Alternatives for MRI in Prostate Cancer Diagnostics—Review of Current Ultrasound-Based Techniques
Abstract
Simple Summary
Abstract
1. Introduction
2. Evidence Acquisition
3. Multiparametric MRI in the Prostate Cancer Diagnostics
4. Ultrasound Techniques in the Prostate Cancer Diagnostics
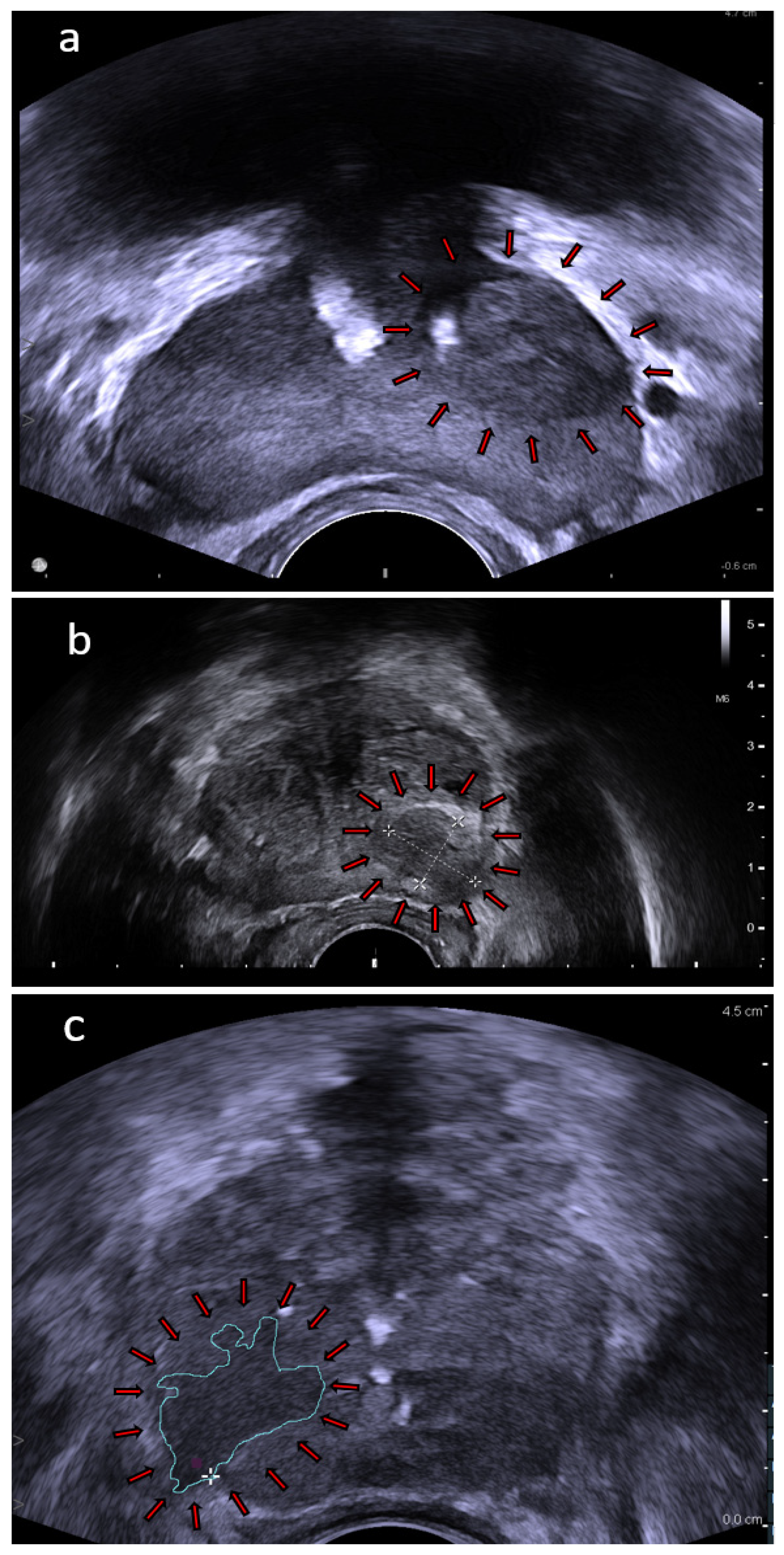
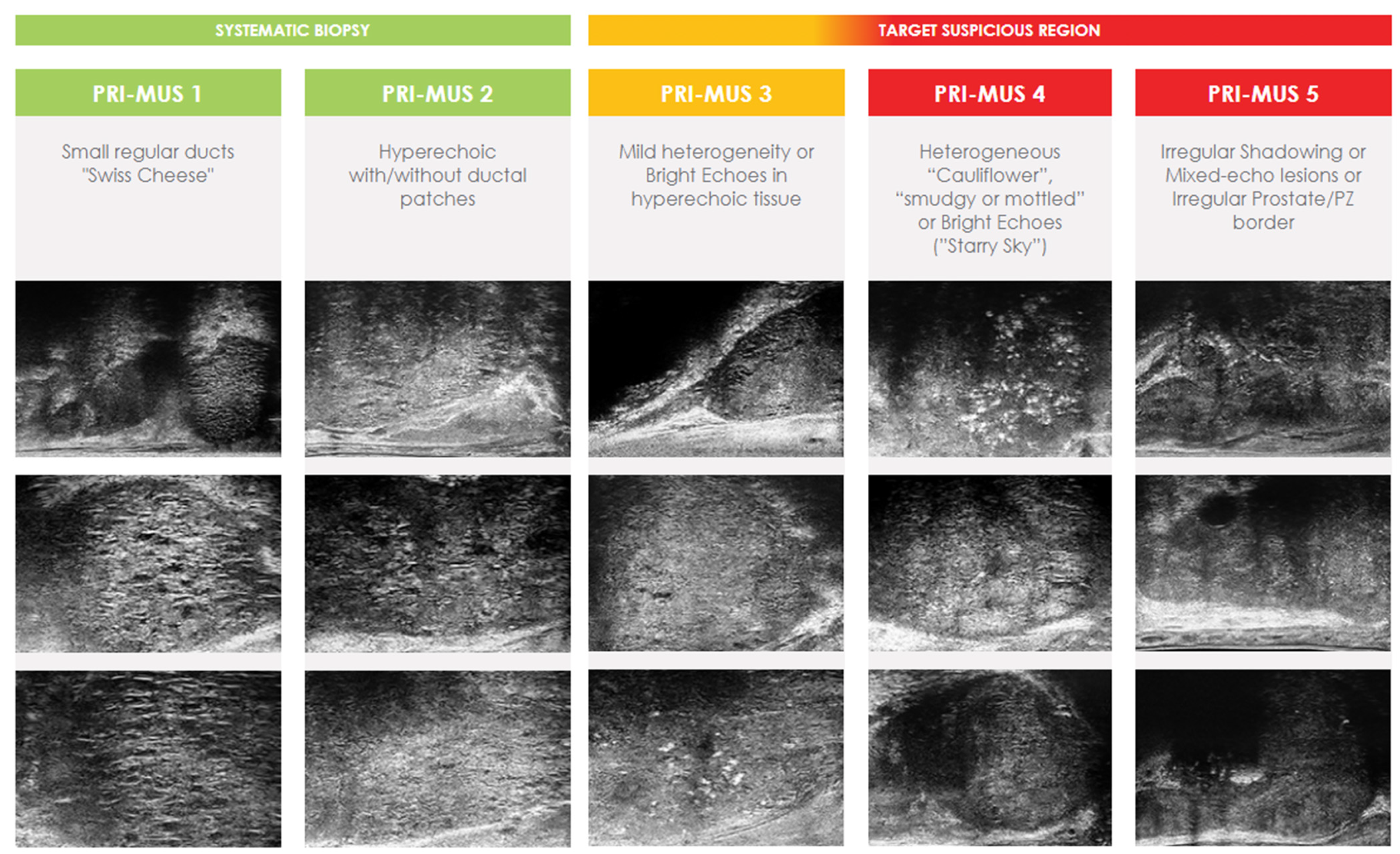
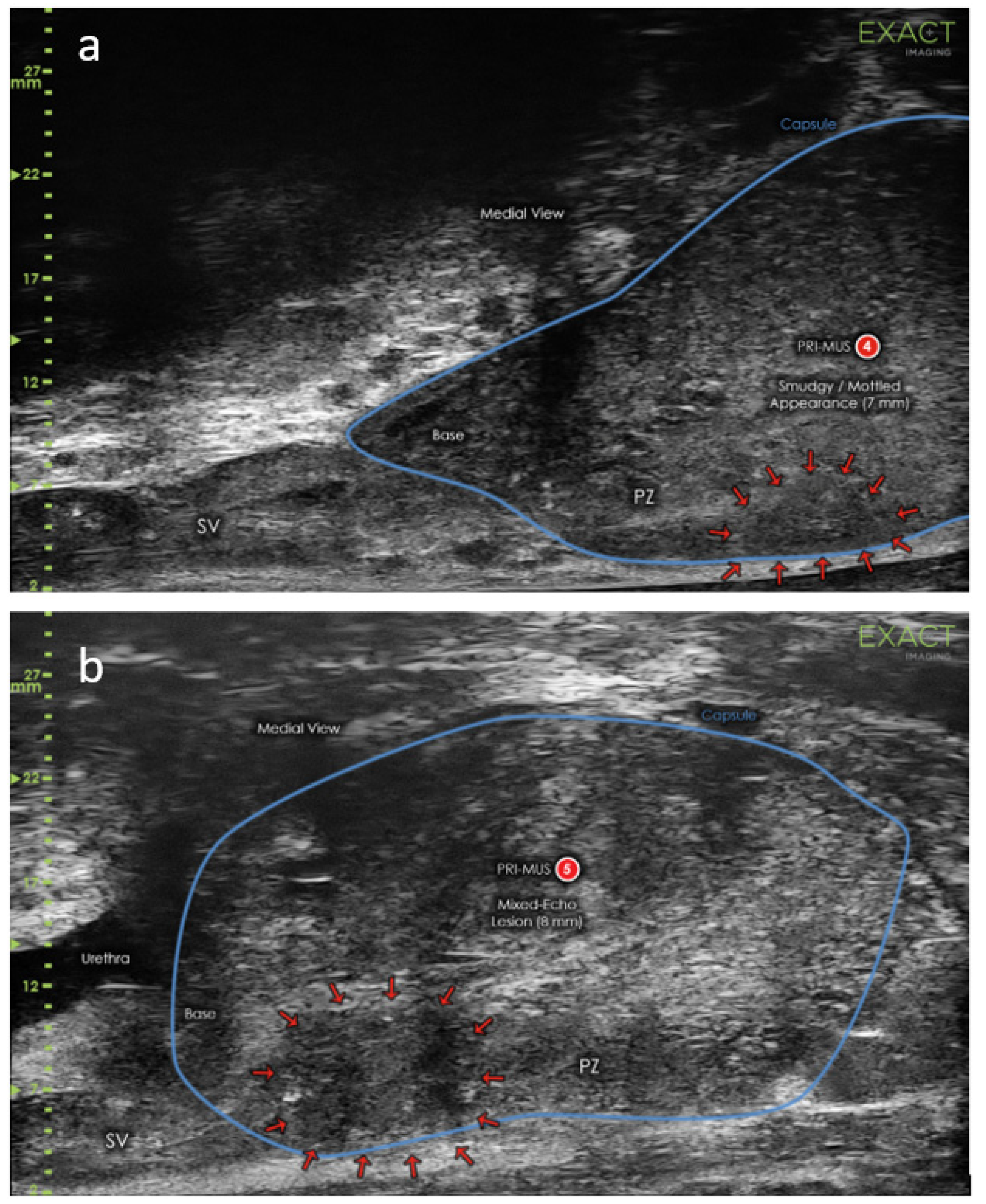
4.1. High Frequency Micro-Ultrasound
4.2. Contrast-Enhanced Ultrasound
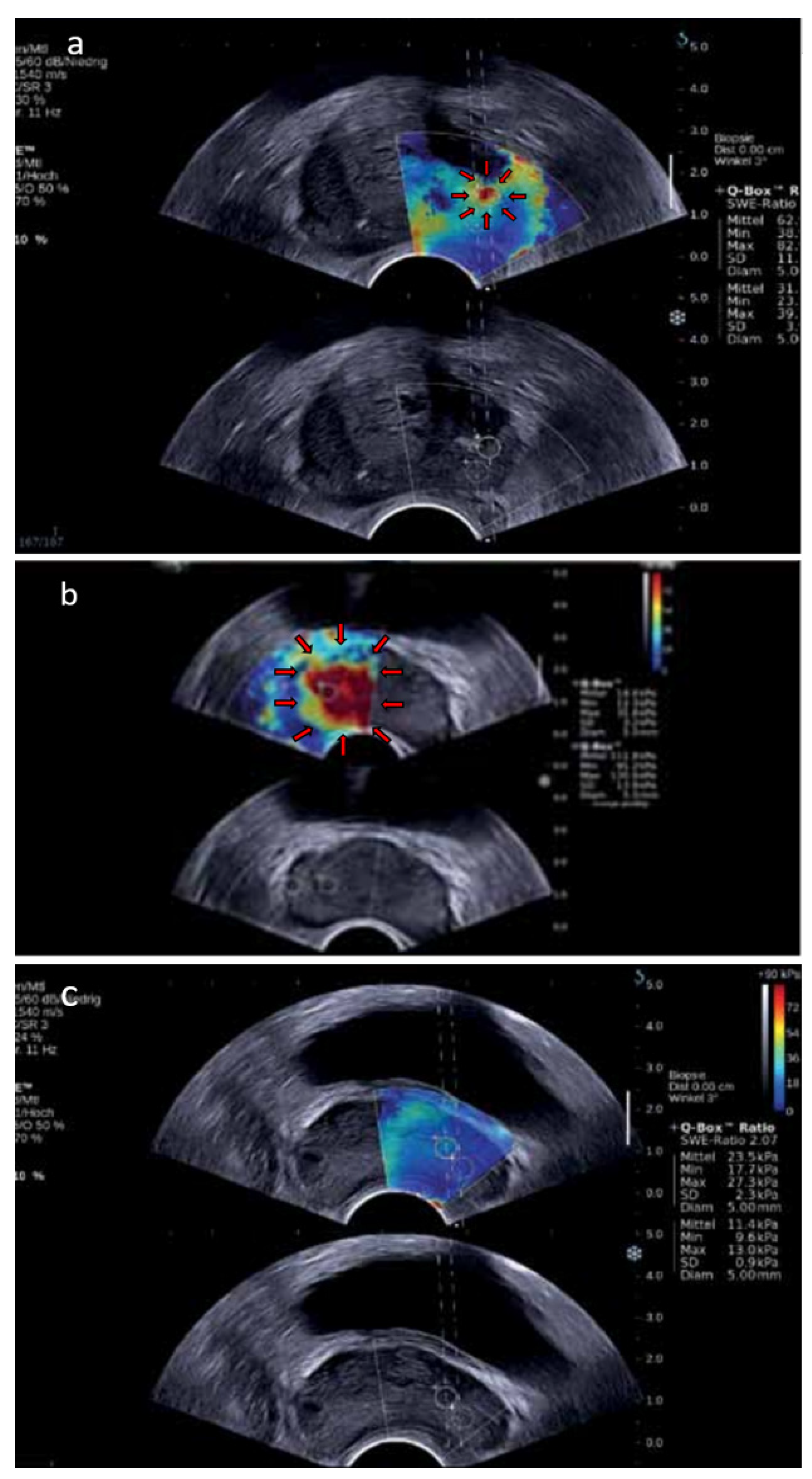
4.3. Shear-Wave Elastography
4.4. Multiparametric Ultrasound
5. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2018. CA A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.S.; Stockert, J.A.; Hackert, V.; Yadav, K.K.; Tewari, A.K. Intratumor Heterogeneity in Prostate Cancer. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2018; Volume 36. [Google Scholar]
- Liu, A.Y.; Roudier, M.P.; True, L.D. Heterogeneity in Primary and Metastatic Prostate Cancer as Defined by Cell Surface CD Profile. Am. J. Pathol. 2004, 165, 1543–1556. [Google Scholar] [CrossRef]
- European Association of Urology. EAU Guidelines on Prostate Cancer. EAU 2019, 5, 30–31. [Google Scholar]
- Schröder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.J.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Määttänen, L.; Lilja, H.; et al. Screening and Prostate Cancer Mortality: Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 Years of Follow-Up. Lancet 2014, 384, 2027–2035. [Google Scholar] [CrossRef]
- Loeb, S.; Bjurlin, M.A.; Nicholson, J.; Tammela, T.L.; Penson, D.F.; Carter, H.B.; Carroll, P.; Etzioni, R. Overdiagnosis and Overtreatment of Prostate Cancer. Eur. Urol. 2014, 65, 1046–1055. [Google Scholar] [CrossRef]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic Accuracy of Multi-Parametric MRI and TRUS Biopsy in Prostate Cancer (PROMIS): A Paired Validating Confirmatory Study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef]
- Loeb, S.; Vellekoop, A.; Ahmed, H.U.; Catto, J.; Emberton, M.; Nam, R.; Rosario, D.J.; Scattoni, V.; Lotan, Y. Systematic Review of Complications of Prostate Biopsy. Eur. Urol. 2013, 64, 876–892. [Google Scholar] [CrossRef]
- Pepe, P.; Aragona, F. Morbidity after Transperineal Prostate Biopsy in 3000 Patients Undergoing 12 vs. 18 vs. More than 24 Needle Cores. Urology 2013, 81, 1142–1146. [Google Scholar] [CrossRef]
- Serefoglu, E.C.; Altinova, S.; Ugras, N.S.; Akincioglu, E.; Asil, E.; Balbay, M.D. How Reliable Is 12-Core Prostate Biopsy Procedure in the Detection of Prostate Cancer? J. Can. Urol. Assoc. 2013, 7, E293. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Mitterberger, M.; Pinggera, G.M.; Horninger, W.; Bartsch, G.; Strasser, H.; Schäfer, G.; Brunner, A.; Halpern, E.J.; Gradl, J.; Pallwein, L.; et al. Comparison of Contrast Enhanced Color Doppler Targeted Biopsy to Conventional Systematic Biopsy: Impact on Gleason Score. J. Urol. 2007, 178, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Halpern, E.J.; Ramey, J.R.; Strup, S.E.; Frauscher, F.; McCue, P.; Gomella, L.O. Detection of Prostate Carcinoma with Contrast-Enhanced Sonography Using Intermittent Harmonic Imaging. Cancer 2005, 104, 2373–2383. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Suh, C.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H. Shear-Wave Elastography for Detection of Prostate Cancer: A Systematic Review and Diagnostic Meta-Analysis. Am. J. Roentgenol. 2017, 209, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, X.; Shi, J.; Huang, Y. Value of Shear Wave Elastography for Diagnosis of Primary Prostate Cancer: A Systematic Review and Meta-Analysis. Med. Ultrason. 2019, 21, 382–388. [Google Scholar] [CrossRef]
- Rohrbach, D.; Wodlinger, B.; Wen, J.; Mamou, J.; Feleppa, E. High-Frequency Quantitative Ultrasound for Imaging Prostate Cancer Using a Novel Micro-Ultrasound Scanner. Ultrasound Med. Biol. 2018, 44, 1341–1354. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.; Jiang, J.; Qi, T.; Liu, W.; Qu, Y.; Guan, W.; Wang, L.; Qi, J. Comparison of Contrast-Enhanced Ultrasound Targeted Biopsy versus Standard Systematic Biopsy for Clinically Significant Prostate Cancer Detection: Results of a Prospective Cohort Study with 1024 Patients. World J. Urol. 2019, 37, 805–811. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Moore, C.M.; Kasivisvanathan, V.; Eggener, S.; Emberton, M.; Fütterer, J.J.; Gill, I.S.; Grubb, R.L.; Hadaschik, B.; Klotz, L.; Margolis, D.J.A.; et al. Standards of Reporting for MRI-Targeted Biopsy Studies (START) of the Prostate: Recommendations from an International Working Group. Eur. Urol. 2013, 64, 544–552. [Google Scholar] [CrossRef]
- O’Connor, L.P.; Lebastchi, A.H.; Horuz, R.; Rastinehad, A.R.; Siddiqui, M.M.; Grummet, J.; Kastner, C.; Ahmed, H.U.; Pinto, P.A.; Turkbey, B. Role of Multiparametric Prostate MRI in the Management of Prostate Cancer. World J. Urol. 2021, 39, 651–659. [Google Scholar] [CrossRef]
- Stabile, A.; Giganti, F.; Rosenkrantz, A.B.; Taneja, S.S.; Villeirs, G.; Gill, I.S.; Allen, C.; Emberton, M.; Moore, C.M.; Kasivisvanathan, V. Multiparametric MRI for Prostate Cancer Diagnosis: Current Status and Future Directions. Nat. Rev. Urol. 2020, 17, 41–61. [Google Scholar] [CrossRef]
