In Search of the Appropriate Anticoagulant-Associated Bleeding Risk Assessment Model for Cancer-Associated Thrombosis Patients
Abstract
:Simple Summary
Abstract
1. Introduction
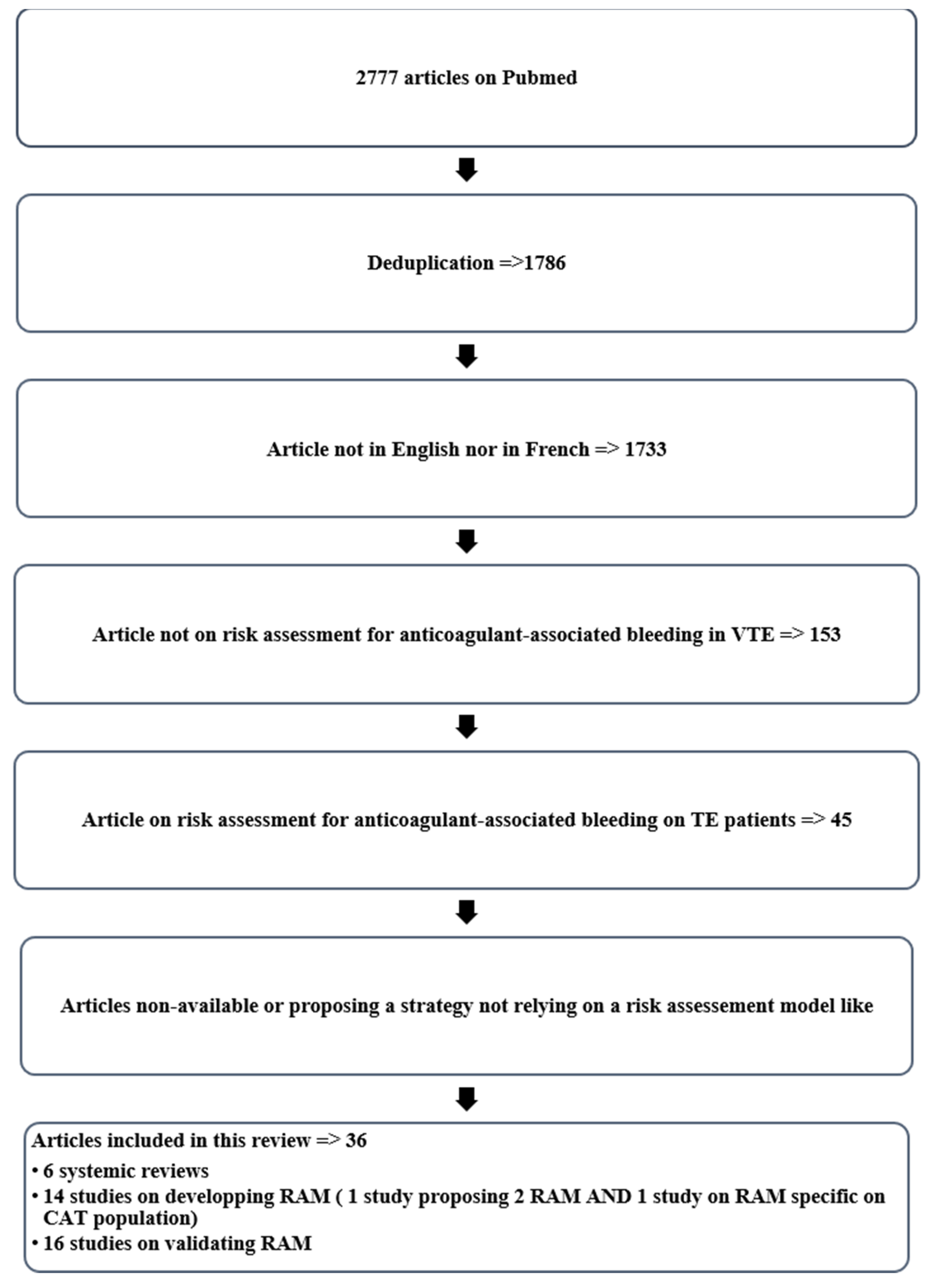
2. Materials and Methods
3. Results
3.1. Description of the Bleeding Risk Assessment Models in VTE Patients
3.2. Description of the Factors Predicting Anticoagulant-Associated Bleeding
3.2.1. Who Are the CAT Patients in Whom We Assess Anticoagulant-Associated Bleeding Risk?
3.2.2. How Do We Assess the Anticoagulant-Associated Bleeding Risk in CAT Patients?
3.2.3. What Is the Anticoagulant-Associated Bleeding Risk?
3.2.4. When Should Anticoagulant-Associated Bleeding Risk Be Assessed in CAT Patients?
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Section/Topic | # | Checklist Item | Reported on Page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 2–3 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 3 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. Prospero N°42022297863 | 3 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 3 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 3 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | Table 2 + page 3 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 3 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 3 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 3 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 4–9; 16–18 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 4–5, Table 3 and Table 4 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | n.a. |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | n.a. |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | n.a. |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 4–5, Figure 1 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | Table 3, Table 4 and Table 5 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 4–9; 16–18 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | Table 4 and Table 5 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | n.a. |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | n.a. |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | n.a. |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 18–21 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 7–9 + Table 3, Table 4 and Table 5 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 7–9 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 4 |
| Study | Risk of Bias (ROB) | Applicability | Overall | ||||||
| Participants | Predictors | Outcome | Analysis | Participants | Predictors | Outcome | ROB | Applicability | |
| Model-derivation studies | |||||||||
| NIEUWENHUIS et al., 1991 [28] | + | − | +/− | − | + | − | − | − | − |
| KUIJER et al., 1999 [33] | + | + | +/− | − | + | + | − | +/− | − |
| RIETE Ruiz-Giménez et al., 2008 [27] | + | + | + | − | + | + | − | + | − |
| EINSTEIN Di Nisio et al., 2016 [37] | − | + | + | − | − | + | − | +/− | − |
| VTE-BLEED Klok et al., 2016 [29] | − | + | + | − | − | + | − | +/− | − |
| ACCP Kearon et al., 2016 [16] | n.a. | + | + | − | n.a. | + | − | +/− | − |
| NIETO 2010 [40] | +/− | + | +/− | − | + | + | − | +/− | − |
| SEILER 2017 [34] | +/− | + | + | − | − | + | − | +/− | − |
| HOKUSAI Di Nisio et al., 2017 [38] | − | + | + | − | − | + | − | +/− | − |
| Skowrońska et al., 2019 [30] | + | + | + | − | + | + | − | + | − |
| MARTINEZ, 2019 [32] | + | + | +/− | − | − | + | − | +/− | − |
| Chopard et al., 2021 [31] | + | + | + | − | + | + | − | + | − |
| Alonso et al., 2021 [39] | + | + | +/− | − | − | + | − | +/− | − |
| CAT-BLEED Winter et al., 2021 [33,41] | +/− | + | + | + | + | + | + | + | + |
| Study | Risk of Bias (ROB) | Applicability | Overall | ||||||
| Participants | Predictors | Outcome | Analysis | Participants | Predictors | Outcome | ROB | Applicability | |
