperthermia in Combination with Radiation Therapy in an Artificial Immune—Tumor-Ecosystem. Cancers 2021, 13, 5764. [Google Scholar] [CrossRef]
- Andersen, M.H. The Balance Players of the Adaptive Immune System. Cancer Res. 2018, 78, 1379–1382. [Google Scholar] [CrossRef]
- Mei, X.; Ten Cate, R.; Van Leeuwen, C.M.; Rodermond, H.M.; De Leeuw, L.; Dimitrakopoulou, D.; Stalpers, L.J.A.; Crezee, J.; Kok, H.P.; Franken, N.A.P.; et al. Radiosensitization by hyperthermia: The effects of temperature, sequence, and time interval in cervical cell lines. Cancers 2020, 12, 582. [Google Scholar] [CrossRef]
- Mondini, M.; Levy, A.; Meziani, L.; Milliat, F.; Deutsch, E. Radiotherapy–immunotherapy combinations—Perspectives and challenges. Mol. Oncol. 2020, 14, 1529–1537. [Google Scholar] [CrossRef]
- Ibuki, Y.; Takahashi, Y.; Tamari, K.; Minami, K.; Seo, Y.; Isohashi, F.; Koizumi, M.; Ogawa, K. Local hyperthermia combined with CTLA-4 blockade induces both local and abscopal effects in a murine breast cancer model. Int. J. Hyperth. 2021, 38, 363–371. [Google Scholar] [CrossRef]
- Kordbacheh, T.; Honeychurch, J.; Blackhall, F.; Faivre-Finn, C.; Illidge, T. Radiotherapy and anti-PD-1/PD-L1 combinations in lung cancer: Building better translational research platforms. Ann. Oncol. 2018, 29, 301–310. [Google Scholar] [CrossRef]
- Derer, A.; Spiljar, M.; Bäumler, M.; Hecht, M.; Fietkau, R.; Frey, B.; Gaipl, U.S. Chemoradiation Increases PD-L1 Expression in Certain Melanoma and Glioblastoma Cells. Front. Immunol. 2016, 7, 610. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Koh, J.; Kim, S.; Jeon, S.-R.; Chie, E.K.; Kim, K.; Kang, G.H.; Han, S.-W.; Kim, T.-Y.; Jeong, S.-Y.; et al. Chemoradiation-Induced Alteration of Programmed Death-Ligand 1 and CD8 + Tumor-Infiltrating Lymphocytes Identified Patients With Poor Prognosis in Rectal Cancer: A Matched Comparison Analysis. Int. J. Radiat. Oncol. 2017, 99, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.Y.S.; Chan, K.-W.; Ni, Y.-B.; Hlaing, T.; Hu, J.; Cheung, S.-Y.; Tse, G.M. Expression and Clinical Significance of Herpes Virus Entry Mediator (HVEM) in Breast Cancer. Ann. Surg. Oncol. 2017, 24, 4042–4050. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.N.; Calabuig-Fariñas, S.; de Mena, M.L.; Torres-Martínez, S.; González, C.G.; García, J.; Ángel, G.; González-Cruz, V.I.; Herrero, C.C. Programmed Death-Ligand 1 (PD-L1) as Immunotherapy Biomarker in Breast Cancer. Cancers 2022, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Breen, W.G.; Leventakos, K.; Dong, H.; Merrell, K.W. Radiation and immunotherapy: Emerging mechanisms of synergy. J. Thorac. Dis. 2020, 12, 7011–7023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, J.; Zheng, L.; Yang, Q.; Chen, X.; Chen, X.; Yu, Y.; Li, F.; Cui, J.; Sun, J. Immune Response on Optimal Timing and Fractionation Dose for Hypofractionated Radiotherapy in Non–Small-Cell Lung Cancer. Front. Mol. Biosci. 2022, 9, 786864. [Google Scholar] [CrossRef]
- Aspeslagh, S.; Postel-Vinay, S.; Rusakiewicz, S.; Soria, J.-C.; Zitvogel, L.; Marabelle, A. Rationale for anti-OX40 cancer immunotherapy. Eur. J. Cancer 2016, 52, 50–66. [Google Scholar] [CrossRef]
- Wimmer, S.; Deloch, L.; Hader, M.; Derer, A.; Grottker, F.; Weissmann, T.; Hecht, M.; Gostian, A.-O.; Fietkau, R.; Frey, B.; et al. Hypofractionated Radiotherapy Upregulates Several Immune Checkpoint Molecules in Head and Neck Squamous Cell Carcinoma Cells Independently of the HPV Status While ICOS-L Is Upregulated Only on HPV-Positive Cells. Int. J. Mol. Sci. 2021, 22, 9114. [Google Scholar] [CrossRef]
- Kok, H.P.; Wust, P.; Stauffer, P.R.; Bardati, F.; van Rhoon, G.C.; Crezee, J. Current state of the art of regional hyperthermia treatment planning: A review. Radiat. Oncol. 2015, 10, 196. [Google Scholar] [CrossRef]
