Physical Activity and Sedentary Behavior in Relation to Cancer Survival: A Narrative Review
Abstract
:Simple Summary
Abstract
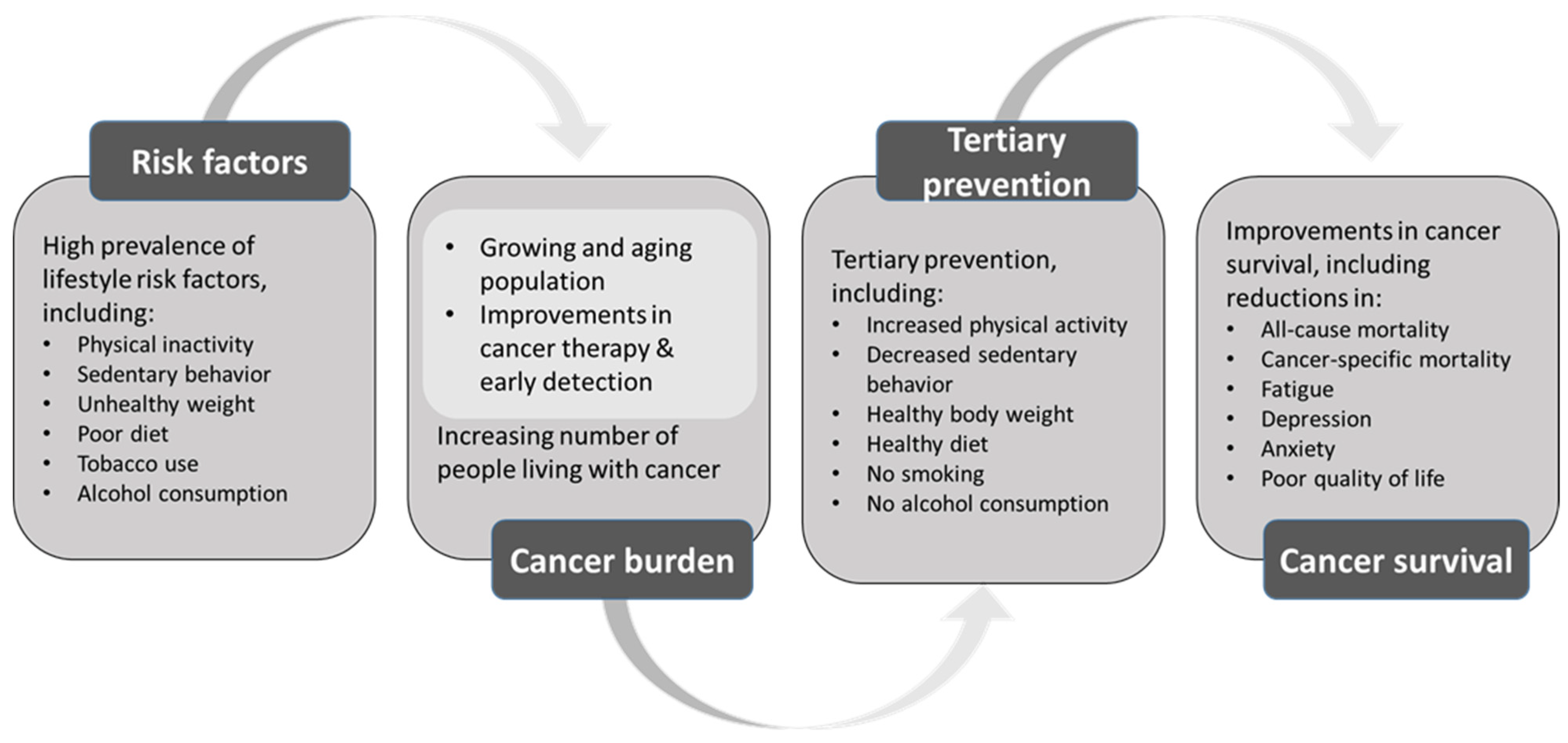
1. Introduction
2. Methods
3. Epidemiologic Evidence on Physical Activity and Cancer Survival
3.1. Definition of Physical Activity and Prevalence among Cancer Survivors
3.2. Physical Activity and Mortality Outcomes in Cancer Survivors
3.3. Physical Activity and Other Health Outcomes in Cancer Survivors
3.4. Barriers, Facilitators, and Other Factors That Determine Levels of Physical Activity in Cancer Survivors
3.5. Quality of Evidence and Future Research Needs
4. Epidemiologic Evidence on Sedentary Behavior and Cancer Survival
4.1. Definition of Sedentary Behavior and Prevalence among Cancer Survivors
4.2. Sedentary Behavior and Mortality Outcomes in Cancer Survivors
4.3. Sedentary Behavior and Other Health Outcomes in Cancer Survivors
4.4. Interventions to Reduce Time Spent Sitting in Cancer Survivors
4.5. Quality of Evidence and Future Research Needs
5. Existing Recommendations for Physical Activity and Sedentary Behavior for Cancer Survivors
6. Conclusions and Opinion
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Schüz, J.; Espina, C.; Villain, P.; Herrero, R.; Leon, M.E.; Minozzi, S.; Romieu, I.; Segnan, N.; Wardle, J.; Wiseman, M.; et al. European Code against Cancer 4th Edition: 12 ways to reduce your cancer risk. Cancer Epidemiol. 2015, 39 (Suppl. 1), S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Leitzmann, M.; Powers, H.; Anderson, A.; Scoccianti, C.; Berrino, F.; Boutron-Ruault, M.-C.; Cecchini, M.; Espina, C.; Key, T.J.; Norat, T.; et al. European Code against Cancer 4th Edition: Physical activity and cancer. Cancer Epidemiol. 2015, 39, S46–S55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedenreich, C.M.; Stone, C.R.; Cheung, W.Y.; Hayes, S.C. Physical Activity and Mortality in Cancer Survivors: A Systematic Review and Meta-Analysis. JNCI Cancer Spectr. 2020, 4, pkz080. [Google Scholar] [CrossRef]
- Lynch, B.M.; Mahmood, S.; Boyle, T. Sedentary Behaviour and Cancer. In Sedentary Behaviour Epidemiology; Leitzmann, M.F., Jochem, C., Schmid, D., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of Physical Activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S516. [Google Scholar] [CrossRef] [Green Version]
- National Cancer Institute. Cancer Survivors and Physical Activity. Available online: https://progressreport.cancer.gov/after/physical_activity (accessed on 4 August 2021).
- Sweegers, M.G.; Boyle, T.; Vallance, J.K.; Chinapaw, M.J.; Brug, J.; Aaronson, N.K.; D’silva, A.; Kampshoff, C.S.; Lynch, B.M.; Nollet, F.; et al. Which cancer survivors are at risk for a physically inactive and sedentary lifestyle? Results from pooled accelerometer data of 1447 cancer survivors. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 1–15. [Google Scholar] [CrossRef]
