Front-Line Therapy for Metastatic Renal Cell Carcinoma: A Perspective on the Current Algorithm and Future Directions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Dual CPI Therapy
3. CPI with VEGFR-Directed Therapy
4. Selecting Front-Line Therapy: General Considerations
5. Special Circumstances
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Negrier, S.; Escudier, B.; Lasset, C.; Douillard, J.-Y.; Savary, J.; Chevreau, C.; Ravaud, A.; Mercatello, A.; Peny, J.; Mousseau, M.; et al. Recombinant Human Interleukin-2, Recombinant Human Interferon Alfa-2a, or Both in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 1998, 338, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Siebels, M.; Negrier, S.; Chevreau, C.; Solska, E.; Desai, A.A.; et al. Sorafenib in Advanced Clear-Cell Renal-Cell Carcinoma. N. Engl. J. Med. 2007, 356, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Cella, D.; Reeves, J.; Hawkins, R.; Guo, J.; Nathan, P.; Staehler, M.; de Souza, P.; Merchan, J.R.; et al. Pazopanib versus Sunitinib in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2013, 369, 722–731. [Google Scholar] [CrossRef] [Green Version]
- Heng, D.Y.C.; Xie, W.; Regan, M.M.; Warren, M.A.; Golshayan, A.R.; Sahi, C.; Eigl, B.J.; Ruether, J.D.; Cheng, T.; North, S.; et al. Prognostic Factors for Overall Survival in Patients with Metastatic Renal Cell Carcinoma Treated with Vascular Endothelial Growth Factor–Targeted Agents: Results from a Large, Multicenter Study. JCO 2009, 27, 5794–5799. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reck, M.; Ciuleanu, T.-E.; Cobo, M.; Schenker, M.; Zurawski, B.; Janoski de Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-Line Nivolumab (NIVO) plus Ipilimumab (IPI) plus Two Cycles of Chemotherapy (Chemo) versus Chemo Alone (4 Cycles) in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC): Two-Year Update from CheckMate 9LA. JCO 2021, 39, 9000. [Google Scholar] [CrossRef]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-Line Nivolumab plus Ipilimumab in Unresectable Malignant Pleural Mesothelioma (CheckMate 743): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Burotto, M.; Choueiri, T.K.; Hammers, H.J.; Plimack, E.R.; Porta, C.G.; George, S.; Powles, T.B.; et al. 661P Conditional Survival and 5-Year Follow-up in CheckMate 214: First-Line Nivolumab + Ipilimumab (N + I) versus Sunitinib (S) in Advanced Renal Cell Carcinoma (ARCC). Ann. Oncol. 2021, 32, S685–S687. [Google Scholar] [CrossRef]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal Cell Carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef]
- Hammers, H.J.; Plimack, E.R.; Infante, J.R.; Rini, B.I.; McDermott, D.F.; Lewis, L.D.; Voss, M.H.; Sharma, P.; Pal, S.K.; Razak, A.R.A.; et al. Safety and Efficacy of Nivolumab in Combination with Ipilimumab in Metastatic Renal Cell Carcinoma: The CheckMate 016 Study. JCO 2017, 35, 3851–3858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heng, D.Y.; Xie, W.; Regan, M.M.; Harshman, L.C.; Bjarnason, G.A.; Vaishampayan, U.N.; Mackenzie, M.; Wood, L.; Donskov, F.; Tan, M.-H.; et al. External Validation and Comparison with Other Models of the International Metastatic Renal-Cell Carcinoma Database Consortium Prognostic Model: A Population-Based Study. Lancet Oncol. 2013, 14, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Albiges, L.; Tannir, N.M.; Burotto, M.; McDermott, D.; Plimack, E.R.; Barthélémy, P.; Porta, C.; Powles, T.; Donskov, F.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib for First-Line Treatment of Advanced Renal Cell Carcinoma: Extended 4-Year Follow-up of the Phase III CheckMate 214 Trial. ESMO Open 2020, 5, e001079. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Powles, T.; Atkins, M.B.; Escudier, B.; McDermott, D.F.; Suarez, C.; Bracarda, S.; Stadler, W.M.; Donskov, F.; Lee, J.L.; et al. Atezolizumab plus Bevacizumab versus Sunitinib in Patients with Previously Untreated Metastatic Renal Cell Carcinoma (IMmotion151): A Multicentre, Open-Label, Phase 3, Randomised Controlled Trial. Lancet 2019, 393, 2404–2415. [Google Scholar] [CrossRef]