| Validation studies | |||||||||
| ACCP | |||||||||
| Scherz et al., 2013 [42] | +/− | + | + | − | +-/ | + | + | +/− | +/− |
| Poli et al., 2013 [44] | +/− | + | + | − | +/− | + | + | +/− | +/− |
| Riva et al., 2014 [46] | + | + | + | − | + | + | − | + | − |
| Palareti et al., 2018 [48] | + | + | + | − | + | + | − | + | − |
| Zhang et al., 2018 [53] | + | + | + | − | + | + | − | + | − |
| Frei et al., 2021 [56] | − | + | + | − | − | + | − | +/− | − |
| De Winter et al., 2021 [36] | + | + | + | − | + | + | − | + | +/− |
| KUIJER | |||||||||
| Scherz et al., 2013 [42] | +/− | + | + | − | +/− | + | + | +/− | − |
| Riva et al., 2014 [46] | + | + | + | − | + | + | − | + | +/− |
| Piovella et al., 2014 [47] | + | + | +/− | − | + | + | − | +/− | − |
| Kline et al., 2016 [45] | + | + | + | − | − | + | − | + | − |
| Zhang et al., 2018 [53] | + | + | + | − | + | + | − | + | − |
| Vedovati et al., 2019 [51] | + | + | + | − | − | + | − | + | − |
| Keller et al., 2021 [54] | + | + | +/− | − | − | + | − | +/− | − |
| Frei et al., 2021 [56] | − | + | + | − | − | + | − | +/− | − |
| De Winter et al., 2021 [36] | + | + | + | − | + | + | − | + | +/− |
| RIETE | |||||||||
| Scherz et al., 2013 [42] | +/− | + | + | − | +/− | + | + | +/− | − |
| Poli et al., 2013 [44] | +/− | + | + | − | +/− | + | + | +/− | +/− |
| Riva et al., 2014 [46] | + | + | + | − | + | + | − | + | +/− |
| Piovella et al., 2014 [47] | + | + | +/− | − | + | + | − | +/− | − |
| Kline et al., 2016 [45] | + | + | + | − | − | + | − | + | − |
| Vedovati et al., 2019 [51] | + | + | + | − | − | + | − | + | − |
| Zhang et al., 2018 [53] | + | + | + | − | + | + | − | + | − |
| Skowrońska et al., 2019 [30] | + | + | + | − | + | + | +/− | + | +/− |
| Mathonier et al., 2021 [55] | + | + | +/− | − | + | + | − | +/− | − |
| Frei et al., 2021 [56] | − | + | + | − | − | + | − | +/− | − |
| De Winter et al., 2021 [36] | + | + | + | − | + | + | − | + | +/− |
| NIETO | |||||||||
| Nieto et al., 2013 [43] | +/− | + | +/− | − | + | + | − | +/− | − |
| MARTNEZ | |||||||||
| De Winter et al., 2021 [36] | + | + | + | − | + | + | − | + | +/− |
| SEILER | |||||||||
| Frei et al., 2021 [56] | − | + | + | − | − | + | − | +/− | − |
| NIEUWENHUIS | |||||||||
| Zhang et al., 2018 [53] | + | + | + | − | + | + | − | + | − |
| HOKUSAI | |||||||||
| De Winter et al., 2021 [36] | + | + | + | − | + | + | − | + | +/− |
| VTE BLEED | |||||||||
| Klok et al., 2017 [50] | + | + | + | − | + | + | − | + | − |
| Rief et al., 2018 [49] | + | + | + | − | + | + | − | + | − |
| Klok et al., 2018 [52] | + | + | + | − | + | + | − | + | − |
| Skowrońska et al., 2019 [30] | + | + | + | − | + | + | +/− | + | +/− |
| Vedovati et al., 2019 [51] | + | + | + | − | − | + | − | + | − |
| Mathonier et al., 2021 [55] | + | + | +/− | − | + | + | − | +/− | − |
| Frei et al., 2021 [56] | − | + | + | − | − | + | − | +/− | − |
| De Winter et al., 2021 [36] | + | + | + | − | + | + | − | + | +/− |
Appendix B
Appendix B.1. Major Bleeding Definition
- Fatal bleeding, and/or;
- Symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome, and/or;
- Bleeding causing a fall in hemoglobin level of 20 g/L or more, or leading to transfusion of two or more units of whole blood or red cells.
Appendix B.2. Clinically Relevant Non-Major Bleeding Definition
- Any sign or symptom of hemorrhage (e.g., more bleeding than would be expected for a clinical circumstance, including bleeding found by imaging alone) that does not fit the criteria for the ISTH definition of major bleeding but does meet at least one of the following criteria:
- Requiring medical intervention by a healthcare professional;
- Leading to hospitalization or increased level of care prompting a face-to-face (i.e., not just a telephone call or electronic communication) evaluation;
- Not a major bleeding.
