Inhibiting Endothelin Receptors with Macitentan Strengthens the Bone Protective Action of RANKL Inhibition and Reduces Metastatic Dissemination in Osteosarcoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. RNA Isolation, Reverse Transcription, and Quantitative PCR
2.3. In Vivo Mouse Models of Osteosarcoma
2.4. IK22-5 RANKL Blocking Antibody Injections
2.5. Macitentan Treatment
2.6. Ifosfamide Treatment
2.7. Micro-CT Analysis
2.8. Histology
2.9. Statistics
3. Results
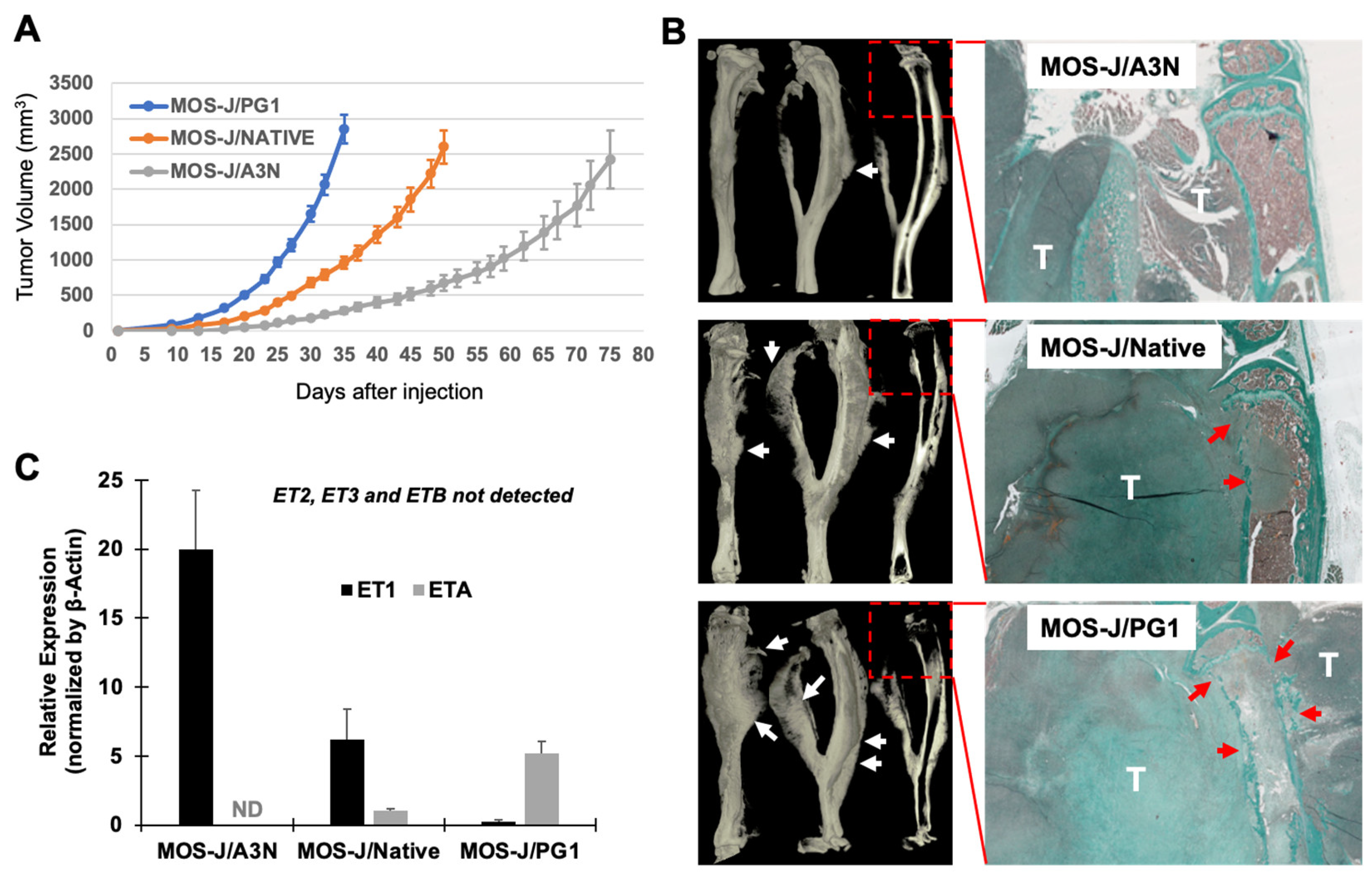
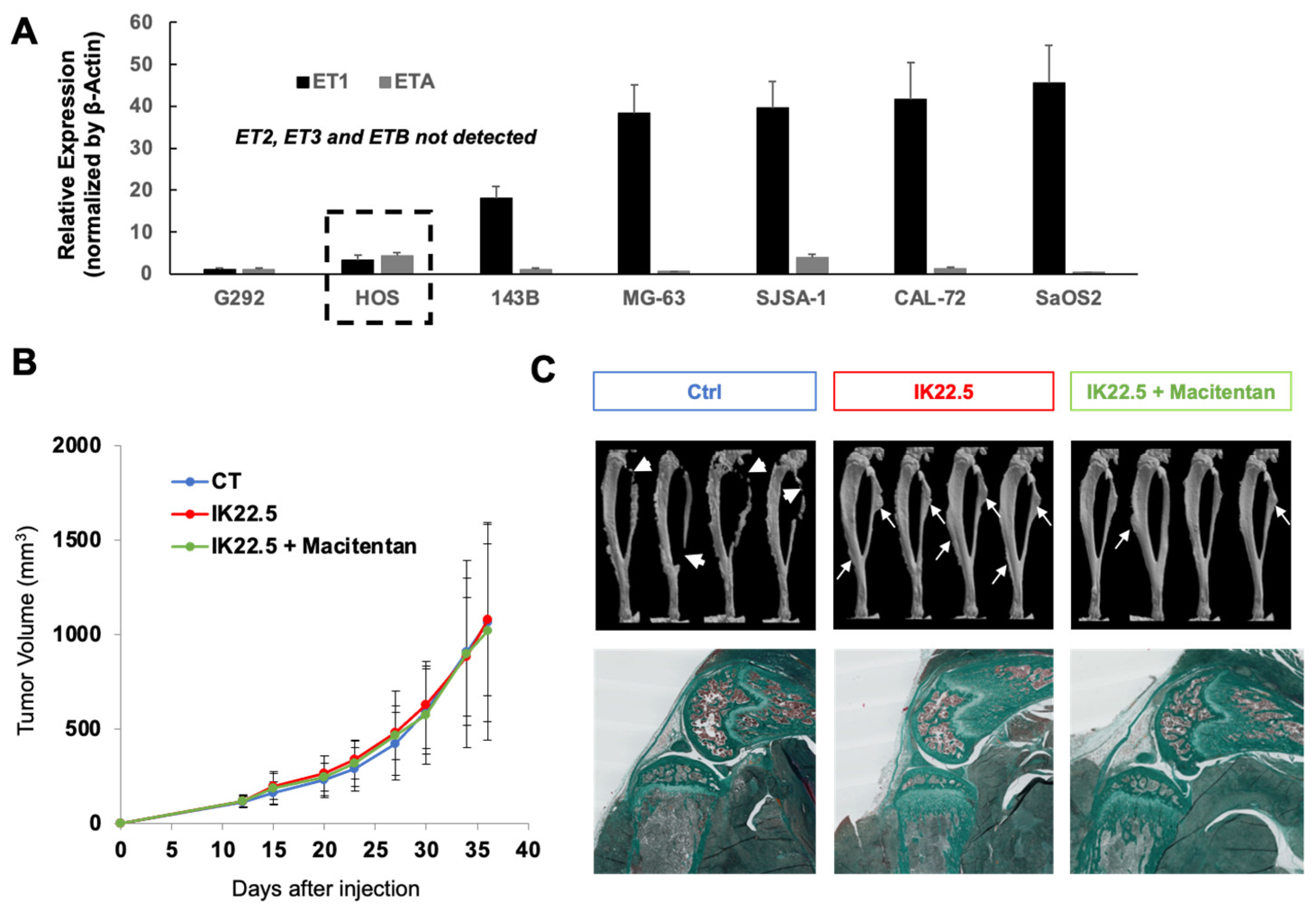
3.1. Correlation between the Aggressivity of the MOS-J Osteosarcoma Model and the Relative Expression of ET1 and ETA
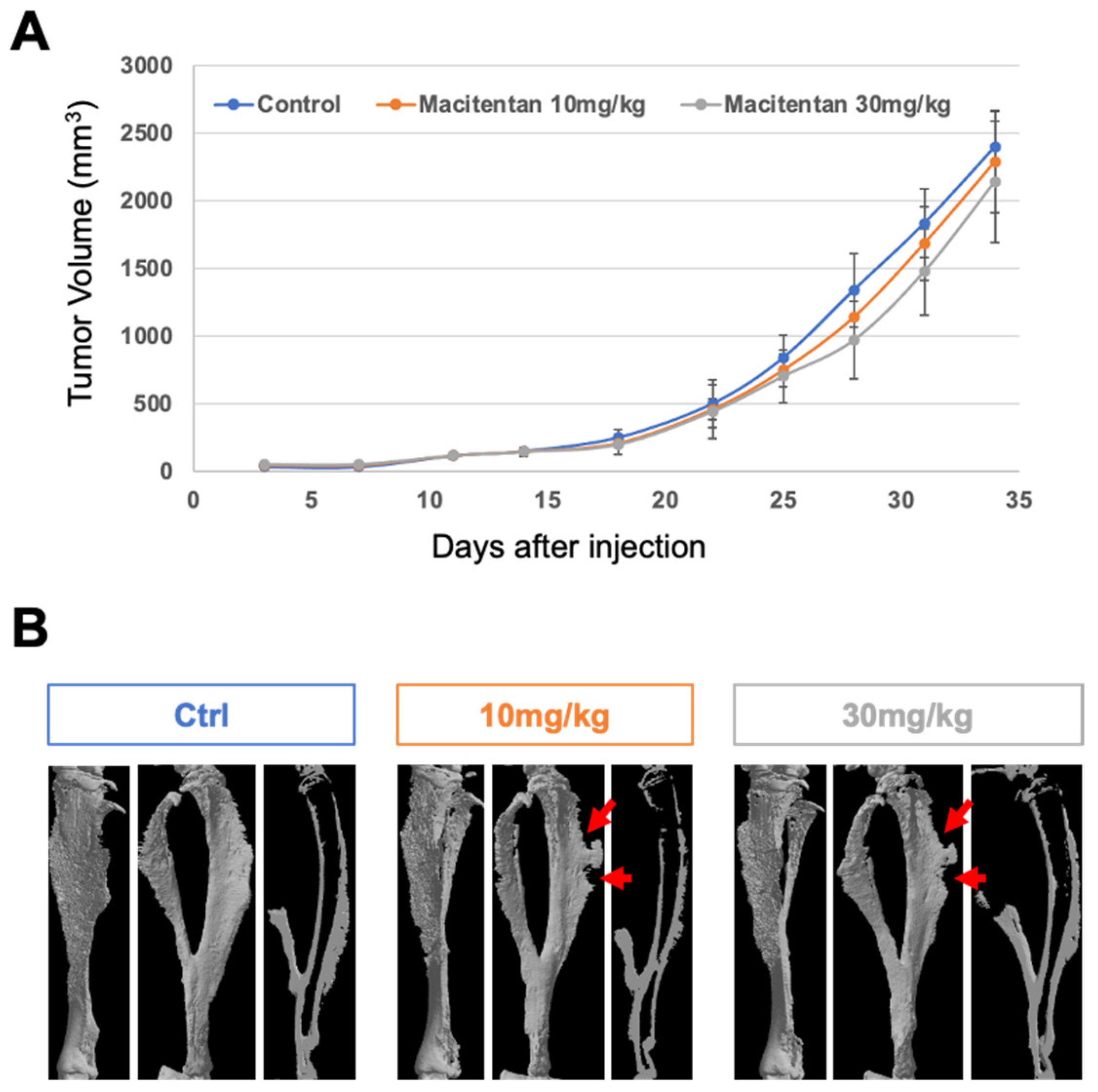
3.2. The Impact of Treatment with Macitentan on the Aggressive Osteosarcoma Model Increased When Associated with RANKL Blockade
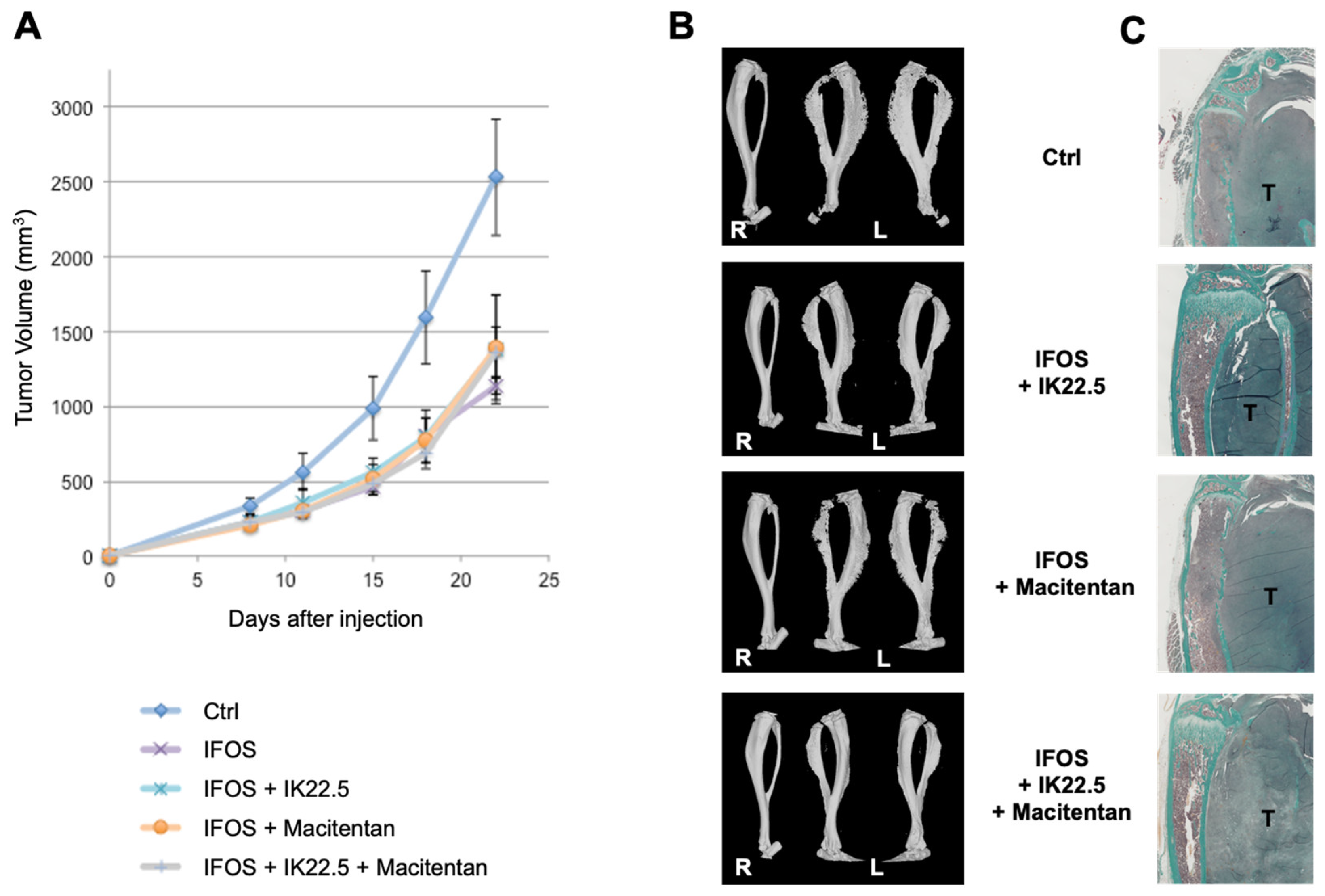
3.3. Macitentan Treatment and RANKL Blockade Alone or Combined Did Not Alter the Response to Conventional Chemotherapy
3.4. Macitentan at a High Dose Reduced the Number and Size of Lung Metastases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smeland, S.; Bielack, S.S.; Whelan, J.; Bernstein, M.; Hogendoorn, P.; Krailo, M.D.; Gorlick, R.; Janeway, K.A.; Ingleby, F.C.; Anninga, J.; et al. Survival and prognosis with osteosarcoma: Outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur. J. Cancer 2019, 109, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Marina, N.M.; Smeland, S.; Bielack, S.S.; Bernstein, M.; Jovic, G.; Krailo, M.D.; Hook, J.M.; Arndt, C.; van den Berg, H.; Brennan, B.; et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): An open-label, international, randomised controlled trial. Lancet Oncol. 2016, 17, 1396–1408. [Google Scholar] [CrossRef]
- Grünewald, T.G.; Alonso, M.; Avnet, S.; Banito, A.; Burdach, S.; Cidre-Aranaz, F.; Di Pompo, G.; Distel, M.; Dorado-Garcia, H.; Garcia-Castro, J.; et al. Sarcoma treatment in the era of molecular medicine. EMBO Mol. Med. 2020, 12, e11131. [Google Scholar] [CrossRef] [PubMed]