- Rini, B.I.; Atkins, M.B.; Escudier, B.; Powles, T.; McDermott, D.F.; Alekseev, B.Y.; Lee, J.-L.; Stroyakovskiy, D.; Rodriguez, C.S.; De Giorgi, U.; et al. Abstract CT188: IMmotion151: Updated Overall Survival (OS) and Exploratory Analysis of the Association of Gene Expression and Clinical Outcomes with Atezolizumab plus Bevacizumab vs. Sunitinib in Patients with Locally Advanced or Metastatic Renal Cell Carcinoma (MRCC). In Clinical Trials; American Association for Cancer Research: Philadelphia, PA, USA, 1 July 2021; p. CT188. [Google Scholar]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Haanen, J.B.A.G.; Larkin, J.; Choueiri, T.K.; Albiges, L.; Rini, B.I.; Atkins, M.B.; Schmidinger, M.; Penkov, K.; Thomaidou, D.; Wang, J.; et al. Efficacy of Avelumab + Axitinib (A + Ax) versus Sunitinib (S) by IMDC Risk Group in Advanced Renal Cell Carcinoma (ARCC): Extended Follow-up Results from JAVELIN Renal 101. JCO 2021, 39, 4574. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Waddell, T.; Gafanov, R.; Pouliot, F.; Nosov, D.; Melichar, B.; Soulieres, D.; Borchiellini, D.; et al. Pembrolizumab (Pembro) plus Axitinib (Axi) versus Sunitinib as First-Line Therapy for Advanced Clear Cell Renal Cell Carcinoma (CcRCC): Results from 42-Month Follow-up of KEYNOTE-426. JCO 2021, 39, 4500. [Google Scholar] [CrossRef]
- Rini, B.I.; Atkins, M.B.; Plimack, E.R.; Soulières, D.; McDermott, R.S.; Bedke, J.; Tartas, S.; Alekseev, B.; Melichar, B.; Shparyk, Y.; et al. Characterization and Management of Treatment-Emergent Hepatic Toxicity in Patients with Advanced Renal Cell Carcinoma Receiving First-Line Pembrolizumab plus Axitinib. Results from the KEYNOTE-426 Trial. Eur. Urol. Oncol. 2021, 458, S2588–S9311. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.; Alekseev, B.; Rha, S.-Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Motzer, R.J.; Rini, B.I.; Haanen, J.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Gravis-Mescam, G.; Uemura, M.; Lee, J.L.; et al. Updated Efficacy Results from the JAVELIN Renal 101 Trial: First-Line Avelumab plus Axitinib versus Sunitinib in Patients with Advanced Renal Cell Carcinoma. Ann. Oncol. 2020, 31, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Approves Nivolumab plus Ipilimumab Combination for Intermediate or Poor-Risk Advanced Renal Cell Carcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-plus-ipilimumab-combination-intermediate-or-poor-risk-advanced-renal-cell (accessed on 2 April 2022).
- FDA. FDA Approves Pembrolizumab plus Axitinib for Advanced Renal Cell Carcinoma. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-plus-axitinib-advanced-renal-cell-carcinoma (accessed on 2 April 2022).
- FDA. FDA Approves Nivolumab plus Cabozantinib for Advanced Renal Cell Carcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-plus-cabozantinib-advanced-renal-cell-carcinoma (accessed on 2 April 2022).
- FDA. FDA Approves Lenvatinib plus Pembrolizumab for Advanced Renal Cell Carcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lenvatinib-plus-pembrolizumab-advanced-renal-cell-carcinoma (accessed on 2 April 2022).