References
- Blom, J.W.; Doggen, C.J.; Osanto, S.; Rosendaal, F.R. Malignancies, Prothrombotic Mutations, and the Risk of Venous Thrombosis. JAMA 2005, 293, 715. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Levine, M.N.; Baker, R.I.; Bowden, C.; Kakkar, A.K.; Prins, M.; Rickles, F.R.; Julian, J.A.; Haley, S.; Kovacs, M.J.; et al. Low-Molecular-Weight Heparin versus a Coumarin for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer. N. Engl. J. Med. 2003, 349, 146–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulder, F.I.; van Es, N.; Kraaijpoel, N.; Di Nisio, M.; Carrier, M.; Duggal, A.; Gaddh, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; et al. Edoxaban for treatment of venous thromboembolism in patient groups with different types of cancer: Results from the Hokusai VTE Cancer study. Thromb. Res. 2020, 185, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBane, R.D., 2nd; Wysokinski, W.E.; Le-Rademacher, J.G.; Zemla, T.; Ashrani, A.; Tafur, A.; Perepu, U.; Anderson, D.; Gundabolu, K.; Kuzma, C.; et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: The ADAM VTE trial. J. Thromb. Haemost. 2020, 18, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Becattini, C.; Bauersachs, R.; Brenner, B.; Campanini, M.; Cohen, A.; Connors, J.M.; Fontanella, A.; Gussoni, G.; Huisman, M.V.; et al. Apixaban versus Dalteparin for the Treatment of Acute Venous Thromboembolism in Patients with Cancer: The Caravaggio Study. Thromb. Haemost. 2018, 118, 1668–1678. [Google Scholar] [CrossRef] [Green Version]
- Young, A.M.; Marshall, A.; Thirlwall, J.; Chapman, O.; Lokare, A.; Hill, C.; Hale, D.; Dunn, J.A.; Lyman, G.H.; Hutchinson, C.; et al. Comparison of an Oral Factor Xa Inhibitor with Low Molecular Weight Heparin in Patients with Cancer with Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J. Clin. Oncol. 2018, 36, 2017–2023. [Google Scholar] [CrossRef]
- Khorana, A.A.; Noble, S.; Lee, A.Y.Y.; Soff, G.; Meyer, G.; O'Connell, C.; Carrier, M. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1891–1894. [Google Scholar] [CrossRef] [Green Version]
- Farge, D.; Frere, C.; Connors, J.M.; Ay, C.; Khorana, A.A.; Munoz, A.; Brenner, B.; Kakkar, A.; Rafii, H.; Solymoss, S.; et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019, 20, e566–e581. [Google Scholar] [CrossRef] [Green Version]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous Thromboembolism Prophylaxis and Treatment in Patients with Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef]
- Cohen, A.T.; Katholing, A.; Rietbrock, S.; Bamber, L.; Martinez, C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer: A population-based cohort study. Thromb. Haemost. 2017, 117, 57–65. [Google Scholar] [CrossRef]
- Lloyd, A.J.; Dewilde, S.; Noble, S.; Reimer, E.; Lee AY, Y. What Impact Does Venous Thromboembolism and Bleeding Have on Cancer Patients’ Quality of Life? Value Health 2018, 21, 449–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connolly, G.C.; Francis, C.W. Cancer-associated thrombosis. Hematology 2013, 2013, 684–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ay, C.; Pabinger, I.; Cohen, A.T. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb. Haemost. 2017, 117, 219–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planquette, B.; Bertoletti, L.; Charles-Nelson, A.; Laporte, S.; Grange, C.; Mahé, I.; Pernod, G.; Elias, A.; Couturaud, F.; Falvo, N.; et al. Rivaroxaban vs Dalteparin in Cancer-Associated Thromboembolism. Chest 2021, 161, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur. Respir. J. 2019, 54, 1901647. [Google Scholar] [PubMed] [Green Version]
- Kearon, C. Antithrombotic Therapy for VTE Disease. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Huisman, M.V. How I assess and manage the risk of bleeding in patients treated for venous thromboembolism. Blood 2020, 135, 724–734. [Google Scholar] [CrossRef] [Green Version]
- de Winter, M.A.; van Es, N.; Büller, H.R.; Visseren FL, J.; Nijkeuter, M. Prediction models for recurrence and bleeding in patients with venous thromboembolism: A systematic review and critical appraisal. Thromb. Res. 2021, 199, 85–96. [Google Scholar] [CrossRef]
- Beyth, R.J.; Quinn, L.M.; Landefeld, C.S. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am. J. Med. 1998, 105, 91–99. [Google Scholar] [CrossRef]
- Gage, B.F.; Yan, Y.; Milligan, P.E.; Waterman, A.D.; Culverhouse, R.; Rich, M.W.; Radford, M.J. Clinical classification schemes for predicting hemorrhage: Results from the National Registry of Atrial Fibrillation (NRAF). Am. Heart J. 2006, 151, 713–719. [Google Scholar] [CrossRef]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; de Vos, C.B.; Crijns, H.J.; Lip, G.Y. A Novel User-Friendly Score (HAS-BLED) To Assess 1-Year Risk of Major Bleeding in Patients with Atrial Fibrillation. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Fang, M.C.; Go, A.S.; Chang, Y.; Borowsky, L.H.; Pomernacki, N.K.; Udaltsova, N.; Singer, D.E. A New Risk Scheme to Predict Warfarin-Associated Hemorrhage. J. Am. Coll. Cardiol. 2011, 58, 395–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jara-Palomares, L.; Jiménez, D.; Bikdeli, B.; Muriel, A.; Rali, P.; Yamashita, Y.; Morimoto, T.; Kimura, T.; Le Mao, R.; Riera-Mestre, A.; et al. Derivation and validation of a clinical prediction rule for thrombolysis-associated major bleeding in patients with acute pulmonary embolism: The BACS score. Eur. Respir. J. 2020, 56, 2002336. [Google Scholar] [CrossRef] [PubMed]