- Heymann, M.-F.; Lezot, F.; Heymann, D. Bisphosphonates in common pediatric and adult bone sarcomas. Bone 2020, 139, 115523. [Google Scholar] [CrossRef] [PubMed]
- Piperno-Neumann, S.; Le Deley, M.-C.; Rédini, F.; Pacquement, H.; Marec-Bérard, P.; Petit, P.; Brisse, H.J.; Lervat, C.; Gentet, J.-C.; Entz-Werlé, N.; et al. Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 1070–1080. [Google Scholar] [CrossRef]
- Navet, B.; Ando, K.; Vargas-Franco, J.W.; Brion, R.; Amiaud, J.; Mori, K.; Yagita, H.; Mueller, C.G.; Verrecchia, F.; Dumars, C.; et al. The Intrinsic and Extrinsic Implications of RANKL/RANK Signaling in Osteosarcoma: From Tumor Initiation to Lung Metastases. Cancers 2018, 10, 398. [Google Scholar] [CrossRef]
- Lézot, F.; Chesneau, J.; Navet, B.; Gobin, B.; Amiaud, J.; Choi, Y.; Yagita, H.; Castaneda, B.; Berdal, A.; Mueller, C.G.; et al. Skeletal consequences of RANKL-blocking antibody (IK22-5) injections during growth: Mouse strain disparities and synergic effect with zoledronic acid. Bone 2015, 73, 51–59. [Google Scholar] [CrossRef]
- Battaglia, S.; Dumoucel, S.; Chesneau, J.; Heymann, M.-F.; Picarda, G.; Gouin, F.; Corradini, N.; Heymann, D.; Redini, F. Impact of oncopediatric dosing regimen of zoledronic acid on bone growth: Preclinical studies and case report of an osteosarcoma pediatric patient. J. Bone Miner. Res. 2011, 26, 2439–2451. [Google Scholar] [CrossRef]
- Lézot, F.; Chesneau, J.; Battaglia, S.; Brion, R.; Castañeda, B.; Farges, J.-C.; Heymann, D.; Rédini, F. Preclinical evidence of potential craniofacial adverse effect of zoledronic acid in pediatric patients with bone malignancies. Bone 2014, 68, 146–152. [Google Scholar] [CrossRef]