- Barentsz, J.O.; Richenberg, J.; Clements, R.; Choyke, P.; Verma, S.; Villeirs, G.; Rouviere, O.; Logager, V.; Fütterer, J.J. ESUR Prostate MR Guidelines 2012. Eur. Radiol. 2012, 22, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Hamoen, E.H.J.; De Rooij, M.; Witjes, J.A.; Barentsz, J.O.; Rovers, M.M. Use of the Prostate Imaging Reporting and Data System (PI-RADS) for Prostate Cancer Detection with Multiparametric Magnetic Resonance Imaging: A Diagnostic Meta-Analysis. Eur. Urol. 2015, 67, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Schnall, M.D.; Shtern, F.; Tempany, C.M.; et al. PI-RADS Prostate Imaging-Reporting and Data System: 2015, Version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef]
- Woo, S.; Suh, C.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H. Diagnostic Performance of Prostate Imaging Reporting and Data System Version 2 for Detection of Prostate Cancer: A Systematic Review and Diagnostic Meta-Analysis. Eur. Urol. 2017, 72, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Ahdoot, M.; Wilbur, A.R.; Reese, S.E.; Lebastchi, A.H.; Mehralivand, S.; Gomella, P.T.; Bloom, J.; Gurram, S.; Siddiqui, M.; Pinsky, P.; et al. MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. N. Engl. J. Med. 2020, 382, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.M.; Rais-Bahrami, S.; Turkbey, B.; George, A.K.; Rothwax, J.; Shakir, N.; Okoro, C.; Raskolnikov, D.; Parnes, H.L.; Linehan, W.M.; et al. Comparison of MR/Ultrasound Fusion-Guided Biopsy with Ultrasound-Guided Biopsy for the Diagnosis of Prostate Cancer. JAMA-J. Am. Med. Assoc. 2015, 313, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, M.; Bianchi, L.; Barbaresi, U.; Vagnoni, V.; Corcioni, B.; Gaudiano, C.; Fiorentino, M.; Giunchi, F.; Chessa, F.; Garofalo, M.; et al. Diagnostic Performance of MRI/TRUS Fusion-Guided Biopsies vs. Systematic Prostate Biopsies in Biopsy-Naïve, Previous Negative Biopsy Patients and Men Undergoing Active Surveillance. Minerva Urol. Nephrol. 2021, 73, 357–366. [Google Scholar] [CrossRef]
- Uno, H.; Taniguchi, T.; Seike, K.; Kato, D.; Takai, M.; Iinuma, K.; Horie, K.; Nakane, K.; Koie, T. The Accuracy of Prostate Cancer Diagnosis in Biopsy-Naive Patients Using Combined Magnetic Resonance Imaging and Transrectal Ultrasound Fusion-Targeted Prostate Biopsy. Transl. Androl. Urol. 2021, 10, 2982. [Google Scholar] [CrossRef]
- Bass, E.J.; Pantovic, A.; Connor, M.J.; Loeb, S.; Rastinehad, A.R.; Winkler, M.; Gabe, R.; Ahmed, H.U. Diagnostic Accuracy of Magnetic Resonance Imaging Targeted Biopsy Techniques Compared to Transrectal Ultrasound Guided Biopsy of the Prostate: A Systematic Review and Meta-Analysis. Prostate Cancer Prostatic Dis. 2021, 24, 1–6. [Google Scholar] [CrossRef]
- Valerio, M.; Donaldson, I.; Emberton, M.; Ehdaie, B.; Hadaschik, B.A.; Marks, L.S.; Mozer, P.; Rastinehad, A.R.; Ahmed, H.U. Detection of Clinically Significant Prostate Cancer Using Magnetic Resonance Imaging-Ultrasound Fusion Targeted Biopsy: A Systematic Review. Eur. Urol. 2015, 68, 8–19. [Google Scholar] [CrossRef]
- Wysock, J.S.; Mendhiratta, N.; Zattoni, F.; Meng, X.; Bjurlin, M.; Huang, W.C.; Lepor, H.; Rosenkrantz, A.B.; Taneja, S.S. Predictive Value of Negative 3T Multiparametric Magnetic Resonance Imaging of the Prostate on 12-Core Biopsy Results. BJU Int. 2016, 118, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; Manfredi, M.; Mele, F.; Cossu, M.; Bollito, E.; Veltri, A.; Cirillo, S.; Regge, D.; Faletti, R.; Passera, R.; et al. Diagnostic Pathway with Multiparametric Magnetic Resonance Imaging Versus Standard Pathway: Results from a Randomized Prospective Study in Biopsy-Naïve Patients with Suspected Prostate Cancer. Eur. Urol. 2017, 72, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Baccaglini, W.; Glina, F.A.; Pazeto, C.L.; Medina, L.G.; Korkes, F.; Bernardo, W.M.; Sotelo, R.; Glina, S.; Marra, G.; Moschini, M.; et al. Accuracy of MRI-Guided Versus Systematic Prostate Biopsy in Patients Under Active Surveillance: A Systematic Review and Meta-Analysis. Clin. Genitourin. Cancer 2021, 19, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ghavimi, S.; Abdi, H.; Waterhouse, J.; Savdie, R.; Chang, S.; Harris, A.; Machan, L.; Gleave, M.; So, A.I.; Goldenberg, L.; et al. Natural History of Prostatic Lesions on Serial Multiparametric Magnetic Resonance Imaging. Can. Urol. Assoc. J. 2018, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Schoots, I.G.; Petrides, N.; Giganti, F.; Bokhorst, L.P.; Rannikko, A.; Klotz, L.; Villers, A.; Hugosson, J.; Moore, C.M. Magnetic Resonance Imaging in Active Surveillance of Prostate Cancer: A Systematic Review. Eur. Urol. 2015, 67, 627–636. [Google Scholar] [CrossRef]
- Elkhoury, F.F.; Simopoulos, D.N.; Marks, L.S. Targeted Prostate Biopsy in the Era of Active Surveillance. Urology 2018, 112, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Schoots, I.G.; Nieboer, D.; Giganti, F.; Moore, C.M.; Bangma, C.H.; Roobol, M.J. Is Magnetic Resonance Imaging-Targeted Biopsy a Useful Addition to Systematic Confirmatory Biopsy in Men on Active Surveillance for Low-Risk Prostate Cancer? A Systematic Review and Meta-Analysis. BJU Int. 2018, 122, 946–958. [Google Scholar] [CrossRef]