- Zhong, S.; Jiang, T.; Ma, T.; Zhang, X.; Tang, J.; Chen, W.; Lv, M.; Zhao, J. Association between physical activity and mortality in breast cancer: A meta-analysis of cohort studies. Eur. J. Epidemiol. 2014, 29, 391–404. [Google Scholar] [CrossRef]
- Lahart, I.; Metsios, G.S.; Nevill, A.; Carmichael, A.R. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015, 54, 635–654. [Google Scholar] [CrossRef]
- Schmid, D.; Leitzmann, M.F. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: A systematic review and meta-analysis. Ann. Oncol. 2014, 25, 1293–1311. [Google Scholar] [CrossRef]
- Lee, J. A Meta-analysis of the Association Between Physical Activity and Breast Cancer Mortality. Cancer Nurs. 2019, 42, 271–285. [Google Scholar] [CrossRef]
- Ibrahim, E.M.; Al-Homaidh, A. Physical activity and survival after breast cancer diagnosis: Meta-analysis of published studies. Med. Oncol. 2011, 28, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Guo, F.; Ye, J.; Li, Y.; Shi, D.; Fang, D.; Guo, J.; Li, L. Pre- and post-diagnosis physical activity is associated with survival benefits of colorectal cancer patients: A systematic review and meta-analysis. Oncotarget 2016, 7, 52095–52103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Wei, S.; Shi, Y.; Pang, S.; Qin, Q.; Yin, J.; Deng, Y.; Chen, Q.; Wei, S.; Nie, S.; et al. The dose–response effect of physical activity on cancer mortality: Findings from 71 prospective cohort studies. Br. J. Sports Med. 2015, 50, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gu, M.; Jing, F.; Cai, S.; Bao, C.; Wang, J.; Jin, M.; Chen, K. Association between physical activity and all cancer mortality: Dose-response meta-analysis of cohort studies. Int. J. Cancer 2015, 138, 818–832. [Google Scholar] [CrossRef] [Green Version]
- Burke, S.; Wurz, A.; Bradshaw, A.; Saunders, S.; West, M.A.; Brunet, J. Physical Activity and Quality of Life in Cancer Survivors: A Meta-Synthesis of Qualitative Research. Cancers 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serdà i Ferrer, B.-C.; van Roekel, E.H.; Lynch, B.M. The Role of Physical Activity in Managing Fatigue in Cancer Survivors. Curr. Nutr. Rep. 2018, 7, 59–69. [Google Scholar] [CrossRef]
- Craft, L.L.; Van Iterson, E.; Helenowski, I.B.; Rademaker, A.W.; Courneya, K.S. Exercise Effects on Depressive Symptoms in Cancer Survivors: A Systematic Review and Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2012, 21, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Elshahat, S.; Treanor, C.; Donnelly, M. Factors influencing physical activity participation among people living with or beyond cancer: A systematic scoping review. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 1–20. [Google Scholar] [CrossRef]