- FDA. FDA Approves Avelumab plus Axitinib for Renal Cell Carcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-avelumab-plus-axitinib-renal-cell-carcinoma (accessed on 2 April 2022).
- Aeppli, S.; Schmaus, M.; Eisen, T.; Escudier, B.; Grünwald, V.; Larkin, J.; McDermott, D.; Oldenburg, J.; Porta, C.; Rini, B.I.; et al. First-Line Treatment of Metastatic Clear Cell Renal Cell Carcinoma: A Decision-Making Analysis among Experts. ESMO Open 2021, 6, 100030. [Google Scholar] [CrossRef] [PubMed]
- Quhal, F.; Mori, K.; Bruchbacher, A.; Resch, I.; Mostafaei, H.; Pradere, B.; Schuettfort, V.M.; Laukhtina, E.; Egawa, S.; Fajkovic, H.; et al. First-Line Immunotherapy-Based Combinations for Metastatic Renal Cell Carcinoma: A Systematic Review and Network Meta-Analysis. Eur. Urol. Oncol. 2021, 4, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Nocera, L.; Karakiewicz, P.I.; Wenzel, M.; Tian, Z.; Shariat, S.F.; Saad, F.; Chun, F.K.H.; Briganti, A.; Kapoor, A.; Lalani, A.-K. Clinical Outcomes and Adverse Events after First-Line Treatment in Metastatic Renal Cell Carcinoma: A Systematic Review and Network Meta-Analysis. J. Urol. 2022, 207, 16–24. [Google Scholar] [CrossRef]
- Powles, T.; Kayani, I.; Sharpe, K.; Lim, L.; Peters, J.; Stewart, G.D.; Berney, D.; Sahdev, A.; Chowdhury, S.; Boleti, E.; et al. A Prospective Evaluation of VEGF-Targeted Treatment Cessation in Metastatic Clear Cell Renal Cancer. Ann. Oncol. 2013, 24, 2098–2103. [Google Scholar] [CrossRef]
- Bedke, J.; Rini, B.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Soulieres, D.; Melichar, B.; et al. Health-Related Quality-of-Life (HRQoL) Analysis from KEYNOTE-426: Pembrolizumab (Pembro) plus Axitinib (Axi) vs. Sunitinib for Advanced Renal Cell Carcinoma (RCC). In Proceedings of the 35th Annual EAU Congress—Virtual (EAU20V), Virtual, 17 July 2020. [Google Scholar]
- Carretero-González, A.; Lora, D.; Martín Sobrino, I.; Sáez Sanz, I.; Bourlon, M.T.; Anido Herranz, U.; Martínez Chanzá, N.; Castellano, D.; de Velasco, G. The Value of PD-L1 Expression as Predictive Biomarker in Metastatic Renal Cell Carcinoma Patients: A Meta-Analysis of Randomized Clinical Trials. Cancers 2020, 12, 1945. [Google Scholar] [CrossRef]
- Salgia, M.; Adashek, J.; Bergerot, P.; Pal, S.K. Non-Clear Cell Renal Cell Carcinoma: Current Management and Best Practice. KCA 2017, 1, 99–105. [Google Scholar] [CrossRef]
- Zhang, T.; Gong, J.; Maia, M.C.; Pal, S.K. Systemic Therapy for Non–Clear Cell Renal Cell Carcinoma. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 337–342. [Google Scholar] [CrossRef]