- Eineluoto, J.T.; Järvinen, P.; Kilpeläinen, T.; Lahdensuo, K.; Kalalahti, I.; Sandeman, K.; Mirtti, T.; Rannikko, A. Patient Experience of Systematic Versus Fusion Prostate Biopsies. Eur. Urol. Oncol. 2018, 1, 202–207. [Google Scholar] [CrossRef]
- Filson, C.P.; Natarajan, S.; Margolis, D.J.A.; Huang, J.; Lieu, P.; Dorey, F.J.; Reiter, R.E.; Marks, L.S. Prostate Cancer Detection with Magnetic Resonance-Ultrasound Fusion Biopsy: The Role of Systematic and Targeted Biopsies. Cancer 2016, 122, 884–892. [Google Scholar] [CrossRef]
- Elkhoury, F.F.; Felker, E.R.; Kwan, L.; Sisk, A.E.; Delfin, M.; Natarajan, S.; Marks, L.S. Comparison of Targeted vs Systematic Prostate Biopsy in Men Who Are Biopsy Naive: The Prospective Assessment of Image Registration in the Diagnosis of Prostate Cancer (PAIREDCAP) Study. JAMA Surg. 2019, 154, 811–818. [Google Scholar] [CrossRef]
- Rouvière, O.; Puech, P.; Renard-Penna, R.; Claudon, M.; Roy, C.; Mège-Lechevallier, F.; Decaussin-Petrucci, M.; Dubreuil-Chambardel, M.; Magaud, L.; Remontet, L.; et al. Use of Prostate Systematic and Targeted Biopsy on the Basis of Multiparametric MRI in Biopsy-Naive Patients (MRI-FIRST): A Prospective, Multicentre, Paired Diagnostic Study. Lancet Oncol. 2019, 20, 100–109. [Google Scholar] [CrossRef]
- Wegelin, O.; van Melick, H.H.E.; Hooft, L.; Bosch, J.L.H.R.; Reitsma, H.B.; Barentsz, J.O.; Somford, D.M. Comparing Three Different Techniques for Magnetic Resonance Imaging-Targeted Prostate Biopsies: A Systematic Review of In-Bore versus Magnetic Resonance Imaging-Transrectal Ultrasound Fusion versus Cognitive Registration. Is There a Preferred Technique? Eur. Urol. 2017, 71, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Schoots, I.G.; Roobol, M.J. From PROMIS to PRO-MRI in Primary Prostate Cancer Diagnosis. Transl. Androl. Urol. 2017, 6, 604. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Ukimura, O.; Kaneko, M.; Matsugasumi, T.; Fujihara, A.; Vourganti, S.; Marks, L.; Sidana, A.; Klotz, L.; Salomon, G.; et al. Moving Away from Systematic Biopsies: Image-Guided Prostate Biopsy (in-Bore Biopsy, Cognitive Fusion Biopsy, MRUS Fusion Biopsy)—Literature Review. World J. Urol. 2021, 39, 677–686. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 1228–1242. [Google Scholar] [CrossRef]
- Yamashiro, J.R.; de Riese, W.T.W. Any Correlation between Prostate Volume and Incidence of Prostate Cancer: A Review of Reported Data for the Last Thirty Years. Res. Rep. Urol. 2021, 13, 749. [Google Scholar] [CrossRef]
- Lophatananon, A.; Light, A.; Burns-Cox, N.; Maccormick, A.; John, J.; Otti, V.; McGrath, J.; Archer, P.; Anning, J.; McCracken, S.; et al. Re-Evaluating the Diagnostic Efficacy of PSA as a Referral Test to Detect Clinically Significant Prostate Cancer in Contemporary MRI-Based Image-Guided Biopsy Pathways. J. Clin. Urol. 2021. [Google Scholar] [CrossRef]
- Pavlovich, C.P.; Cornish, T.C.; Mullins, J.K.; Fradin, J.; Mettee, L.Z.; Connor, J.T.; Reese, A.C.; Askin, F.B.; Luck, R.; Epstein, J.I.; et al. High-Resolution Transrectal Ultrasound: Pilot Study of a Novel Technique for Imaging Clinically Localized Prostate Cancer. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2014; Volume 32. [Google Scholar] [CrossRef]
- Ghai, S.; Eure, G.; Fradet, V.; Hyndman, M.E.; McGrath, T.; Wodlinger, B.; Pavlovich, C.P. Assessing Cancer Risk on Novel 29 MHz Micro-Ultrasound Images of the Prostate: Creation of the Micro-Ultrasound Protocol for Prostate Risk Identification. J. Urol. 2016, 196, 562–569. [Google Scholar] [CrossRef]
- Pavlovich, C.P.; Hyndman, M.E.; Eure, G.; Ghai, S.; Caumartin, Y.; Herget, E.; Young, J.D.; Wiseman, D.; Caughlin, C.; Gray, R.; et al. A Multi-institutional Randomized Controlled Trial Comparing First-generation Transrectal High-resolution Micro-ultrasound with Conventional Frequency Transrectal Ultrasound for Prostate Biopsy. BJUI Compass 2021, 2, 126–133. [Google Scholar] [CrossRef]
- Abouassaly, R.; Klein, E.A.; El-Shefai, A.; Stephenson, A. Impact of Using 29 MHz High-Resolution Micro-Ultrasound in Real-Time Targeting of Transrectal Prostate Biopsies: Initial Experience. World J. Urol. 2020, 38, 1201–1206. [Google Scholar] [CrossRef]
- Odriozola, A.A.; Sanchez, A.; de La Cruz, I.; Pereira, J.G.; Gamarra, M.; Urdaneta, F.; Mora, J.; Ibarluzea, G. Initial Results Comparing 29 MHz Micro-Ultrasound with Multi-Parametric MRI for Targeted Prostate Biopsy: Relative Sensitivity to Clinically Significant Prostate Cancer. Eur. Urol. Suppl. 2018, 17, E901. [Google Scholar] [CrossRef]
- Eure, G.; Fanney, D.; Lin, J.; Wodlinger, B.; Ghai, S. Comparison of Conventional Transrectal Ultrasound, Magnetic Resonance Imaging, and Micro-Ultrasound for Visualizing Prostate Cancer in an Active Surveillance Population: A Feasibility Study. Can. Urol. Assoc. J. 2019, 13, E70–E77. [Google Scholar] [CrossRef] [PubMed]
- Lughezzani, G.; Saita, A.; Lazzeri, M.; Paciotti, M.; Maffei, D.; Lista, G.; Hurle, R.; Buffi, N.M.; Guazzoni, G.; Casale, P. Comparison of the Diagnostic Accuracy of Micro-Ultrasound and Magnetic Resonance Imaging/Ultrasound Fusion Targeted Biopsies for the Diagnosis of Clinically Significant Prostate Cancer. Eur. Urol. Oncol. 2019, 2, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Claros, O.R.; Tourinho-Barbosa, R.R.; Fregeville, A.; Gallardo, A.C.; Muttin, F.; Carneiro, A.; Stabile, A.; Moschini, M.; Macek, P.; Cathala, N.; et al. Comparison of Initial Experience with Transrectal Magnetic Resonance Imaging Cognitive Guided Micro-Ultrasound Biopsies versus Established Transperineal Robotic Ultrasound Magnetic Resonance Imaging Fusion Biopsies for Prostate Cancer. J. Urol. 2020, 203, 918–925. [Google Scholar] [CrossRef]