- Frey, B.; Mika, J.; Jelonek, K.; Cruz-Garcia, L.; Roelants, C.; Testard, I.; Cherradi, N.; Lumniczky, K.; Polozov, S.; Napieralska, A.; et al. Systemic modulation of stress and immune parameters in patients treated for prostate adenocarcinoma by intensity-modulated radiation therapy or stereotactic ablative body radiotherapy. Strahlenther. Onkol. 2020, 196, 1018–1033. [Google Scholar] [CrossRef]
- Zhou, J.-G.; Donaubauer, A.-J.; Frey, B.; Becker, I.; Rutzner, S.; Eckstein, M.; Sun, R.; Ma, H.; Schubert, P.; Schweizer, C.; et al. Prospective development and validation of a liquid immune profile-based signature (LIPS) to predict response of patients with recurrent/metastatic cancer to immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e001845. [Google Scholar] [CrossRef] [PubMed]
- Oei, A.; Kok, H.; Oei, S.; Horsman, M.; Stalpers, L.; Franken, N.; Crezee, J. Molecular and biological rationale of hyperthermia as radio- and chemosensitizer. Adv. Drug Deliv. Rev. 2020, 163–164, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, J.; Grau, C.; Lindegaard, J.; Horsman, M. The potential of using hyperthermia to eliminate radioresistant hypoxic cells. Radiother. Oncol. 1991, 20, 113–116. [Google Scholar] [CrossRef]
- Ademaj, A.; Veltsista, D.P.; Ghadjar, P.; Marder, D.; Oberacker, E.; Ott, O.J.; Wust, P.; Puric, E.; Hälg, R.A.; Rogers, S.; et al. Clinical Evidence for Thermometric Parameters to Guide Hyperthermia Treatment. Cancers 2022, 14, 625. [Google Scholar] [CrossRef]
- Demaria, S.; Golden, E.B.; Formenti, S.C. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol. 2015, 1, 1325–1332. [Google Scholar] [CrossRef]
- Yamamoto, N.; Matsumoto, K.; Hagiwara, S.; Saito, M.; Furue, H.; Shigetomi, T.; Narita, Y.; Mitsudo, K.; Tohnai, I.; Kobayashi, T.; et al. Optimization of hyperthermia and dendritic cell immunotherapy for squamous cell carcinoma. Oncol. Rep. 2011, 25, 1525–1532. [Google Scholar] [CrossRef]
- Rückert, M.; Flohr, A.-S.; Hecht, M.; Gaipl, U.S. Radiotherapy and the immune system: More than just immune suppression. Stem Cells 2021, 39, 1155–1165. [Google Scholar] [CrossRef]

| Marker | Fluorochrome | Manufacturer |
|---|---|---|
| PD-L1 (CD274) | BV 605 | Biolegend |
| PD-L2 (CD273) | APC | Biolegend |
| ICOS-L (CD275) | BV 421 | BD Horizon |
| HVEM (CD270) | APC | Biolegend |
| TNFRSF9 (CD137-L) | BV 421 | Biolegend |
| OX40-L (CD252) | PE | Biolegend |
| Live/Dead | Zombie NIR | Biolegend |
| Marker | Fluorochrome | Manufacturer |
|---|---|---|
| CD70 | FITC | Biolegend |
| CD83 | PE-Cy7 | eBioscience |
| CD80 | APC | Miltenyi Biotec (MACS) |
| Live/Dead | Zombie Yellow | Biolegend |
| CD11c | V450 | Biolegend |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sengedorj, A.; Hader, M.; Heger, L.; Frey, B.; Dudziak, D.; Fietkau, R.; Ott, O.J.; Scheidegger, S.; Barba, S.M.; Gaipl, U.S.; et al. The Effect of Hyperthermia and Radiotherapy Sequence on Cancer Cell Death and the Immune Phenotype of Breast Cancer Cells. Cancers 2022, 14, 2050. https://doi.org/10.3390/cancers14092050
Sengedorj A, Hader M, Heger L, Frey B, Dudziak D, Fietkau R, Ott OJ, Scheidegger S, Barba SM, Gaipl US, et al. The Effect of Hyperthermia and Radiotherapy Sequence on Cancer Cell Death and the Immune Phenotype of Breast Cancer Cells. Cancers. 2022; 14(9):2050. https://doi.org/10.3390/cancers14092050
Chicago/Turabian StyleSengedorj, Azzaya, Michael Hader, Lukas Heger, Benjamin Frey, Diana Dudziak, Rainer Fietkau, Oliver J. Ott, Stephan Scheidegger, Sergio Mingo Barba, Udo S. Gaipl, and et al. 2022. "The Effect of Hyperthermia and Radiotherapy Sequence on Cancer Cell Death and the Immune Phenotype of Breast Cancer Cells" Cancers 14, no. 9: 2050. https://doi.org/10.3390/cancers14092050
APA StyleSengedorj, A., Hader, M., Heger, L., Frey, B., Dudziak, D., Fietkau, R., Ott, O. J., Scheidegger, S., Barba, S. M., Gaipl, U. S., & Rückert, M. (2022). The Effect of Hyperthermia and Radiotherapy Sequence on Cancer Cell Death and the Immune Phenotype of Breast Cancer Cells. Cancers, 14(9), 2050. https://doi.org/10.3390/cancers14092050