- Moons, K.G.; de Groot, J.A.; Bouwmeester, W.; Vergouwe, Y.; Mallett, S.; Altman, D.G.; Reitsma, J.B.; Collins, G.S. Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies: The CHARMS Checklist. PLoS Med. 2014, 11, e1001744. [Google Scholar] [CrossRef] [PubMed]
- Kaatz, S.; Ahmad, D.; Spyropoulos, A.C.; Schulman, S.; The Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2015, 13, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Kearon, C.; The Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients: Definitions of major bleeding in clinical studies. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Ruíz-Giménez, N.; Suárez, C.; González, R.; Nieto, J.A.; Todolí, J.A.; Samperiz, A.L.; Monreal, M.; RIETE Investigators. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE Registry. Thromb. Haemost. 2008, 100, 26–31. [Google Scholar] [CrossRef]
- Nieuwenhuis, H.K.; Albada, J.; Banga, J.D.; Sixma, J.J. Identification of risk factors for bleeding during treatment of acute venous thromboembolism with heparin or low molecular weight heparin. Blood 1991, 78, 2337–2343. [Google Scholar] [CrossRef] [Green Version]
- Klok, F.A.; Hösel, V.; Clemens, A.; Yollo, W.D.; Tilke, C.; Schulman, S.; Lankeit, M.; Konstantinides, S.V. Prediction of bleeding events in patients with venous thromboembolism on stable anticoagulation treatment. Eur. Respir. J. 2016, 48, 1369–1376. [Google Scholar] [CrossRef]
- Skowrońska, M.; Furdyna, A.; Ciurzyński, M.; Pacho, S.; Bienias, P.; Palczewski, P.; Kurnicka, K.; Jankowski, K.; Lipińska, A.; Uchacz, K.; et al. D-dimer levels enhance the discriminatory capacity of bleeding risk scores for predicting in-hospital bleeding events in acute pulmonary embolism. Eur. J. Intern. Med. 2019, 69, 8–13. [Google Scholar] [CrossRef]
- Chopard, R.; Piazza, G.; Falvo, N.; Ecarnot, F.; Besutti, M.; Capellier, G.; Schiele, F.; Badoz, M.; Meneveau, N. An Original Risk Score to Predict Early Major Bleeding in Acute Pulmonary Embolism. Chest 2021, 160, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.; Katholing, A.; Wallenhorst, C.; Cohen, A.T. Prediction of significant bleeding during vitamin K antagonist treatment for venous thromboembolism in outpatients. Br. J. Haematol. 2020, 189, 524–533. [Google Scholar] [CrossRef]
- Kuijer, P.M.M.; Hutten, B.A.; Prins, M.H.; Büller, H.R. Prediction of the Risk of Bleeding During Anticoagulant Treatment for Venous Thromboembolism. Arch. Intern. Med. 1999, 159, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seiler, E.; Limacher, A.; Mean, M.; Beer, H.J.; Osterwalder, J.; Frauchiger, B.; Righini, M.; Aschwanden, M.; Matter, C.M.; Banyai, M.; et al. Derivation and validation of a novel bleeding risk score for elderly patients with venous thromboembolism on extended anticoagulation. Thromb. Haemost. 2017, 117, 1930–1936. [Google Scholar] [CrossRef]
- de Winter, M.A.; Dorresteijn, J.A.N.; Ageno, W.; Ay, C.; Beyer-Westendorf, J.; Coppens, M.; Klok, F.A.; Moustafa, F.; Riva, N.; Ruiz Artacho, P.C.; et al. External Validation of Existing Risk Scores and Development of a New Risk Score [abstract]. Res. Pract. Thromb Haemost 2021, 5. [Google Scholar]
- de Winter, M.A.; Dorresteijn, J.A.N.; Ageno, W.; Ay, C.; Beyer-Westendorf, J.; Coppens, M.; Klok, F.A.; Moustafa, F.; Riva, N.; Ruiz Artacho, P.C.; et al. Estimating Bleeding Risk in Patients with Cancer-Associated Thrombosis: Evaluation of Existing Risk Scores and Development of a New Risk Score. Thromb. Haemost. 2021. [Google Scholar] [CrossRef]
- Di Nisio, M.D.; Ageno, W.; Rutjes AW, S.; Pap, A.F.; Büller, H.R. Risk of major bleeding in patients with venous thromboembolism treated with rivaroxaban or with heparin and vitamin K antagonists. Thromb. Haemost. 2016, 115, 424–432. [Google Scholar] [PubMed]
- Di Nisio, M.; Raskob, G.; Büller, H.R.; Grosso, M.A.; Zhang, G.; Winters, S.M.; Cohen, A. Prediction of major and clinically relevant bleeding in patients with VTE treated with edoxaban or vitamin K antagonists. Thromb. Haemost. 2017, 117, 784–793. [Google Scholar]
- Alonso, A.; Norby, F.L.; MacLehose, R.F.; Zakai, N.A.; Walker, R.F.; Adam, T.J.; Lutsey, P.L. Claims-Based Score for the Prediction of Bleeding in a Contemporary Cohort of Patients Receiving Oral Anticoagulation for Venous Thromboembolism. J. Am. Heart Assoc. 2021, 10, e021227. [Google Scholar] [CrossRef]
- Nuñez, M.J.; Villalba, J.C.; Cebrián, E.; Visoná, A.; Lopez-Jimenez, L.; Núñez, M.; Szwebel, T.A.; Luque, J.M.; Jaras, M.J.; Monreal, M.; et al. Fatal bleeding in patients receiving anticoagulant therapy for venous thromboembolism: Findings from the RIETE registry: Fatal bleeding during anticoagulant therapy. J. Thromb. Haemost. 2010, 8, 1216–1222. [Google Scholar]
- Ageno, W.; Mantovani, L.G.; Haas, S.; Kreutz, R.; Monje, D.; Schneider, J.; van Eickels, M.; Gebel, M.; Zell, E.; Turpie, A.G. Safety and effectiveness of oral rivaroxaban versus standard anticoagulation for the treatment of symptomatic deep-vein thrombosis (XALIA): An international, prospective, non-interventional study. Lancet Haematol. 2016, 3, e12–e21. [Google Scholar] [CrossRef]
- Scherz, N.; Méan, M.; Limacher, A.; Righini, M.; Jaeger, K.; Beer, H.J.; Frauchiger, B.; Osterwalder, J.; Kucher, N.; Matter, C.M.; et al. Prospective, multicenter validation of prediction scores for major bleeding in elderly patients with venous thromboembolism. J. Thromb. Haemost. 2013, 11, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Nieto, J.A.; Solano, R.; Trapero Iglesias, N.; Ruiz-Giménez, N.; Fernández-Capitán, C.; Valero, B.; Tiberio, G.; Bura-Riviere, A.; Monreal, M.; RIETE Investigators. Validation of a score for predicting fatal bleeding in patients receiving anticoagulation for venous thromboembolism. Thromb. Res. 2013, 132, 175–179. [Google Scholar] [CrossRef]