- Socarrás, M.E.R.; Rivas, J.G.; Rivera, V.C.; Elbers, J.R.; González, L.L.; Mercado, I.M.; del Alamo, J.F.; del Dago, P.J.; Sancha, F.G. Prostate Mapping for Cancer Diagnosis: The Madrid Protocol. Transperineal Prostate Biopsies Using Multiparametric Magnetic Resonance Imaging Fusion and Micro-Ultrasound Guided Biopsies. J. Urol. 2020, 204, 726–733. [Google Scholar] [CrossRef]
- Cornud, F.; Lefevre, A.; Flam, T.; Dumonceau, O.; Galiano, M.; Soyer, P.; Camparo, P.; Barral, M. MRI-directed high-frequency (29MhZ) TRUS-guided biopsies: Initial results of a single-center study. Eur. Radiol. 2020, 30, 4838–4846. [Google Scholar] [CrossRef]
- Wiemer, L.; Hollenbach, M.; Heckmann, R.; Kittner, B.; Plage, H.; Reimann, M.; Asbach, P.; Friedersdorff, F.; Schlomm, T.; Hofbauer, S.; et al. Evolution of Targeted Prostate Biopsy by Adding Micro-Ultrasound to the Magnetic Resonance Imaging Pathway. Eur. Urol. Focus 2020, 7, 1292–1299. [Google Scholar] [CrossRef]
- Klotz, L.; Lughezzani, G.; Maffei, D.; Sánchez, A.; Pereira, J.G.; Staerman, F.; Cash, H.; Luger, F.; Lopez, L.; Sanchez-Salas, R.; et al. Comparison of Micro-Ultrasound and Multiparametric Magnetic Resonance Imaging for Prostate Cancer: A Multicenter, Prospective Analysis. Can. Urol. Assoc. J. 2020, 15, E11–E16. [Google Scholar] [CrossRef]
- Lughezzani, G.; Maffei, D.; Saita, A.; Paciotti, M.; Diana, P.; Buffi, N.M.; Colombo, P.; Elefante, G.M.; Hurle, R.; Lazzeri, M.; et al. Diagnostic Accuracy of Microultrasound in Patients with a Suspicion of Prostate Cancer at Magnetic Resonance Imaging: A Single-Institutional Prospective Study. Eur. Urol. Focus 2021, 7, 1019–1026. [Google Scholar] [CrossRef]
- Sountoulides, P.; Pyrgidis, N.; Polyzos, S.A.; Mykoniatis, I.; Asouhidou, E.; Papatsoris, A.; Dellis, A.; Anastasiadis, A.; Lusuardi, L.; Hatzichristou, D. Micro-Ultrasound-Guided vs Multiparametric Magnetic Resonance Imaging-Targeted Biopsy in the Detection of Prostate Cancer: A Systematic Review and Meta-Analysis. J. Urol. 2021, 205, 1254–1262. [Google Scholar] [CrossRef]
- You, C.; Li, X.; Du, Y.; Peng, L.; Wang, H.; Zhang, X.; Wang, A. The Micro-Ultrasound Guided Prostate Biopsy in Detection of Prostate Cancer: A Systematic Review and Meta-Analysis. J. Endourol. 2022, 36, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Avolio, P.P.; Lughezzani, G.; Paciotti, M.; Maffei, D.; Uleri, A.; Frego, N.; Hurle, R.; Lazzeri, M.; Saita, A.; Guazzoni, G.; et al. The Use of 29 MHz Transrectal Micro-Ultrasound to Stratify the Prostate Cancer Risk in Patients with PI-RADS III Lesions at Multiparametric MRI: A Single Institutional Analysis. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 832.e1–832.e7. [Google Scholar] [CrossRef]
- Klotz, L.; Andriole, G.; Cash, H.; Cooperberg, M.; Crawford, E.D.; Emberton, M.; Gomez-Sancha, F.; Klein, E.; Lughezzani, G.; Marks, L.; et al. Optimization of Prostate Biopsy-Micro-Ultrasound versus MRI (OPTIMUM): A 3-Arm Randomized Controlled Trial Evaluating the Role of 29 MHz Micro-Ultrasound in Guiding Prostate Biopsy in Men with Clinical Suspicion of Prostate Cancer. Contemp. Clin. Trials 2021, 112, 106618. [Google Scholar] [CrossRef] [PubMed]
- Greis, C. Technology Overview: SonoVue (Bracco, Milan). Eur. Radiol. Suppl. 2004, 14, P11–P15. [Google Scholar] [CrossRef]
- Piscaglia, F.; Bolondi, L.; Aiani, L.; Luigi Angeli, M.; Arienti, V.; Barozzi, L.; Basilico, R.; Bertolotto, M.; Biasini, E.; Busilacchi, P.; et al. The Safety of Sonovue® in Abdominal Applications: Retrospective Analysis of 23188 Investigations. Ultrasound Med. Biol. 2006, 32, 1369–1375. [Google Scholar] [CrossRef]
- Tang, C.; Fang, K.; Guo, Y.; Li, R.; Fan, X.; Chen, P.; Chen, Z.; Liu, Q.; Zou, Y. Safety of Sulfur Hexafluoride Microbubbles in Sonography of Abdominal and Superficial Organs: Retrospective Analysis of 30,222 Cases. J. Ultrasound Med. 2017, 36, 531–538. [Google Scholar] [CrossRef]
- Sidhu, P.S.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Der Med.-Eur. J. Ultrasound 2018, 39, e2–e44. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Nolsoe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) in the Liver-Update 2020-WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultraschall Der Med. 2020, 41, 562–585. [Google Scholar]
- Russo, G.; Mischi, M.; Scheepens, W.; de La Rosette, J.J.; Wijkstra, H. Angiogenesis in Prostate Cancer: Onset, Progression and Imaging. BJU Int. 2012, 110, E794–E808. [Google Scholar] [CrossRef]
- Jung, E.M.; Weber, M.A.; Wiesinger, I. Contrast-Enhanced Ultrasound Perfusion Imaging of Organs. Radiologe 2021, 61, 19–28. [Google Scholar] [CrossRef]