- Pal, S.K.; Ali, S.M.; Yakirevich, E.; Geynisman, D.M.; Karam, J.A.; Elvin, J.A.; Frampton, G.M.; Huang, X.; Lin, D.I.; Rosenzweig, M.; et al. Characterization of Clinical Cases of Advanced Papillary Renal Cell Carcinoma via Comprehensive Genomic Profiling. Eur. Urol. 2018, 73, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Tangen, C.; Thompson, I.M.; Balzer-Haas, N.; George, D.J.; Heng, D.Y.C.; Shuch, B.; Stein, M.; Tretiakova, M.; Humphrey, P.; et al. A Comparison of Sunitinib with Cabozantinib, Crizotinib, and Savolitinib for Treatment of Advanced Papillary Renal Cell Carcinoma: A Randomised, Open-Label, Phase 2 Trial. Lancet 2021, 397, 695–703. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clincal Practice Guidelines in Oncology—Kidney Cancer; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2022. [Google Scholar]
- Pal, S.K.; McGregor, B.; Suárez, C.; Tsao, C.-K.; Kelly, W.; Vaishampayan, U.; Pagliaro, L.; Maughan, B.L.; Loriot, Y.; Castellano, D.; et al. Cabozantinib in Combination with Atezolizumab for Advanced Renal Cell Carcinoma: Results from the COSMIC-021 Study. JCO 2021, 39, 3725–3736. [Google Scholar] [CrossRef]
- Lee, C.-H.; Voss, M.H.; Carlo, M.I.; Chen, Y.-B.; Reznik, E.; Knezevic, A.; Lefkowitz, R.A.; Shapnik, N.; Tassone, D.; Dadoun, C.; et al. Nivolumab plus Cabozantinib in Patients with Non-Clear Cell Renal Cell Carcinoma: Results of a Phase 2 Trial. JCO 2021, 39, 4509. [Google Scholar] [CrossRef]
- Hutson, T.E.; Michaelson, M.D.; Kuzel, T.M.; Agarwal, N.; Molina, A.M.; Hsieh, J.J.; Vaishampayan, U.N.; Xie, S.; Bapat, U.; Jain, R.K.; et al. A Phase II Study of Lenvatinib plus Everolimus in Patients with Advanced Non-Clear Cell Renal Cell Carcinoma (NccRCC). JCO 2020, 38, 685. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Chang, Y.-H.; Hajek, J.; Symeonides, S.N.; Lee, J.L.; Sarwar, N.; et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 385, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Albiges, L.; Suarez Rodriguez, C.; Liu, B.; Doss, J.; Khurana, S.; Scheffold, C.; Voss, M.H.; Choueiri, T.K. CONTACT-03: Randomized, Open-Label Phase III Study of Atezolizumab plus Cabozantinib versus Cabozantinib Monotherapy Following Progression on/after Immune Checkpoint Inhibitor (ICI) Treatment in Patients with Advanced/Metastatic Renal Cell Carcinoma. JCO 2021, 39, TPS370. [Google Scholar] [CrossRef]
- Meza, L.; Chawla, N.S.; Giannarini, G.; Pal, S.K. Cytoreductive Nephrectomy in 2021: Obsolete. Eur. Urol. Open Sci. 2022, 36, 44–46. [Google Scholar] [CrossRef]
- Jeong, I.G.; Khandwala, Y.S.; Kim, J.H.; Han, D.H.; Li, S.; Wang, Y.; Chang, S.L.; Chung, B.I. Association of Robotic-Assisted vs. Laparoscopic Radical Nephrectomy with Perioperative Outcomes and Health Care Costs, 2003 to 2015. JAMA 2017, 318, 1561. [Google Scholar] [CrossRef]
- Southwest Oncology Group. Phase III Trial of Immunotherapy-Based Combination Therapy with or without Cytoreductive Nephrectomy for Metastatic Renal Cell Carcinoma (PROBE Trial); Clinicaltrials.gov: Bethesda, MD, USA, 2021.
- Donskov, F. Multicenter Randomized Phase III Trial of Deferred Cytoreductive Nephrectomy in Synchronous Metastatic Renal Cell Carcinoma Receiving Checkpoint Inhibitors: A DaRenCa and NoRenCa Trial Evaluating the Impact of Surgery or No Surgery. The NORDIC-SUN-Trial; Clinicaltrials.gov: Bethesda, MD, USA, 2020.