- Vargas-Franco, J.W.; Castaneda, B.; Gama, A.; Mueller, C.G.; Heymann, D.; Rédini, F.; Lézot, F. Genetically-achieved disturbances to the expression levels of TNFSF11 receptors modulate the effects of zoledronic acid on growing mouse skeletons. Biochem. Pharmacol. 2019, 168, 133–148. [Google Scholar] [CrossRef]
- Isawa, M.; Karakawa, A.; Sakai, N.; Nishina, S.; Kuritani, M.; Chatani, M.; Negishi-Koga, T.; Sato, M.; Inoue, M.; Shimada, Y.; et al. Biological Effects of Anti-RANKL Antibody and Zoledronic Acid on Growth and Tooth Eruption in Growing Mice. Sci. Rep. 2019, 9, 19895. [Google Scholar] [CrossRef] [PubMed]
- Gama, A.; Perea, L.; Yepes, C.; Betancur, J.J.; Vargas, J.; Amiaud, J.; Babajko, S.; Lezot, F.; Castaneda, B. Effets de l’inhibition post-natale de RANKL sur l’éruption et la formation radiculaire des molaires de souris C57BL/6. L’Orthodontie Française 2019, 90, 55–63. [Google Scholar] [CrossRef]
- Gama, A.; Maman, L.; Vargas-Franco, J.W.; Omar, R.; Royer, B.B.-L.; Yagita, H.; Babajko, S.; Berdal, A.; Acevedo, A.C.; Heymann, D.; et al. Primary Retention of Molars and RANKL Signaling Alteration during Craniofacial Growth. J. Clin. Med. 2020, 9, 898. [Google Scholar] [CrossRef]
- Stark, Z.; Savarirayan, R. Osteopetrosis. Orphanet J. Rare Dis. 2009, 4, 5. [Google Scholar] [CrossRef]
- Kristianto, J.; Johnson, M.G.; Afzal, R.; Blank, R.D. Endothelin Signaling in Bone. Endocrinol. Metab. Clin. N. Am. 2016, 46, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.G.; Konicke, K.; Kristianto, J.; Gustavson, A.; Garbo, R.; Wang, X.; Yuan, B.; Blank, R.D. Endothelin signaling regulates mineralization and posttranscriptionally regulates SOST in TMOb cells via miR 126-3p. Physiol. Rep. 2017, 5, e13088. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.-W.; Wang, X.; Jiang, X.-Q.; Xu, L.-Q.; Pan, H.-Y. In vivo and in vitro study of osteogenic potency of endothelin-1 on bone marrow-derived mesenchymal stem cells. Exp. Cell Res. 2017, 357, 25–32. [Google Scholar] [CrossRef]