- Roy, C.; Buy, X.; Lang, H.; Saussine, C.; Jacqmin, D. Contrast Enhanced Color Doppler Endorectal Sonography of the Prostate: Efficiency for Detecting Peripheral Zone Tumors and Role for Biopsy Procedure. J. Urol. 2003, 170, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Mitterberger, M.J.; Aigner, F.; Horninger, W.; Ulmer, H.; Cavuto, S.; Halpern, E.J.; Frauscher, F. Comparative Efficiency of Contrast-Enhanced Colour Doppler Ultrasound Targeted versus Systematic Biopsy for Prostate Cancer Detection. Eur. Radiol. 2010, 20, 2791–2796. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Liu, L.; Shen, L.; Cai, J.R.; Xu, L.; Xiang, L.H. Comparison of Contrast-Enhanced Ultrasound Targeted Biopsies versus Standard Systematic Biopsies for Prostate Cancer Correction in Different PSA Value Groups in Rural China. Clin. Hemorheol. Microcirc. 2021, 77, 295–301. [Google Scholar] [CrossRef]
- Li, Y.; Tang, J.; Fei, X.; Gao, Y. Diagnostic Performance of Contrast Enhanced Ultrasound in Patients with Prostate Cancer. A Meta-Analysis. Acad. Radiol. 2013, 20, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wu, S.; Huang, L. Contrast-Enhanced Ultrasound Evaluation of the Prostate before Transrectal Ultrasound-Guided Biopsy Can Improve Diagnostic Sensitivity: A STARD-Compliant Article. Medicine 2020, 99, e19946. [Google Scholar] [CrossRef]
- Kuenen, M.P.J.; Mischi, M.; Wijkstra, H. Contrast-Ultrasound Diffusion Imaging for Localization of Prostate Cancer. IEEE Trans. Med. Imaging 2011, 30, 1493–1502. [Google Scholar] [CrossRef]
- van Sloun, R.J.; Demi, L.; Postema, A.W.; de la Rosette, J.J.; Wijkstra, H.; Mischi, M. Ultrasound-Contrast-Agent Dispersion and Velocity Imaging for Prostate Cancer Localization. Med. Image Anal. 2017, 35, 610–619. [Google Scholar] [CrossRef]
- Postema, A.W.; Frinking, P.J.A.; Smeenge, M.; de Reijke, T.M.; de La Rosette, J.J.M.C.H.; Tranquart, F.; Wijkstra, H. Dynamic Contrast-Enhanced Ultrasound Parametric Imaging for the Detection of Prostate Cancer. BJU Int. 2015, 117, 598–603. [Google Scholar] [CrossRef]
- Postema, A.W.; Gayet, M.C.W.; van Sloun, R.J.G.; Wildeboer, R.R.; Mannaerts, C.K.; Savci-Heijink, C.D.; Schalk, S.G.; Kajtazovic, A.; van der Poel, H.; Mulders, P.F.A.; et al. Contrast-Enhanced Ultrasound with Dispersion Analysis for the Localization of Prostate Cancer: Correlation with Radical Prostatectomy Specimens. World J. Urol. 2020, 38, 2811–2818. [Google Scholar] [CrossRef]
- Mannaerts, C.K.; Engelbrecht, M.R.W.; Postema, A.W.; van Kollenburg, R.A.A.; Hoeks, C.M.A.; Savci-Heijink, C.D.; van Sloun, R.J.G.; Wildeboer, R.R.; de Reijke, T.M.; Mischi, M.; et al. Detection of Clinically Significant Prostate Cancer in Biopsy-Naïve Men: Direct Comparison of Systematic Biopsy, Multiparametric MRI- and Contrast-Ultrasound-Dispersion Imaging-Targeted Biopsy. BJU Int. 2020, 126, 481–493. [Google Scholar] [CrossRef]
- Niessen, C.; Jung, E.M.; Beyer, L.; Pregler, B.; Dollinger, M.; Haimerl, M.; Scheer, F.; Stroszczynski, C.; Wiggermann, P. Percutaneous Irreversible Electroporation (IRE) of Prostate Cancer: Contrast-Enhanced Ultrasound (CEUS) Findings. Clin. Hemorheol. Microcirc. 2015, 61, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.M.; Engel, M.; Wiggermann, P.; Schicho, A.; Lerchbaumer, M.; Stroszczynski, C.; Fischer, T.; Wiesinger, I. Contrast Enhanced Ultrasound (CEUS) with Parametric Imaging after Irreversible Electroporation (IRE) of the Prostate to Assess the Success of Prostate Cancer Treatment. Clin. Hemorheol. Microcirc. 2021, 77, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Apfelbeck, M.; Chaloupka, M.; Schlenker, B.; Stief, C.G.; Clevert, D.A. Follow-up after Focal Therapy of the Prostate with High Intensity Focused Ultrasound (HIFU) Using Contrast Enhanced Ultrasound (CEUS) in Combination with MRI Image Fusion. Clin. Hemorheol. Microcirc. 2019, 73, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, F.; Martins, M.; Regusci, S.; Jichlinski, P.; Meuwly, J.Y.; Lucca, I.; Valerio, M. The Utility of Intraoperative Contrast-Enhanced Ultrasound in Detecting Residual Disease after Focal HIFU for Localized Prostate Cancer. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 846.e1–846.e7. [Google Scholar] [CrossRef] [PubMed]
- Moschouris, H.; Stamatiou, K.; Malagari, K.; Marmaridou, K.; Kladis-Kalentzis, K.; Kiltenis, M.; Papadogeorgopoulos, N.; Tsavari, A.; Manoloudaki, K. The Value of Contrast-Enhanced Ultrasonography in Detection of Prostatic Infarction after Prostatic Artery Embolization for the Treatment of Symptomatic Benign Prostatic Hyperplasia. Diagn. Interv. Radiol. 2019, 25, 134–143. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, Y.Q.; Zhu, Y.K.; Yao, X.H.; Qi, J. Factors Influencing the Degree of Enhancement of Prostate Cancer on Contrast-Enhanced Transrectal Ultrasonography: Correlation with Biopsy and Radical Prostatectomy Specimens. Br. J. Radiol. 2012, 85, e979–e986. [Google Scholar] [CrossRef]
- Baur, A.D.J.; Schwabe, J.; Rogasch, J.; Maxeiner, A.; Penzkofer, T.; Stephan, C.; Rudl, M.; Hamm, B.; Jung, E.M.; Fischer, T. A Direct Comparison of Contrast-Enhanced Ultrasound and Dynamic Contrast-Enhanced Magnetic Resonance Imaging for Prostate Cancer Detection and Prediction of Aggressiveness. Eur. Radiol. 2018, 28, 1949–1960. [Google Scholar] [CrossRef]
- Maxeiner, A.; Fischer, T.; Schwabe, J.; Baur, A.D.J.; Stephan, C.; Peters, R.; Slowinski, T.; von Laffert, M.; Marticorena Garcia, S.R.; Hamm, B.; et al. Contrast-Enhanced Ultrasound (CEUS) and Quantitative Perfusion Analysis in Patients with Suspicion for Prostate Cancer. Ultraschall Med. 2019, 40, 340–348. [Google Scholar] [CrossRef]