- Choueiri, T.K.; Albiges, L.; Powles, T.; Scheffold, C.; Wang, F.; Motzer, R.J. A Phase III Study (COSMIC-313) of Cabozantinib in Combination with Nivolumab and Ipilimumab in Patients with Previously Untreated Advanced Renal Cell Carcinoma of Intermediate or Poor-Risk. JCO 2020, 38, TPS5102. [Google Scholar] [CrossRef]
- Jonasch, E.; Donskov, F.; Iliopoulos, O.; Rathmell, W.K.; Narayan, V.K.; Maughan, B.L.; Oudard, S.; Else, T.; Maranchie, J.K.; Welsh, S.J.; et al. Belzutifan for Renal Cell Carcinoma in von Hippel–Lindau Disease. N. Engl. J. Med. 2021, 385, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Approves Belzutifan for Cancers Associated with von Hippel-Lindau Disease. 2022. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-belzutifan-cancers-associated-von-hippel-lindau-disease (accessed on 2 April 2022).
- Merck Sharp & Dohme Corp. An Open-Label, Randomized Phase 3 Study to Evaluate Efficacy and Safety of Pembrolizumab (MK-3475) in Combination with Belzutifan (MK-6482) and Lenvatinib (MK-7902), or MK-1308A in Combination with Lenvatinib, versus Pembrolizumab and Lenvatinib, as First-Line Treatment in Participants with Advanced Clear Cell Renal Cell Carcinoma (CcRCC); Merck Sharp & Dohme Corp.: Kenilworth, NJ, USA, 2021. [Google Scholar]
- Tannir, N.M.; Agarwal, N.; Pal, S.K.; Cho, D.C.; Formiga, M.; Guo, J.; George, D.J.; Tagliaferri, M.A.; Singel, S.M.; O’Keeffe, B.A.; et al. PIVOT-09: A Phase III Randomized Open-Label Study of Bempegaldesleukin (NKTR-214) plus Nivolumab versus Sunitinib or Cabozantinib (Investigator’s Choice) in Patients with Previously Untreated Advanced Renal Cell Carcinoma (RCC). JCO 2020, 38, TPS763. [Google Scholar] [CrossRef]
- CoImmune. A Phase 2b Randomized Trial of Autologous Dendritic Cell Immunotherapy (CMN-001) Plus Standard Treatment of Advanced Renal Cell Carcinoma; CoImmune: Durham, NC, USA, 2019. [Google Scholar]
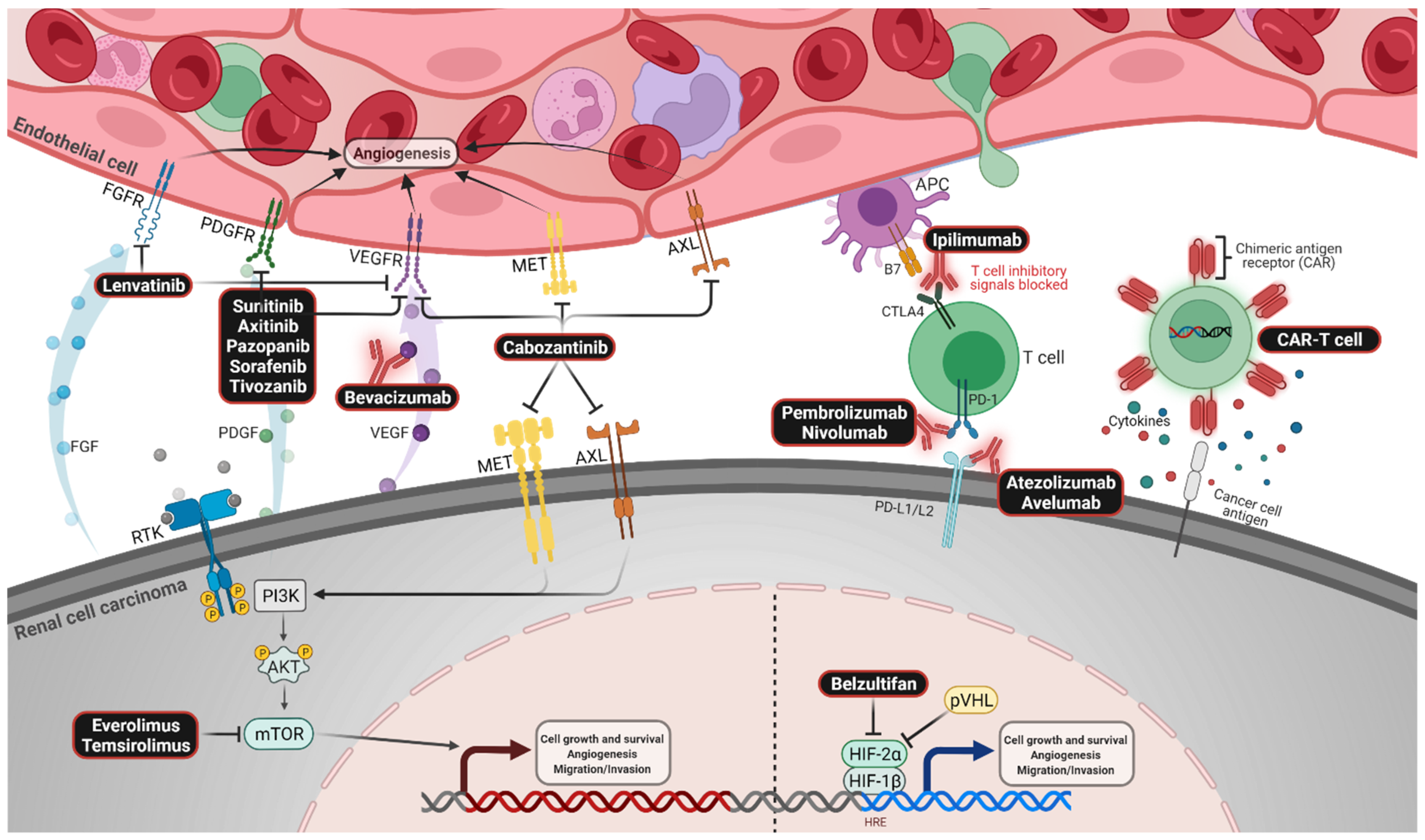