- Michie, S.; Van Stralen, M.M.; West, R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement. Sci. 2011, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Browall, M.; Mijwel, S.; Rundqvist, H.; Wengström, Y. Physical Activity During and After Adjuvant Treatment for Breast Cancer: An Integrative Review of Women’s Experiences. Integr. Cancer Ther. 2018, 17, 16–30. [Google Scholar] [CrossRef] [Green Version]
- Livsey, L.; Lewis, K. Breast cancer survivors’ perceptions of participating in a supervised exercise intervention: An exploratory review of the literature. Women Health 2017, 58, 1017–1036. [Google Scholar] [CrossRef] [PubMed]
- Lavallee, J.; Abdin, S.; Faulkner, J.; Husted, M. Barriers and facilitators to participating in physical activity for adults with breast cancer receiving adjuvant treatment: A qualitative metasynthesis. Psycho-Oncology 2019, 28, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M.; On Behalf Of SBRN Terminology Consensus Project Participants. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, C.; Freisling, H.; Leitzmann, M.F.; Taljaard-Krugell, C.; Jacobs, I.; Kruger, H.S.; Smuts, C.M.; Pieters, M. Diet and sedentary behaviour in relation to cancer survival. A report from the national health and nutrition examination survey linked to the U.S. mortality registry. Clin. Nutr. 2020, 39, 3489–3496. [Google Scholar] [CrossRef]
- Cao, C.; Friedenreich, C.M.; Yang, L. Association of Daily Sitting Time and Leisure-Time Physical Activity With Survival Among US Cancer Survivors. JAMA Oncol. 2022, 8, 395. [Google Scholar] [CrossRef]
- Swain, C.T.V.; Nguyen, N.H.; Eagles, T.; Vallance, J.K.; Boyle, T.; Lahart, I.M.; Lynch, B.M. Postdiagnosis sedentary behavior and health outcomes in cancer survivors: A systematic review and meta-analysis. Cancer 2019, 126, 861–869. [Google Scholar] [CrossRef]
- Schmid, D.; Matthews, C.E.; Leitzmann, M.F. Physical activity and sedentary behavior in relation to mortality among renal cell cancer survivors. PLoS ONE 2018, 13, e0198995. [Google Scholar] [CrossRef] [Green Version]
- Friedenreich, C.M.; Wang, Q.; Neilson, H.K.; Kopciuk, K.A.; McGregor, S.E.; Courneya, K.S. Physical Activity and Survival After Prostate Cancer. Eur. Urol. 2016, 70, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Schmid, D.; Behrens, G.; Arem, H.; Hart, C.; Herr, W.; Jochem, C.; Matthews, C.E.; Leitzmann, M.F. Pre- and post-diagnosis physical activity, television viewing, and mortality among hematologic cancer survivors. PLoS ONE 2018, 13, e0192078. [Google Scholar] [CrossRef] [Green Version]
- Guinan, E.; Hussey, J.; Broderick, J.; Lithander, F.E.; O’donnell, D.; Kennedy, M.J.; Connolly, E.M. The effect of aerobic exercise on metabolic and inflammatory markers in breast cancer survivors—A pilot study. Support. Care Cancer 2013, 21, 1983–1992. [Google Scholar] [CrossRef]