- Bercoff, J.; Chaffai, S.; Tanter, M.; Sandrin, L.; Catheline, S.; Fink, M.; Gennisson, J.L.; Meunier, M. In Vivo Breast Tumor Detection Using Transient Elastography. Ultrasound Med. Biol. 2003, 29, 1387–1396. [Google Scholar] [CrossRef]
- Bercoff, J.; Pernot, M.; Tanter, M.; Fink, M. Monitoring Thermally-Induced Lesions with Supersonic Shear Imaging. Ultrason. Imaging 2004, 26, 71–84. [Google Scholar] [CrossRef]
- Ahmad, S.; Cao, R.; Varghese, T.; Bidaut, L.; Nabi, G. Transrectal Quantitative Shear Wave Elastography in the Detection and Characterisation of Prostate Cancer. Surg. Endosc. 2013, 27, 3280–3287. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G.; Cosgrove, D.; Brock, M.; Cantisani, V.; Correas, J.M.; Postema, A.W.; Salomon, G.; Tsutsumi, M.; Xu, H.X.; Dietrich, C.F. WFUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography: Part 5. Prostate. Ultrasound Med. Biol. 2016, 43, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Boehm, K.; Salomon, G.; Beyer, B.; Schiffmann, J.; Simonis, K.; Graefen, M.; Budaeus, L. Shear Wave Elastography for Localization of Prostate Cancer Lesions and Assessment of Elasticity Thresholds: Implications for Targeted Biopsies and Active Surveillance Protocols. J. Urol. 2015, 193, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G.; Memo, R.; Schaub, C.R. Shear Wave Ultrasound Elastography of the Prostate: Initial Results. Ultrasound Q. 2012, 28, 13–20. [Google Scholar] [CrossRef] [PubMed]
- McNeal, J.E. The Zonal Anatomy of the Prostate. Prostate 1981, 2, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Rouvière, O.; Melodelima, C.; Hoang Dinh, A.; Bratan, F.; Pagnoux, G.; Sanzalone, T.; Crouzet, S.; Colombel, M.; Mège-Lechevallier, F.; Souchon, R. Stiffness of Benign and Malignant Prostate Tissue Measured by Shear-Wave Elastography: A Preliminary Study. Eur. Radiol. 2016, 27, 1858–1866. [Google Scholar] [CrossRef]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.H.; Cosgrove, D.; et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 1: Basic Principles and Terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef]
- Barr, R.G.; Nakashima, K.; Amy, D.; Cosgrove, D.; Farrokh, A.; Schafer, F.; Bamber, J.C.; Castera, L.; Choi, B.I.; Chou, Y.H.; et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 2: Breast. Ultrasound Med. Biol. 2015, 41, 1148–1160. [Google Scholar] [CrossRef]
- Ferraioli, G.; Filice, C.; Castera, L.; Choi, B.I.; Sporea, I.; Wilson, S.R.; Cosgrove, D.; Dietrich, C.F.; Amy, D.; Bamber, J.C.; et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 3: Liver. Ultrasound Med. Biol. 2015, 41, 1161–1179. [Google Scholar] [CrossRef]
- Cosgrove, D.; Barr, R.; Bojunga, J.; Cantisani, V.; Chammas, M.C.; Dighe, M.; Vinayak, S.; Xu, J.M.; Dietrich, C.F. WFUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography: Part 4. Thyroid. Ultrasound Med. Biol. 2017, 43, 4–26. [Google Scholar] [CrossRef]
- Correas, J.M.; Tissier, A.M.; Khairoune, A.; Vassiliu, V.; Méjean, A.; Hélénon, O.; Memo, R.; Barr, R.G. Prostate Cancer: Diagnostic Performance of Real-Time Shear-Wave Elastography. Radiology 2015, 275, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ruan, L.; Ren, W.; Dun, G.; Liu, J.; Zhang, Y.; Wan, Q. Stiffness of Prostate Gland Measured by Transrectal Real-Time Shear Wave Elastography for Detection of Prostate Cancer: A Feasibility Study. Br. J. Radiol. 2019, 92, 20180970. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Qiu, S.; Chang, T.; Jin, K.; Bao, Y.; Yang, L.; Wei, Q. The Role of Real-Time Elastography-Targeted Biopsy in the Detection and Diagnosis of Prostate Cancer: A Systematic Review and Meta-Analysis. Medicine 2018, 97, e0220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, P.; Yin, B.; Fei, X.; Xu, X.W.; Song, Y.S. Transrectal Shear Wave Elastography Combined with Transition Zone Biopsy for Detecting Prostate Cancer. Zhonghua Nan Ke Xue 2015, 21, 610–614. [Google Scholar]
- Shoji, S.; Hashimoto, A.; Nakamura, T.; Hiraiwa, S.; Sato, H.; Sato, Y.; Tajiri, T.; Miyajima, A. Novel Application of Three-Dimensional Shear Wave Elastography in the Detection of Clinically Significant Prostate Cancer. Biomed. Rep. 2018, 8, 373–377. [Google Scholar] [CrossRef]
- Koh, J.; Jung, D.C.; Oh, Y.T.; Yoo, M.G.; Noh, S.; Han, K.H.; Rha, K.H.; Choi, Y.D.; Hong, S.J. Additional Targeted Biopsy in Clinically Suspected Prostate Cancer: Prospective Randomized Comparison between Contrast-Enhanced Ultrasound and Sonoelastography Guidance. Ultrasound Med. Biol. 2015, 41, 2836–2841. [Google Scholar] [CrossRef]
- Sidhu, P.S. Multiparametric Ultrasound (MPUS) Imaging: Terminology Describing the Many Aspects of Ultrasonography. Ultraschall Der Med.-Eur. J. Ultrasound 2015, 36, 315–317. [Google Scholar] [CrossRef]
- Postema, A.; Mischi, M.; de la Rosette, J.; Wijkstra, H. Multiparametric Ultrasound in the Detection of Prostate Cancer: A Systematic Review. World J. Urol. 2015, 33, 1651–1659. [Google Scholar] [CrossRef]
- Wildeboer, R.R.; Panfilova, A.P.; Mischi, M.; Wijkstra, H. Imaging Modalities in Focal Therapy: Multiparametric Ultrasound. Arch. Esp. De Urol. 2016, 69, 281–290. [Google Scholar]