| Trial Name | CheckMate 214 [9,10] | KEYNOTE-426 [18,20] | CheckMate 9ER [22] | CLEAR [23] | JAVELIN Renal 101 [17,19,24] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment arm | Ipilimumab + Nivolumab n = 550 | Sunitinib n = 546 | Axitinib + Pembrolizumab n = 432 | Sunitinib n = 429 | Cabozantinib + Nivolumab n = 323 | Sunitinib n = 328 | Lenvatinib + Pembrolizumab n = 355 | Lenvatinib + Everolimus n = 357 | Sunitinib n = 357 | Axitinib + Avelumab n = 270 | Sunitinib n = 290 | |
| Median follow-up | 67.7 mo | 42.8 mo | 18.1 mo | 26.6 mo | ITT: NRE c PD-L1(+): ~19 mo | |||||||
| PFS | Median, mo | ITT: 12.3 I/P: 11.6 | ITT: 12.3 I/P: 8.3 | 15.7 | 11.1 | 16.6 | 8.3 | 23.9 | 14.7 | 9.2 | ITT c: 13.9 PD-L1(+): 13.8 | ITT c: 8.5 PD-L1(+): 7.0 |
| HR, (95% CI) | ITT: 0.86 (0.73–1.01); p = 0.0628 I/P: 0.73 (0.61–0.87); p = 0.0004 | 0.68 (0.58–0.80) p < 0.0001 | 0.51 (0.41–0.64) p < 0.001 | L + P vs. S: 0.39 (0.32–0.49) p < 0.001 L + E vs. S: 0.65 (0.53–0.80) p < 0.001 | ITT c: 0.69 (0.56–0.84) p < 0.0001 PD-L1(+): 0.62 (0.49–0.77) p < 0.0001 | |||||||
| OS | Median, mo | ITT: 55.7 I/P: 47.0 | ITT: 38.4 I/P: 26.6 | 45.7 | 40.1 | NR (NE) | NR (22.6-NE) | NR | NR | NR | ITT c: NE PD-L1 (+): NE | ITT c: 37.8 PD-L1 (+): 29.6 |
| HR, (95% CI) | ITT: 0.72 (0.62–0.85); p < 0.0001 I/P: 0.68 (0.58–0.81); p < 0.0001 | 0.73 (0.60–0.88) p < 0.001 | 0.60 (0.40–0.89) a p = 0.001 | L + P vs. S: 0.66 (0.49–0.88) p = 0.005 L + E vs. S: 1.15 (0.88–1.50) p = 0.30 | ITT c: 0.67 (0.57–0.79) p = 0.0116 PD-L1(+): 0.83 (0.506–1.151) p = 0.1301 | |||||||
| ORR (%) | ITT: 39 I/P: 42.0 | ITT: 32 I/P: 27.0 | 60.4 | 39.6 | 55.7 | 27.1 | 71.0 | 53.5 | 36.1 | ITT: 52.5 PD-L1(+): 55.9 | ITT:27.3 PD-L1(+): 27.2 | |
| TRAEs b (%) | Fatigue: 37 Pruritus: 28 Diarrhea: 27 | Diarrhea: 52 Fatigue: 49 PPE: 43 | Diarrhea: 54.3 HTN: 44.5 Fatigue: 38.5 | Diarrhea: 44.9 HTN: 45.4 Fatigue: 37.9 | Diarrhea: 63.8 PPE:40.0 HTN: 34.7 | Diarrhea: 47.2 PPE:40.6 HTN: 40.6 | Diarrhea: 61.4 HTN: 55.4 Hypothyroidism: 47.2 | Diarrhea: 66.5 Stomatitis: 47.6 HTN: 45.6 | Diarrhea: 49.4 HTN: 41.5 Stomatitis: 38.5 | Diarrhea: 62.2 HTN: 49.5 Fatigue 41.5 | Diarrhea: 47.6 Fatigue: 40.1 Nausea: 39.2 | |
| FDA approval | I/P: April 16, 2018 [25] | April 19, 2019 [26] | January 22, 2021 [27] | L + P: August 10, 2021 [28] | May 14, 2019 [29] | |||||||