- Poli, D.; Antonucci, E.; Testa, S.; Cosmi, B.; Palareti, G.; Ageno, W.; FCSA Italian Federation of Anticoagulation Clinics. The predictive ability of bleeding risk stratification models in very old patients on vitamin K antagonist treatment for venous thromboembolism: Results of the prospective collaborative EPICA study. J. Thromb. Haemost. 2013, 11, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Riva, N.; Bellesini, M.; Di Minno, M.N.; Mumoli, N.; Pomero, F.; Franchini, M.; Fantoni, C.; Lupoli, R.; Brondi, B.; Borretta, V.; et al. Poor predictive value of contemporary bleeding risk scores during long-term treatment of venous thromboembolism: A multicentre retrospective cohort study. Thromb. Haemost. 2014, 112, 511–521. [Google Scholar]
- Piovella, C.; Dalla Valle, F.; Trujillo-Santos, J.; Pesavento, R.; López, L.; Font, L.; Valle, R.; Nauffal, D.; Monreal, M.; Prandoni, P.; et al. Comparison of four scores to predict major bleeding in patients receiving anticoagulation for venous thromboembolism: Findings from the RIETE registry. Intern. Emerg. Med. 2014, 9, 847–852. [Google Scholar] [CrossRef]
- Kline, J.A.; Jimenez, D.; Courtney, D.M.; Ianus, J.; Cao, L.; Lensing, A.W.; Prins, M.H.; Wells, P.S. Comparison of Four Bleeding Risk Scores to Identify Rivaroxaban-treated Patients with Venous Thromboembolism at Low Risk for Major Bleeding. Acad. Emerg. Med. 2016, 23, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Klok, F.A.; Barco, S.; Konstantinides, S.V. External validation of the VTE-BLEED score for predicting major bleeding in stable anticoagulated patients with venous thromboembolism. Thromb. Haemost. 2017, 117, 1164–1170. [Google Scholar] [CrossRef]
- Palareti, G.; Antonucci, E.; Mastroiacovo, D.; Ageno, W.; Pengo, V.; Poli, D.; Testa, S.; Tosetto, A.; Prandoni, P. The American College of Chest Physician score to assess the risk of bleeding during anticoagulation in patients with venous thromboembolism. J. Thromb. Haemost. 2018, 16, 1994–2002. [Google Scholar] [CrossRef]
- Rief, P.; Raggam, R.B.; Hafner, F.; Avian, A.; Hackl, G.; Cvirn, G.; Brodmann, M.; Gary, T. Calculation of HAS-BLED Score Is Useful for Early Identification of Venous Thromboembolism Patients at High Risk for Major Bleeding Events: A Prospective Outpatients Cohort Study. Semin. Thromb. Hemost. 2018, 44, 348–352. [Google Scholar]
- Zhang, Z.; Lei, J.; Zhai, Z.; Yang, Y.; Wan, J.; Xie, W.; Wang, C. Comparison of prediction value of four bleeding risk scores for pulmonary embolism with anticoagulation: A real-world study in Chinese patients. Clin. Respir. J. 2019, 13, 139–147. [Google Scholar] [CrossRef]
- Klok, F.A.; Barco, S.; Turpie, A.G.G.; Haas, S.; Kreutz, R.; Mantovani, L.G.; Gebel, M.; Herpers, M.; Bugge, J.P.; Kostantinides, S.V.; et al. Predictive value of venous thromboembolism (VTE)-BLEED to predict major bleeding and other adverse events in a practice-based cohort of patients with VTE: Results of the XALIA study. Br. J. Haematol. 2018, 183, 457–465. [Google Scholar] [CrossRef]
- Vedovati, M.C.; Mancuso, A.; Pierpaoli, L.; Paliani, U.; Conti, S.; Ascani, A.; Galeotti, G.; Di Filippo, F.; Caponi, C.; Agnelli, G.; et al. Prediction of major bleeding in patients receiving DOACs for venous thromboembolism: A prospective cohort study. Int. J. Cardiol. 2020, 301, 167–172. [Google Scholar] [CrossRef]
- Keller, K.; Münzel, T.; Hobohm, L.; Ostad, M.A. Predictive value of the Kuijer score for bleeding and other adverse in-hospital events in patients with venous thromboembolism. Int. J. Cardiol. 2021, 329, 179–184. [Google Scholar] [CrossRef]
- Mathonier, C.; Meneveau, N.; Besutti, M.; Ecarnot, F.; Falvo, N.; Guillon, B.; Schiele, F.; Chopard, R. Available Bleeding Scoring Systems Poorly Predict Major Bleeding in the Acute Phase of Pulmonary Embolism. J. Clin. Med. 2021, 10, 3615. [Google Scholar] [CrossRef]
- Frei, A.N.; Stalder, O.; Limacher, A.; Méan, M.; Baumgartner, C.; Rodondi, N.; Aujesky, D. Comparison of Bleeding Risk Scores in Elderly Patients Receiving Extended Anticoagulation with Vitamin K Antagonists for Venous Thromboembolism. Thromb. Haemost. 2021, 121, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- van Es, N.; Coppens, M.; Schulman, S.; Middeldorp, S.; Büller, H.R. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: Evidence from phase 3 trials. Blood 2014, 124, 1968–1975. [Google Scholar] [CrossRef]
- Holbrook, A.; Benipal, H.; Paterson, J.M.; Martins, D.; Greaves, S.; Lee, M.; Gomes, T. Adverse event rates associated with oral anticoagulant treatment early versus later after hospital discharge in older adults: A retrospective population-based cohort study. CMAJ Open 2021, 9, E364–E375. [Google Scholar] [CrossRef]
- Rubboli, A. Incidence, clinical impact and risk of bleeding during oral anticoagulation therapy. World J. Cardiol. 2011, 3, 351. [Google Scholar] [CrossRef]
- Carrier, M. Systematic Review: Case-Fatality Rates of Recurrent Venous Thromboembolism and Major Bleeding Events Among Patients Treated for Venous Thromboembolism. Ann. Intern. Med. 2010, 152, 578. [Google Scholar] [CrossRef]
- Ensor, J.; Riley, R.D.; Moore, D.; Snell, K.I.; Bayliss, S.; Fitzmaurice, D. Systematic review of prognostic models for recurrent venous thromboembolism (VTE) post-treatment of first unprovoked VTE. BMJ Open 2016, 6, e011190. [Google Scholar] [CrossRef] [PubMed]