- Pinto, B.; Dunsiger, S.; Stein, K. Does a peer-led exercise intervention affect sedentary behavior among breast cancer survivors? Psycho-Oncology 2016, 26, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Survivors of Breast and Other Cancers. Available online: http://dietandcancerreport.org (accessed on 18 March 2022).
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J. Clin. 2022, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J. Clin. Oncol. 2016, 34, 611–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, E.; LaMonte, S.J.; Erb, N.L.; Beckman, K.L.; Sadeghi, N.; Hutcheson, K.; Stubblefield, M.D.; Abbott, D.M.; Fisher, P.S.; Stein, K.D.; et al. American Cancer Society Head and Neck Cancer Survivorship Care Guideline. CA Cancer J. Clin. 2016, 66, 203–239. [Google Scholar] [CrossRef] [PubMed]
- Resnick, M.J.; Lacchetti, C.; Bergman, J.; Hauke, R.J.; Hoffman, K.E.; Kungel, T.M.; Morgans, A.K.; Penson, D.F. Prostate Cancer Survivorship Care Guideline: American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J. Clin. Oncol. 2015, 33, 1078–1085. [Google Scholar] [CrossRef] [Green Version]
- Meyerhardt, J.A.; Mangu, P.B.; Flynn, P.J.; Korde, L.; Loprinzi, C.L.; Minsky, B.D.; Petrelli, N.J.; Ryan, K.; Schrag, D.H.; Wong, S.L.; et al. Follow-Up Care, Surveillance Protocol, and Secondary Prevention Measures for Survivors of Colorectal Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J. Clin. Oncol. 2013, 31, 4465–4470. [Google Scholar] [CrossRef]
- Denlinger, C.S.; Sanft, T.; Moslehi, J.J.; Overholser, L.; Armenian, S.; Baker, K.S.; Broderick, G.; Demark-Wahnefried, W.; Friedman, D.L.; Goldman, M.; et al. NCCN Guidelines Insights: Survivorship, Version 2.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 1016–1023. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2018; pp. 56–65. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jochem, C.; Leitzmann, M. Physical Activity and Sedentary Behavior in Relation to Cancer Survival: A Narrative Review. Cancers 2022, 14, 1720. https://doi.org/10.3390/cancers14071720
Jochem C, Leitzmann M. Physical Activity and Sedentary Behavior in Relation to Cancer Survival: A Narrative Review. Cancers. 2022; 14(7):1720. https://doi.org/10.3390/cancers14071720
Chicago/Turabian StyleJochem, Carmen, and Michael Leitzmann. 2022. "Physical Activity and Sedentary Behavior in Relation to Cancer Survival: A Narrative Review" Cancers 14, no. 7: 1720. https://doi.org/10.3390/cancers14071720
APA StyleJochem, C., & Leitzmann, M. (2022). Physical Activity and Sedentary Behavior in Relation to Cancer Survival: A Narrative Review. Cancers, 14(7), 1720. https://doi.org/10.3390/cancers14071720