| Title | Trial ID | Intervention | Histology | Phase | Sample Size | Primary Endpoint | Status |
|---|---|---|---|---|---|---|---|
| MK-6482–012 | NCT04736706 | Pembrolizumab + lenvatinib + belzutifan vs. Pembrolizumab + lenvatinib + quavonlimab vs. Pembrolizumab + lenvatinib | Clear cell RCC | 3 | 1431 | PFS and OS | Recruiting |
| COSMIC 313 | NCT03937219 | Nivolumab + ipilimumab + cabozantinib vs. Nivolumab + ipilimumab + placebo | Clear cell RCC | 3 | 840 | PFS | Active, not recruiting |
| A Study of AK104 Plus Axitinib in Advanced/Metastatic Clear Cell RCC | NCT05256472 | AK104 + axitinib | Clear cell RCC | 2 | 40 | ORR | Not yet recruiting |
| Dendritic Cell Immunotherapy Plus Standard Treatment of Advanced RCC | NCT04203901 | Nivolumab + ipilimumab +/− CMN-001 followed by lenvatinib + everolimus | Clear cell RCC | 2b | 120 | OS | Recruiting |
| PDIGREE Alliance A031704 | NCT03793166 | Nivolumab + ipilimumab followed by Nivolumab vs. Nivolumab + cabozantinib | Clear cell RCC +/− Sarcomatoid features | 3 | 1046 | OS | Recruiting |
| PIVOT-09 | NCT03729245 | Bempegaldesleukin + nivolumab vs. Sunitinib or cabozantinib | Clear cell RCC +/− Sarcomatoid features | 3 | 623 | ORR and OS | Active, not recruiting |
| Checkmate 8Y8 | NCT03873402 | Nivolumab + ipilimumab vs. Nivolumab + placebo | Clear cell RCC +/− Sarcomatoid features | 3 | 437 | PFS and ORR | Active, not recruiting |
| PIVOT IO 011 | NCT04540705 | Part 1 Nivolumab + Bempegaldesleukin + Axitinib Nivolumab + Bempegaldesleukin + Cabozantinib Part 2 Nivolumab + Bempegaldesleukin + Cabozantinib vs. Nivolumab + Cabozantinib | Clear cell RCC +/− Sarcomatoid features | 1/2 | 251 | Safety, DLT, ORR | Recruiting |
| Study to Evaluate the Efficacy and Safety of Toripalimab in Combination with Axitinib Versus Sunitinib Monotherapy in Advanced RCC | NCT04394975 | Toripalimab + axitinib vs. sunitinib | Clear cell RCC +/− Sarcomatoid features | 3 | 380 | PFS | Recruiting |
| PROBE study | NCT04510597 | Immunotherapy-based combination +/− cytoreductive nephrectomy | Any RCC histology (Except collecting duct carcinoma) | 3 | 364 | OS | Recruiting |