- van Es, N.; Wells, P.S.; Carrier, M. Bleeding risk in patients with unprovoked venous thromboembolism: A critical appraisal of clinical prediction scores. Thromb. Res. 2017, 152, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, N.E.M.; Ridker, P.M.; Dorresteijn, J.A.N.; Visseren, F.L.J. The prediction of therapy-benefit for individual cardiovascular disease prevention: Rationale, implications, and implementation. Curr. Opin. Lipidol. 2018, 29, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Moons, K.G.M.; Wolff, R.F.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S. PROBAST: A Tool to Assess Risk of Bias and Applicability of Prediction Model Studies: Explanation and Elaboration. Ann. Intern. Med. 2019, 170, W1–W33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Criteria | Question |
|---|---|
| Bleeding risk assessment | Risk of bleeding during anticoagulation |
| Participants | All adult patients with VTE |
| Risk factors | Patients’ demographics, cancers, comorbidities, concomitant treatments, physiological variables, laboratory measurements, genetics, and history of bleeding before the index event |
| Outcome to be predicted | Bleeding, ISTH major bleeding, fatal bleeding, and clinically relevant non-major bleeding |
| Venous Thromboembolism | Prediction | Bleeding |
|---|---|---|
| Venous thromboembolism [MeSH] OR | Clinical prediction rule [MeSH] OR | Bleeding [MeSH] |
| Pulmonary embolism [MeSH] OR | Risk management model [MeSH] OR | OR |
| Venous thrombosis [MeSH] OR | Prognostic score [MeSH] OR | Hemorrhage [MeSH] OR hemorrhage [MeSH] |
| Deep vein thrombosis [MeSH] OR | Prediction score [MeSH] | - |
| Characteristics | ACCP [16] | EINSTEIN [37] | HOKUSAI [38] | Kuijer [33] | Martinez [32] | Nieuwenhuis [28] | RIETE [27] | VTE-BLEED [29] | Seiler [34] | CAT-BLEED [33] | Alonso [39] | Nieto [40] | Chopard [31] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographic characteristics | |||||||||||||
| Age | X | X | X | X | X | X | X | X | X | ||||
| Sex (Female, F or Male, M) | X | (F) | (F) | (M) | (M) | (F) | |||||||
| BMI | X | X | |||||||||||
| Race | X | ||||||||||||
| Bleeding risk factors | |||||||||||||
| Alcohol abuse | X | X | X | ||||||||||
| History of bleeding | X | X | X | X | X | X | X | X | X | ||||
| Kidney and/or liver failure | X | X | X | X | X | X | |||||||
| Diabetes mellitus | X | X | |||||||||||
| Uncontrolled hypertension. (+/− Male) | X | X | X | ||||||||||
| Recent surgical procedure | X | X | |||||||||||
| Antiplatelet therapy and NSAIDs | X | X | X | X | |||||||||
| Poor anticoagulant control | X | X | |||||||||||
| Frequent falls, previous stroke, dementia | X | X | |||||||||||
| Recent trauma | X | X | |||||||||||
| Cancer history | |||||||||||||
| (active) Cancer or metastatic cancer | X | X | X | X | X | X | X | X | X | ||||
| Genitourinary cancer | X | ||||||||||||
| Gastrointestinal cancer and Edoxaban treatment | X | ||||||||||||
| Anticancer therapy with gastrointestinal toxicity | X | ||||||||||||
| Index events | |||||||||||||
| Pulmonary embolism as index event | X | X | |||||||||||
| Distal DVT | X | ||||||||||||
| Other comorbidities | |||||||||||||
| Comorbidity + decrease in functional capacity/immobility | X | X | X | ||||||||||
| Cardiovascular disease (stroke/coronaropathy/peripheral arterial disease) | X | X | X | X | |||||||||
| Syncope | X | ||||||||||||
| Tobacco and COPD | X | X | |||||||||||
| Biological parameters | |||||||||||||
| Anemia/Hemoglobin | X | X | X | X | X | X | X | X | |||||
| INR/abnormal prothrombin time | X | X | X | ||||||||||
| Thrombopenia | X | X | X | X | |||||||||
| D-dimer | X * | X * | |||||||||||
| Drugs | |||||||||||||
| Rivaroxaban | X | ||||||||||||
| Apixaban | X | ||||||||||||
| VKA | X | ||||||||||||
| Reference | Type of Sources | Follow-Up (Months) | Time of Inclusion | Anticoagulant Type | Number of Patients | Number of Patients with Active Cancer (%) | Bleeding Outcome | Number of Bleedings | OR/HR/RR/β-Coefficient Cancer | Limiting Exclusion Criteria |
|---|---|---|---|---|---|---|---|---|---|---|
| NIEUWENHUIS Nieuwenhuis et al., 1991 [28] | Randomized, controlled trial | <1 | Baseline | LMWH | 96 | 64 (32.9%) | Major bleeding (death, interruption of treatment, transfusion, a decrease of >2.42 g/dL) and minor bleeding (= non major bleeding) | 23 Major bleedings | RR: 0.9 | - |
| UFH | 98 | |||||||||
| KUIJER Kuijer et al., 1999 [33] | Randomized controlled trial | 3 | Baseline | LMWH | 510 | 119 (23%) | All bleeding episodes during anticoagulation, Major bleeding (critical site, interruption of treatment, transfusion, a decrease of >2.42 g/dL) | 16 Major bleedings (46 total bleeding) | OR: 2.2 (/) | - |
| UFH | 511 | 113 (22%) | 12 Major bleedings (47 total bleeding) | |||||||
| RIETE Ruiz-Giménez et al., 2008 [27] | Prospective cohort | 3 | Baseline | LMWH/ UFH or VKA or Cava filter | 13,057 (derivation sample) and 6572 (validation sample) | 2 756 (21.1% of the derivation sample) and 1321 (20 % of the validation sample) | Major bleeding (ISTH) during anticoagulation | 111 Major bleedings and 337 Non major bleeding | OR: 2.1 (1.7–2.6) | - |
| NIETO Nieto et al., 2010 [40] | Prospective cohort | 3 | Baseline | Thrombolytic/LMWH/ UFH or VKA or Cava filter | 24395 | 5063 (20.8%) | Fatal bleeding | 135 Fatal bleeding | OR: 2.87 (2.04–4.03) | Patients currently participating in a therapeutic clinical trial with a blinded therapy |