- Lee, M.-S.; Wang, J.; Yuan, H.; Jiao, H.; Tsai, T.-L.; Squire, M.W.; Li, W.-J. Endothelin-1 differentially directs lineage specification of adipose- and bone marrow–derived mesenchymal stem cells. FASEB J. 2018, 33, 996–1007. [Google Scholar] [CrossRef]
- Selej, M.; Romero, A.J.; Channick, R.N.; Clozel, M. Development of macitentan for the treatment of pulmonary arterial hypertension. Ann. N. Y. Acad. Sci. 2015, 1358, 68–81. [Google Scholar] [CrossRef]
- Davenport, A.P.; Hyndman, K.A.; Dhaun, N.; Southan, C.; Kohan, D.E.; Pollock, J.S.; Pollock, D.M.; Webb, D.J.; Maguire, J.J. Endothelin. Pharmacol. Rev. 2016, 68, 357–418. [Google Scholar] [CrossRef]
- Joliat, M.J.; Umeda, S.; Lyons, B.L.; Lynes, M.A.; Shultz, L.D. Establishment and characterization of a new osteogenic cell line (MOS-J) from a spontaneous C57BL/6J mouse osteosarcoma. Vivo 2002, 16, 223–228. [Google Scholar]
- Navet, B.; Vargas-Franco, J.W.; Gama, A.; Amiaud, J.; Choi, Y.; Yagita, H.; Mueller, C.G.; Rédini, F.; Heymann, D.; Castaneda, B.; et al. Maternal RANKL Reduces the Osteopetrotic Phenotype of Null Mutant Mouse Pups. J. Clin. Med. 2018, 7, 426. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Odgren, P.R.; Kim, D.-K.; Marks, S.C.; Choi, Y. Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc. Natl. Acad. Sci. USA 2000, 97, 10905–10910. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, S.; Nakajima, A.; Ikeda, K.; Aoki, K.; Ohya, K.; Akiba, H.; Yagita, H.; Okumura, K. Amelioration of bone loss in collagen-induced arthritis by neutralizing anti-RANKL monoclonal antibody. Biochem. Biophys. Res. Commun. 2006, 347, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Renema, N.; Lezot, F.; Ory, B.; Walkley, C.R.; Grigoriadis, A.E.; Heymann, D. Small animal models for the study of bone sarcoma pathogenesis:characteristics, therapeutic interests and limitations. J. Bone Oncol. 2018, 12, 7–13. [Google Scholar] [CrossRef]