- Tay, K.J.; Schulman, A.A.; Sze, C.; Tsivian, E.; Polascik, T.J. New Advances in Focal Therapy for Early Stage Prostate Cancer. Expert Rev. Anticancer Ther. 2017, 17, 737–743. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, J.; Luo, Y.; Wang, Y.; Wu, M.; Memmott, B.; Gao, J. Diagnostic Performance of Multiparametric Transrectal Ultrasound in Localized Prostate Cancer: A Comparative Study with Magnetic Resonance Imaging. J. Ultrasound Med. 2019, 38, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Pepe, P.; Pepe, L.; Panella, P.; Pennisi, M. Can Multiparametric Ultrasound Improve Cognitive MRI/TRUS Fusion Prostate Biopsy. Arch. Ital. Di Urol. E Androl. 2020, 92, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Drudi, F.M.; Cantisani, V.; Angelini, F.; Ciccariello, M.; Messineo, D.; Ettorre, E.; Liberatore, M.; Scialpi, M. Multiparametric MRI versus Multiparametric US in the Detection of Prostate Cancer. Anticancer Res. 2019, 39, 3101–3110. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Ye, X.; Zhang, L.; Sun, Y.; Ni, Z.; Liu, H.; Xu, J.; Dong, F. Evaluation of the Performance of the Ultrasound (US) Elastographic Q-Analysis Score Combined With the Prostate Imaging Reporting and Data System for Malignancy Risk Stratification in Prostate Nodules Based on Transrectal US–Magnetic Resonance Imaging Fusion Imaging. J. Ultrasound Med. 2019, 38, 2991–2998. [Google Scholar] [CrossRef]
- Ding, Z.; Song, D.; Wu, H.; Tian, H.; Ye, X.; Liang, W.; Xu, J.; Dong, F. Development and Validation of a Nomogram Based on Multiparametric Magnetic Resonance Imaging and Elastography-Derived Data for the Stratification of Patients with Prostate Cancer. Quant. Imaging Med. Surg. 2021, 11, 3252–3262. [Google Scholar] [CrossRef]
- Lanza, G.M.; Wickline, S.A. Targeted Ultrasonic Contrast Agents for Molecular Imaging and Therapy. Curr. Probl. Cardiol. 2003, 28, 625–653. [Google Scholar] [CrossRef]
- Baier, J.; Rix, A.; Kiessling, F. Molecular Ultrasound Imaging. In Recent Results in Cancer Research; Springer: New York, NY, USA, 2020; Volume 216. [Google Scholar]
- Marshall, D.; Pedley, R.B.; Boden, J.A.; Boden, R.; Melton, R.G.; Begent, R.H.J. Polyethylene Glycol Modification of a Galactosylated Streptavidin Clearing Agent: Effects on Immunogenicity and Clearance of a Biotinylated Anti-Tumour Antibody. Br. J. Cancer 1996, 73, 565–572. [Google Scholar] [CrossRef][Green Version]
- Tardy, I.; Pochon, S.; Theraulaz, M.; Emmel, P.; Passantino, L.; Tranquart, F.; Schneider, M. Ultrasound Molecular Imaging of VEGFR2 in a Rat Prostate Tumor Model Using BR55. Investig. Radiol. 2010, 45, 573–578. [Google Scholar] [CrossRef]
- Smeenge, M.; Tranquart, F.; Mannaerts, C.K.; de Reijke, T.M.; van de Vijver, M.J.; Laguna, M.P.; Pochon, S.; de La Rosette, J.J.M.C.H.; Wijkstra, H. First-in-Human Ultrasound Molecular Imaging with a VEGFR2-Specific Ultrasound Molecular Contrast Agent (BR55) in Prostate Cancer a Safety and Feasibility Pilot Study. Investig. Radiol. 2017, 52, 419–427. [Google Scholar] [CrossRef]
- Warram, J.M.; Sorace, A.G.; Saini, R.; Umphrey, H.R.; Zinn, K.R.; Hoyt, K. A Triple-Targeted Ultrasound Contrast Agent Provides Improved Localization to Tumor Vasculature. J. Ultrasound Med. 2011, 30, 921–931. [Google Scholar] [CrossRef]
- Anderson, C.R.; Hu, X.; Zhang, H.; Tlaxca, J.; Declèves, A.E.; Houghtaling, R.; Sharma, K.; Lawrence, M.; Ferrara, K.W.; Rychak, J.J. Ultrasound Molecular Imaging of Tumor Angiogenesis with an Integrin Targeted Microbubble Contrast Agent. Investig. Radiol. 2011, 46, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, N.; Ren, Y.; Foygel, K.; Rosenberg, J.; Willmann, J.K. Tumor Angiogenic Marker Expression Levels during Tumor Growth: Longitudinal Assessment with Molecularly Targeted Microbubbles and US Imaging. Radiology 2011, 258, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.K.; Lutz, A.M.; Paulmurugan, R.; Patel, M.R.; Chu, P.; Rosenberg, J.; Gambhir, S.S. Dual-Targeted Contrast Agent for US Assessment of Tumor Angiogenesis in Vivo. Radiology 2008, 248, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.X.; Wang, W.P.; Wen, J.X.; Lin, L.W.; Exner, A.A.; Guan, P.S.; Chen, X.J. Dual-Targeted Microbubbles Specific to Integrin AVβ3 and Vascular Endothelial Growth Factor Receptor 2 for Ultrasonography Evaluation of Tumor Angiogenesis. Ultrasound Med. Biol. 2018, 44, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Palmowski, M.; Peschke, P.; Huppert, J.; Hauff, P.; Reinhardt, M.; Maurer, M.; Karger, C.P.; Scholz, M.; Semmler, W.; Huber, P.E.; et al. Molecular Ultrasound Imaging of Early Vascular Response in Prostate Tumors Irradiated with Carbon Ions. Neoplasia 2009, 11, 856–863. [Google Scholar] [CrossRef]
- Turco, S.; Frinking, P.; Wildeboer, R.; Arditi, M.; Wijkstra, H.; Lindner, J.R.; Mischi, M. Contrast-Enhanced Ultrasound Quantification: From Kinetic Modeling to Machine Learning. Ultrasound Med. Biol. 2020, 46, 518–543. [Google Scholar] [CrossRef]