| EINSTEIN Di Nisio et al., 2016 [37] | Randomized controlled trial | 3 to 12 | I within the 3 weeks, and after the first 3 weeks, overall study | RIVAROXABAN | 4130 | 232 (5.6%) | Major bleeding (ISTH) during anticoagulation | 40 Major bleedings | HR: 3.47 (1.79–6.7) in the 3 first weeks and HR: 2.49 (1.54–4.03) for the entire study | Creatinine clearance < 30 mL/minute/Clinically significant liver disease/Active bleeding or a high risk of bleeding contraindicating anticoagulant treatment/Uncontrolled high blood pressure/ Life-expectancy of <3 months |
| LMWH/VKA | 4116 | 196 (4.8%) | 72 Major bleedings | |||||||
| VTE-BLEED Klok et al., 2016 [29] | Randomized, controlled trial | 6 | 1 month, overall study | DABIGATRAN | 2553 | 114 (2.2%) | Major bleeding (ISTH) and CRNMB (ISTH) during anticoagulation | 37 Major bleedings and 101 CRNMB | OR: 4.18 (2.50–7.02) | High risk of bleeding/Liver disease/Creatinine clearance < 30 mL per minute/Life expectancy of less than 6 months/Requirement for long-term antiplatelet therapy > 100 mg of aspirin |
| VKA | 2554 | - | 51 Major bleedings and 167 CRNMB | - | ||||||
| ACCP Kearon et al., 2016 [16] | Meta-analysis of 9 studies | 6 | - | LMWH and VKA | 3637 | - | Major bleeding (ISTH) during anticoagulation | - | RR: 0.96 (0.65–1.42) | - |
| SEILER Seiler et al., 2017 [34] | Prospective cohort | 36 | Baseline | VKA | 1003 | 71 (<1%) | Major bleeding (ISTH) during anticoagulation | 66 Major bleedings (743 bleedings) | β-coefficient: 0.56 (−0.18–1.3) | Terminal illness/Catheter-related thrombosis |
| HOKUSAI Di Nisio et al., 2017 [38] | Randomized, controlled trial | 3 to 12 | Baseline | LMWH/ EDOXABAN | 4118 | 109 (4.59%) | Major bleeding (ISTH) during anticoagulation | 56 Major bleedings | OR: 3.86 (1.50–9.92) | Cancer for which long-term treatment with LMWH was anticipated/ Aspirin at a dose > 100 mg daily or dual antiplatelet therapy/ Creatinine clearance < 30 mL/min |
| LMWH/VKA | 4122 | 99 (3.03%) | 66 Major bleedings | OR: 2.17 (0.67–7.06) | ||||||
| LMWH | 522 | 511 (97.5%) | - | - | - | - | ||||
| Skowrońska et al., 2019 [30] | Prospective cohort | 0.5 | Baseline | Thrombolysis, UHF, LMWH, FONDAPARINUX, | 310 | 57 (18.3%) | Major bleeding (ISTH) and CRNMB (ISTH) during anticoagulation that occurred during the hospital stay | 18 Major bleedings and 17 CRNMB | - | - |
| RIVAROXABAN, VKA | ||||||||||
| Prospective cohort | Baseline | Combination therapy (VKA + LMWH) | ||||||||
| MARTINEZ Martinez et al., 2019 [32] | Prospective cohort | 3 | Baseline | VKA | 10,010 | 746 (7.45%) | Major bleeding and CRNMB resulting in hospitalization (CRNMB-H) | 344 Major bleedings and 3 112 CRNMB-H | Early post-VTE active cancer sHR: 7.92 (4.33–14.49) Persisting active cancer sHR: 1.69 (0.99–2.88) | ≥2 VKA prescriptions before the initial VTE diagnosis |
| Chopard et al., 2021 [31] | Prospective cohort | 1 | Baseline | UFH, LMWH, DOAC, cava filters, thrombolysis | 2754 | 507 (18%) | Major bleeding (ISTH) during anticoagulation | 82 Major bleedings | - | - |
| Alonso et al., 2021 [39] | Database from 2011 to 2017 | 6 | Initially 4 weeks, after VTE | VKA | 116,319 | 18 (<1%) | Hospitalization for intracranial hemorrhage, gastrointestinal bleeding, or other major bleeding as defined by the International Classification of Diseases, (9th and 10th) | 2294 bleedings | HR: 1.43 (1.30–1.47) | Patient using dabigatran 1141 |
| RIVAROXABAN | 37,214 | 16 (<1%) | ||||||||
| APIXABAN | 11,901 | 17 (<1%) | ||||||||
| CAT-BLEED Winter et al., 2021 [33,41] | Randomized, controlled trial | 6 | Baseline | EDOXABAN | 524 | 513 (98.3%) | Major bleeding (ISTH) during anticoagulation | 39 Major bleedings and 110 CRNMB | Genitourinary cancer sHR: 2.48 (1.14–5.38) | Active bleeding/ Aspirin at a dose > 100 mg daily or dual antiplatelet therapy/ Creatinine clearance < 30 mL/min/ Clinically significant liver disease/ Uncontrolled high blood pressure/ ECOG 3–4/Life expectancy < 3 month/ Platelet count < 50,000 |
| LMWH | 522 | 511 (97.5%) | Gastrointestinal cancer edoxaban treatment sHR: 2.20 (1.07–4.53) Regionally advanced or metastatic cancer sHR: 1.21 (0.82–1.80) |
| Reference | Bleeding Model | AF Model | Type of Sources | Follow Up (Months) | Anticoagulant Type | Number of Patients | Bleeding Outcome | Number of Major Bleeding | Number of Patient with Active Cancer (%) | Bleeding Outcome in Cancer Patients | OR/HR/RR/β-Coefficient Cancer | Limiting Exclusion Criteria | Conclusion of the Author on the Validation in Clinical Practice |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scherz et al., 2013 [42] | ACCP, Kuijer, RIETE | OBRI | Prospective cohort, multicenter | 3 | Thrombolysis, UHF, LMWH, FONDAPARINUX | 663 | Major bleeding (ISTH) during anticoagulation | 28 | 98 (14.6%) | 9 | - | Patients <65 y.o. | - |
| VKA | |||||||||||||
| Nieto et al., 2013 [43] | Nieto | - | Prospective cohort, multicenter (RIETE) | - | Thrombolysis/LMWH/ UFH or VKA or Cava filter | 15,206 | Fatal bleeding | 52 | 3468 (22.8%) | 29 | - | Patients currently participating in a therapeutic clinical trial with a blinded therapy | better for predicting gastrointestinal than intracranial fatal bleeding |
| Poli et al., 2013 [44] | ACCP 2012, RIETE | ATRIA, HAS-BLED, HEMORR2HAGES, OBRI, | Prospective cohort (EPICA); 27 hospitals in Italy | 24 | VKA | 887 | Major bleeding (ISTH) during anticoagulation | 47 | 110 (10.1%) | 11 | 1.1 (0.6–2.3) | Judged too frail | No |
| Riva et al., 2014 [45] | ACCP 2012, Kuijer, RIETE | ATRIA, HAS-BLED, HEMORR2HAGES, Shireman | Retrospective cohort; anticoagulation clinics of 5 hospitals in Italy | 12 | VKA | 681 | Major bleeding (ISTH) and CRNMB (ISTH) during anticoagulation | 50 | 78 (11.4%) | / | - | - | No |