- Mödder, U.I.; Oursler, M.J.; Khosla, S.; Monroe, D.G. Wnt10b activates the wnt, notch, and NFκB pathways in u2os osteosarcoma cells. J. Cell. Biochem. 2011, 112, 1392–1402. [Google Scholar] [CrossRef]
- Zhao, Y.; Liao, Q.; Zhu, Y.; Long, H. Endothelin-1 Promotes Osteosarcoma Cell Invasion and Survival against Cisplatin-induced Apoptosis. Clin. Orthop. Relat. Res. 2011, 469, 3190–3199. [Google Scholar] [CrossRef]
- Neumann, Z.; Pondenis, H.; Masyr, A.; Byrum, M.; Wycislo, K.; Fan, T. The Association of Endothelin-1 Signaling with Bone Alkaline Phosphatase Expression and Protumorigenic Activities in Canine Osteosarcoma. J. Veter-Intern. Med. 2015, 29, 1584–1594. [Google Scholar] [CrossRef]
- Sakurai, T.; Morimoto, H.; Kasuya, Y.; Takuwa, Y.; Nakauchi, H.; Masaki, T.; Goto, K. Level of ETB receptor mRNA is down-regulated by endothelins through decreasing the intracellular stability of mRNA molecules. Biochem. Biophys. Res. Commun. 1992, 186, 342–347. [Google Scholar] [CrossRef]
- Nambi, P.; Wu, H.L.; Lipshutz, D.; Prabhakar, U. Identification and characterization of endothelin receptors on rat osteoblastic osteosarcoma cells: Down-regulation by 1,25-dihydroxy-vitamin D3. Mol. Pharmacol. 1995, 47, 266–271. [Google Scholar]
- Asada, S.; Kasuya, Y.; Sakurai, T.; Masaki, T.; Goto, K. Endothelin-1-induced downregulation of ETB receptor mRNA: Participation of cAMP. J. Cardiovasc. Pharmacol. 1995, 26, S272–S275. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Stern, P.H. EndothelinB receptor activation enhances parathyroid hormone-induced calcium signals in UMR-106 cells. J. Bone Miner. Res. 2009, 10, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Semler, D.E.; Ohlstein, E.H.; Nambi, P.; Slater, C.; Stern, P.H. Endothelin-1-evoked calcium transients in UMR-106 osteoblastic osteosarcoma cells are mediated through endothelin-A and endothelin-B receptors. J. Pharmacol. Exp. Ther. 1995, 272, 1052–1058. [Google Scholar] [PubMed]