| MK-3475-03A | NCT04626479 | Pembrolizumab/Quavonlimab + Lenvatinib vs. Favezelimab/Pembrolizumab+ Lenvatinib vs. Pembrolizumab + Belzutifan + Lenvatinib vs. Pembrolizumab + Lenvatinib | Any RCC histology | 1b/2 | 390 | DLT, AE, ORR | Recruiting |
| MK-3475-B61/ KEYNOTE-B61 | NCT04704219 | Pembrolizumab + Lenvatinib | Non-clear cell RCC | 2 | 152 | ORR | Active, not recruiting |
| A Study of Nivolumab In Combination With Cabozantinib in Patients With Non-Clear Cell RCC | NCT03635892 | Cabozantinib + nivolumab | Non-clear cell RCC | 2 | 97 | ORR | Recruiting |
| PAXIPEM | NCT05096390 | Axitinib +/− pembrolizumab | Type 2 or mixed pRCC | 3 | 72 | ORR | Not yet recruiting |
| The LENKYN Trial | NCT04267120 | Pembrolizumab + Lenvatinib | Non-clear cell RCC | 2 | 34 | ORR | Recruiting |
| ALTER-UC-001 | NCT05124431 | Anlotinib + everolimus | Non-clear cell RCC | 2 | 30 | ORR | Not yet recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govindarajan, A.; Castro, D.V.; Zengin, Z.B.; Salgia, S.K.; Patel, J.; Pal, S.K. Front-Line Therapy for Metastatic Renal Cell Carcinoma: A Perspective on the Current Algorithm and Future Directions. Cancers 2022, 14, 2049. https://doi.org/10.3390/cancers14092049
Govindarajan A, Castro DV, Zengin ZB, Salgia SK, Patel J, Pal SK. Front-Line Therapy for Metastatic Renal Cell Carcinoma: A Perspective on the Current Algorithm and Future Directions. Cancers. 2022; 14(9):2049. https://doi.org/10.3390/cancers14092049
Chicago/Turabian StyleGovindarajan, Ameish, Daniela V. Castro, Zeynep B. Zengin, Sabrina K. Salgia, Jalen Patel, and Sumanta K. Pal. 2022. "Front-Line Therapy for Metastatic Renal Cell Carcinoma: A Perspective on the Current Algorithm and Future Directions" Cancers 14, no. 9: 2049. https://doi.org/10.3390/cancers14092049
APA StyleGovindarajan, A., Castro, D. V., Zengin, Z. B., Salgia, S. K., Patel, J., & Pal, S. K. (2022). Front-Line Therapy for Metastatic Renal Cell Carcinoma: A Perspective on the Current Algorithm and Future Directions. Cancers, 14(9), 2049. https://doi.org/10.3390/cancers14092049