| Piovella et al., 2014 [46] | RIETE, KUIJER | mOBRI | Prospective cohort, multicenter (RIETE) | 3 | Thrombolysis, UHF, LMWH, | 8717 | Major bleeding = clinically overt with a need for transfusion of at least two units of red blood cells/retroperitoneal or intracranial/ permanent discontinuation of treatment/ fatal | 82 | 1807 (20.7%) | 22 | - | - | Slightly better performance of the RIETE |
| OBRI | RIVAROXABAN, VKA | ||||||||||||
| Kline et al., 2016 [47] | RIETE, KUIJER | mOBRI OBRI | Pooled data of EINSTEIN PE and EINSTEIN DVT | 3 to 12 | RIVAROXABAN | 4130 | Major bleeding (ISTH) during anticoagulation | 40 | 232 (5.6%) | - | - | - | Good performance for RIETE |
| Klok et al., 2017 [48] | VTE-BLEED | - | RCT (HOKUSAI VTE) international study | 3 to 12 | VKA | 3903 | Major bleeding (ISTH) during chronic, stable anticoagulation (>30 days) | 40 | 181 (31%) | 6 | - | - | Yes |
| Palareti et al., 2018 [49] | ACCP 2016 | - | Prospective cohort (START2) in multiple hospitals in Italy | >12 | VKA DOAC (subtype not specified) | 2263 | Major bleeding (ISTH) and CRNMB (ISTH) during anticoagulation | 48 | 175 (23.4%) | 4 | HR = 1.0 (0.4–3.0) | - | No |
| Rief et al., 2018 [50] | VTE-BLEED | HAS-BLED | Prospective cohort study, 1 hospital in Austria | 12 | LMWH, VKA, APIXABAN, RIVAROXABAN, EDOXABAN, | 111 | Major bleeding (ISTH) during anticoagulation | 4 | 12 (11%) | - | - | - | Did not discuss validity of the VTE bleed |
| Zhang et al., 2018 [51] | ACCP, Kuijer, RIETE, NIEUWENHUIS | - | Prospective cohort | 3 | VKA, LMWH | 563 | Major bleeding (ISTH) and CRNMB (ISTH) during anticoagulation | 16 | 70 (12.4%) | - | - | - | Good performance of the ACCP |
| Klok et al., 2018 [52] | VTE-BLEED | - | RCT (Xalia); multiple hospitals in 12 countries | >12 | LMWH RIVAROXABAN | 4457 | Major bleeding (ISTH) during anticoagulation | 39 | 500 (11%) | - | HR = 1.0 (0.61–1.7) | - | Yes |
| Vedovati et al., 2019 [53] | Kuijer, RIETE, VTE-BLEED, | HAS-BLED, ATRIA | Prospective cohort | >12 | APIXABAN, RIVAROXABAN, EDOXABAN, DABIGATRAN | 1034 | Major bleeding (ISTH definition) during anticoagulation | 26 | 164 (15.9%) | 5 | HR = 1.930 (0.721–5.170) | - | No |
| Skowrońska et al., 2019 [30] | VTE-BLEED, RIETE | HEMORR2HAGES, HAS-BLED | PE-aWARE registry | 0.5 | Thrombolysis, UHF, LMWH, FONDAPARINUX, | 310 | Major bleeding (ISTH) and CRNMB (ISTH) during anticoagulation that occurred during the hospital stay | 17 | 56 (18.1%) | 11 | - | - | Good performance at identifying Acute PE patients at risk of in-hospital bleeding complication of the VTE bleed |
| RIVAROXABAN, VKA | |||||||||||||
| Combination therapy (VKA + LMWH) | |||||||||||||
| Keller et al., 2021 [54] | KUIJER | - | Nationwide German registry | - | DOAC, VKA | 1,204,895 | Hospitalization for intracranial hemorrhage, gastrointestinal bleeding, or other major bleeding as defined by the International Classification of Diseases | - | 25885 (2.1%) | - | - | - | Good performance at predicting in hospital major bleeding |
| Mathonier et al., 2021 [55] | VTE-BLEED, RIETE | ORBIT, HEMORR2HAGES, ATRIA, HAS-BLED | BFC-FRANCE registry | 0.25 | UFH, LMWH, FONDAPARINUX, VKA and DOACs | 2754 | Major bleeding (ISTH) that occurred during the hospital stay | 82 | 507 (18.4%) | 17 | OR= 4.7 (3.2–6.8) | - | No |
| Frei et al., 2021 [56] | VTE-BLEED, Seiler, Kuijer, RIETE, ACCP, | OBRI, HEMORR2HAGES, HAS-BLED, ATRIA | Prospective, multicenter SWIss venous Thromboembolism COhort study 65+ (SWITCO 65+) | 36 | VKA | 743 | Major bleeding (ISTH) and CRNMB (ISTH) during anticoagulation | 45 | 10 (1.3%) | 16 | - | Terminal illness, catheter-related thrombosis, age under 65 | No |
| De Winter et al., 2021 [36] | VTE-BLEED, RIETE, Martinez, Kuijer, HOKUSAI, ACCP | HAS-BLED | HOKUSAI VTE cancer post hoc analysis | >12 | EDOXABAN, LMWH | 1046 | Major bleeding (ISTH) and CRNMB (ISTH) during anticoagulation that occurred during the hospital stay | 39 | 1024 (97.8%) | 39 | - | - | No good performance of the existing RAM in CAT population |
| Ambivalent Prediction Factors of Anticoagulant-Associated Bleeding and Recurrent VTE |
|---|
| Age |
| Sex (Female or Male) |
| BMI |
| PE as the index VTE |
| History of cardiovascular disease (stroke/coronaropathy/peripheral arterial disease) |
| Cancer site |
| Cancer stage |
| Chemotherapy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poénou, G.; Tolédano, E.; Helfer, H.; Plaisance, L.; Happe, F.; Versini, E.; Diab, N.; Djennaoui, S.; Mahé, I. In Search of the Appropriate Anticoagulant-Associated Bleeding Risk Assessment Model for Cancer-Associated Thrombosis Patients. Cancers 2022, 14, 1937. https://doi.org/10.3390/cancers14081937
Poénou G, Tolédano E, Helfer H, Plaisance L, Happe F, Versini E, Diab N, Djennaoui S, Mahé I. In Search of the Appropriate Anticoagulant-Associated Bleeding Risk Assessment Model for Cancer-Associated Thrombosis Patients. Cancers. 2022; 14(8):1937. https://doi.org/10.3390/cancers14081937
Chicago/Turabian StylePoénou, Géraldine, Emmanuel Tolédano, Hélène Helfer, Ludovic Plaisance, Florent Happe, Edouard Versini, Nevine Diab, Sadji Djennaoui, and Isabelle Mahé. 2022. "In Search of the Appropriate Anticoagulant-Associated Bleeding Risk Assessment Model for Cancer-Associated Thrombosis Patients" Cancers 14, no. 8: 1937. https://doi.org/10.3390/cancers14081937
APA StylePoénou, G., Tolédano, E., Helfer, H., Plaisance, L., Happe, F., Versini, E., Diab, N., Djennaoui, S., & Mahé, I. (2022). In Search of the Appropriate Anticoagulant-Associated Bleeding Risk Assessment Model for Cancer-Associated Thrombosis Patients. Cancers, 14(8), 1937. https://doi.org/10.3390/cancers14081937