- Suzuki, A.; Shinoda, J.; Watanabe-Tomita, Y.; Ozaki, N.; Oiso, Y.; Kozawa, O. ETA receptor mediates the signaling of endothelin-1 in osteoblast-like cells. Bone 1997, 21, 143–146. [Google Scholar] [CrossRef]
- Someya, A.; Yuyama, H.; Fujimori, A.; Ukai, M.; Fukushima, S.; Sasamata, M. Effect of YM598, a selective endothelin ETA receptor antagonist, on endothelin-1-induced bone formation. Eur. J. Pharmacol. 2006, 543, 14–20. [Google Scholar] [CrossRef]
- Clines, G.A.; Mohammad, K.S.; Bao, Y.; Stephens, O.W.; Suva, L.J.; Shaughnessy, J.D., Jr.; Fox, J.W.; Chirgwin, J.M.; Guise, T.A. Dickkopf Homolog 1 Mediates Endothelin-1-Stimulated New Bone Formation. Mol. Endocrinol. 2007, 21, 486–498. [Google Scholar] [CrossRef]
- Sin, A.; Tang, W.; Wen, C.; Chung, S.; Chiu, K. The emerging role of endothelin-1 in the pathogenesis of subchondral bone disturbance and osteoarthritis. Osteoarthr. Cartil. 2014, 23, 516–524. [Google Scholar] [CrossRef]
- Shioide, M.; Noda, M. Endothelin modulates osteopontin and osteocalcin messenger ribonucleic acid expression in rat osteoblastic osteosarcoma cells. J. Cell. Biochem. 1993, 53, 176–180. [Google Scholar] [CrossRef]
- Clines, G.A.; Mohammad, K.S.; Grunda, J.M.; Clines, K.L.; Niewolna, M.; McKenna, C.R.; McKibbin, C.R.; Yanagisawa, M.; Suva, L.J.; Chirgwin, J.M.; et al. Regulation of postnatal trabecular bone formation by the osteoblast endothelin A receptor. J. Bone Miner. Res. 2011, 26, 2523–2536. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Yang, L.; Li, Z.; Ogata, Y. Endothelin-1 regulates rat bone sialoprotein gene transcription. J. Oral Sci. 2010, 52, 221–229. [Google Scholar] [CrossRef][Green Version]
- Marion, A.; Dieudonné, F.-X.; Patiño-Garcia, A.; Lecanda, F.; Marie, P.J.; Modrowski, D. Calpain-6 is an endothelin-1 signaling dependent protective factor in chemoresistant osteosarcoma. Int. J. Cancer 2011, 130, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wu, Y.; Peng, D. Astrocyte elevated gene-1 regulates