- Wildeboer, R.R.; Postema, A.W.; Demi, L.; Kuenen, M.P.J.; Wijkstra, H.; Mischi, M. Multiparametric Dynamic Contrast-Enhanced Ultrasound Imaging of Prostate Cancer. Eur. Radiol. 2016, 27, 3226–3234. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, F.; Zhou, X.; Guo, Y.; Tang, F.; Ren, F.; Guo, J.; Ji, S. A Deep Learning Approach for Targeted Contrast-Enhanced Ultrasound Based Prostate Cancer Detection. IEEE/ACM Trans. Comput. Biol. Bioinform. 2018, 16, 1794–1801. [Google Scholar] [CrossRef]
- Wildeboer, R.R.; Mannaerts, C.K.; van Sloun, R.J.G.; Budäus, L.; Tilki, D.; Wijkstra, H.; Salomon, G.; Mischi, M. Automated Multiparametric Localization of Prostate Cancer Based on B-Mode, Shear-Wave Elastography, and Contrast-Enhanced Ultrasound Radiomics. Eur. Radiol. 2019, 30, 806–815. [Google Scholar] [CrossRef]
- Dias, A.B.; O’Brien, C.; Correas, J.-M.; Ghai, S. Multiparametric Ultrasound and Micro-Ultrasound in Prostate Cancer: A Comprehensive Review. Br. J. Radiol. 2022, 95, 20210633. [Google Scholar] [CrossRef]
- Ström, P.; Kartasalo, K.; Olsson, H.; Solorzano, L.; Delahunt, B.; Berney, D.M.; Bostwick, D.G.; Evans, A.J.; Grignon, D.J.; Humphrey, P.A.; et al. Artificial Intelligence for Diagnosis and Grading of Prostate Cancer in Biopsies: A Population-Based, Diagnostic Study. Lancet Oncol. 2020, 21, 222–232. [Google Scholar] [CrossRef]
- Ferro, M.; de Cobelli, O.; Vartolomei, M.D.; Lucarelli, G.; Crocetto, F.; Barone, B.; Sciarra, A.; del Giudice, F.; Muto, M.; Maggi, M.; et al. Prostate Cancer Radiogenomics—from Imaging to Molecular Characterization. Int. J. Mol. Sci. 2021, 22, 9971. [Google Scholar] [CrossRef] [PubMed]
| Study Authors | Year | Number of Patients | FBx CDR | SBx CDR | FBx and SBx Combined CDR |
|---|---|---|---|---|---|
| Kasivisvanathan et al. [11] | 2018 | 500 | 38% | 26% | - |
| Bass et al. * [30] | 2021 | 8456 | 83% * | 63% * | - |
| Porpiglia et al. [33] | 2017 | 212 | 60.5% | 29.5% | - |
| Baccaglini et al. [34] | 2020 | 741 | 31% | 30% | - |
| Ahdoot et al. [26] | 2020 | 2103 | 52% | 53% | 62% |
| Filson et al. [40] | 2020 | 825 | 28% | 24% | 35% |
| Elkhoury et al. [41] | 2019 | 300 | 62% | 60% | 70% |
| Rouviere et al. [42] | 2019 | 251 | 32% | 29% | 37% |
| Risk Group | Grade Group | Gleason Score | Gleason Pattern |
|---|---|---|---|
| Low/Very Low | 1 | ≤6 | ≤3 + 3 |
| Intermediate (Favorable/Unfavorable) | 2 3 | 7 7 | 3 + 4 4 + 3 |
| High/Very High | 4 5 | 8 9 or 10 | 4 + 4, 3 + 5, 5 + 3 5 + 4, 5 + 4 or 5 + 5 |
| Study Authors | Year | Number of Patients (MicroUS-Bx/FBx) | Sensitivity MicroUS-Bx/FBx | Specificity MicroUS/mpMRI | CDR MicroUS-Bx/FBx |
|---|---|---|---|---|---|
| Astobieta Odriozola et al. [53] | 2018 | 35 | 95%/57% | 40%/91% | 57%/34% |
| Eure et al. [54] | 2019 | 9 | 89%/56% | x/x | 89%/56% |
| Abouassaly et al. [52] | 2020 | 67/19 | 95%/80% | x/x | 30%/42% |
| Claros et al. [56] | 2020 | 47/222 | x/x | x/x | 38%/23% |
| Klotz et al. [60] | 2020 | 1040 | 94%/90% | 22%/23% | 37%/36% |
| Cornud et al. [58] | 2020 | 118 | 100%/94% | 23%/x | 51.4%/46% |
| Rodriguez Socarras et al. [57] | 2020 | 194 | 99%/86% | 29.3%/x | 41%/36% |
| Lughezzani et al. [61] | 2021 | 320 | 87%/87% | 26%/x | 32%/32% |
| Wiemer et al. [59] | 2021 | 159 | 95%/71% | 15%/x | 47%/35% |
| Study Authors | Year | Number of Patients | CEUS-Bx Sensitivity | SBx Sensitivity | CEUS-Bx CDR | SBx CDR |
|---|---|---|---|---|---|---|
| Mitterberger et al. [74] | 2010 | 1776 | 85% | 73% | 27% | 23% |
| Yunkai et al. [17] | 2019 | 1024 | 90% | 79% | 29% | 25% |
| Lu et al. [75] | 2021 | 186 | 91% | 100% | 58% | 63% |
| Imaging Technique | TRUS | MicroUS | SWE | CEUS | |
|---|---|---|---|---|---|
| Variable | |||||
| Wave Type | ultrasound wave | ultrasound wave | shear wave | ultrasound wave | |
| Wave Frequency | 5–12 MHz | 29 MHz | 100–600 Hz | 5–12 MHz | |
| Wave Penetration Depth | 8–12 cm | 6 cm | 3–4 cm | 8–12 cm | |
| Main Measured Parameter | wave impedance [kg/(m2s)] | wave impedance [kg/(m2s)] | Young’s modulus (stiffness) [kPa] | perfusion intensity [mL/g] | |
| Guidelines for Prostate Image Interpretation | NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer | PRI-MUS protocol | WFUMB guidelines | - | |
| Contrast Agent | - | - | - | microbubbles | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurwin, A.; Kowalczyk, K.; Knecht-Gurwin, K.; Stelmach, P.; Nowak, Ł.; Krajewski, W.; Szydełko, T.; Małkiewicz, B. Alternatives for MRI in Prostate Cancer Diagnostics—Review of Current Ultrasound-Based Techniques. Cancers 2022, 14, 1859. https://doi.org/10.3390/cancers14081859
Gurwin A, Kowalczyk K, Knecht-Gurwin K, Stelmach P, Nowak Ł, Krajewski W, Szydełko T, Małkiewicz B. Alternatives for MRI in Prostate Cancer Diagnostics—Review of Current Ultrasound-Based Techniques. Cancers. 2022; 14(8):1859. https://doi.org/10.3390/cancers14081859
Chicago/Turabian StyleGurwin, Adam, Kamil Kowalczyk, Klaudia Knecht-Gurwin, Paweł Stelmach, Łukasz Nowak, Wojciech Krajewski, Tomasz Szydełko, and Bartosz Małkiewicz. 2022. "Alternatives for MRI in Prostate Cancer Diagnostics—Review of Current Ultrasound-Based Techniques" Cancers 14, no. 8: 1859. https://doi.org/10.3390/cancers14081859
APA StyleGurwin, A., Kowalczyk, K., Knecht-Gurwin, K., Stelmach, P., Nowak, Ł., Krajewski, W., Szydełko, T., & Małkiewicz, B. (2022). Alternatives for MRI in Prostate Cancer Diagnostics—Review of Current Ultrasound-Based Techniques. Cancers, 14(8), 1859. https://doi.org/10.3390/cancers14081859