osteosarcoma cell invasion and chemoresistance via endothelin-1/endothelin A receptor signaling. Oncol. Lett. 2012, 5, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, B.; Wang, M.; Ni, J. Endothelin-1 gene polymorphisms and risk of chemoresistant pediatric osteosarcoma. Pediatr. Blood Cancer 2013, 61, 612–617. [Google Scholar] [CrossRef]
- Liu, B.; Wu, Y.; Zhou, Y.; Peng, D. Endothelin A Receptor Antagonism Enhances Inhibitory Effects of Anti-Ganglioside GD2 Monoclonal Antibody on Invasiveness and Viability of Human Osteosarcoma Cells. PLoS ONE 2014, 9, e93576. [Google Scholar] [CrossRef] [PubMed]
- Felx, M.; Guyot, M.-C.; Isler, M.; Turcotte, R.E.; Doyon, J.; Khatib, A.-M.; Leclerc, S.; Moreau, A.; Moldovan, F. Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving the transcription factor NF-κB in human osteosarcoma. Clin. Sci. 2006, 110, 645–654. [Google Scholar] [CrossRef]
- Zang, X.; Zhou, Y.; Huang, Z.; Zhang, C. Endothelin-1 Single Nucleotide Polymorphisms and Risk of Pulmonary Metastatic Osteosarcoma. PLoS ONE 2013, 8, e73349. [Google Scholar] [CrossRef]
- Liao, Q.; Li, Y.; Li, K.; Zhong, D.; Weng, X.; Mi, M. Knockdown of endothelin A receptor expression inhibits osteosarcoma pulmonary metastasis in an orthotopic xenograft mouse model. Mol. Med. Rep. 2012, 5, 1391–1395. [Google Scholar] [CrossRef]


| Mouse and Human | ||
| Primers | Sequences | Amplicon |
| ET1-Fw ET1-Rv | 5′-ACT TCT GCC ACC TGG ACA TC-3′ 5′-CCA GCA CTT CTT GTC TTT TTG G-3′ | 142 pb |
| ETA-Fw ETA-Rv | 5′-TAT TTT GTG AGC AAG AAA TT-3′ 5′-GGG GAC CGA GGT CAT-3′ | 55 pb |
| ETB-Fw ETB-Rv | 5′-GGT CCC AAT ATC TTG ATC G-3′ 5′-CAA CAG CTC GAT ATC TGT CA-3′ | 171 pb |
| Mouse | ||
| Primers | Sequences | Amplicon |
| ET2-Fw ET2-Rv | 5′-CCT GGC TTG ACA AGG AAT GT-3′ 5′-CTT CGA TGG CAG AAG GTA GC-3′ | 181 pb |
| ET3-Fw ET3-Rv | 5′-CCC TGG TGA GAG GAT TGT GT-3′ 5′-CTG GGA GCT TTC TGG AAC TG-3′ | 295 pb |
| βACT-Fw βACT -Rv | 5′-CTA AGG CCA ACC GTG AAA AG-3′ 5′-ACC AGA GGC ATA CAG GGA CA-3′ | 140 pb |
| Human | ||
| Primers | Sequences | Amplicon |
| ET2-Fw ET2-Rv | 5′-GCT ATG GTC TCC GTG CCT AC-3′ 5′-GCC GTA AGG AGC TGT CTG TT-3′ | 243 pb |
| ET3-Fw ET3-Rv | 5′-TCA ACA CTC CCG AAC AGA CG-3′ 5′-TGA CGT CCA GAG TTT GGG TG-3′ | 186 pb |
| βACT-Fw βACT -Rv | 5′-CCT CGC CTT TGC CGA TCC-3′ 5′-AGG ATG CCT CTC TTG CTC TG -3′ | 243 pb |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Garcia, J.; Vargas-Franco, J.W.; Royer, B.B.-L.; Cochonneau, D.; Amiaud, J.; Heymann, M.-F.; Heymann, D.; Lézot, F. Inhibiting Endothelin Receptors with Macitentan Strengthens the Bone Protective Action of RANKL Inhibition and Reduces Metastatic Dissemination in Osteosarcoma. Cancers 2022, 14, 1765. https://doi.org/10.3390/cancers14071765
Muñoz-Garcia J, Vargas-Franco JW, Royer BB-L, Cochonneau D, Amiaud J, Heymann M-F, Heymann D, Lézot F. Inhibiting Endothelin Receptors with Macitentan Strengthens the Bone Protective Action of RANKL Inhibition and Reduces Metastatic Dissemination in Osteosarcoma. Cancers. 2022; 14(7):1765. https://doi.org/10.3390/cancers14071765
Chicago/Turabian StyleMuñoz-Garcia, Javier, Jorge William Vargas-Franco, Bénédicte Brounais-Le Royer, Denis Cochonneau, Jérôme Amiaud, Marie-Françoise Heymann, Dominique Heymann, and Frédéric Lézot. 2022. "Inhibiting Endothelin Receptors with Macitentan Strengthens the Bone Protective Action of RANKL Inhibition and Reduces Metastatic Dissemination in Osteosarcoma" Cancers 14, no. 7: 1765. https://doi.org/10.3390/cancers14071765
APA StyleMuñoz-Garcia, J., Vargas-Franco, J. W., Royer, B. B.-L., Cochonneau, D., Amiaud, J., Heymann, M.-F., Heymann, D., & Lézot, F. (2022). Inhibiting Endothelin Receptors with Macitentan Strengthens the Bone Protective Action of RANKL Inhibition and Reduces Metastatic Dissemination in Osteosarcoma. Cancers, 14(7), 1765. https://doi.org/10.3390/cancers14071765









