Quantitative Multiparametric Ultrasound (mpUS) in the Assessment of Inconclusive Cervical Lymph Nodes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
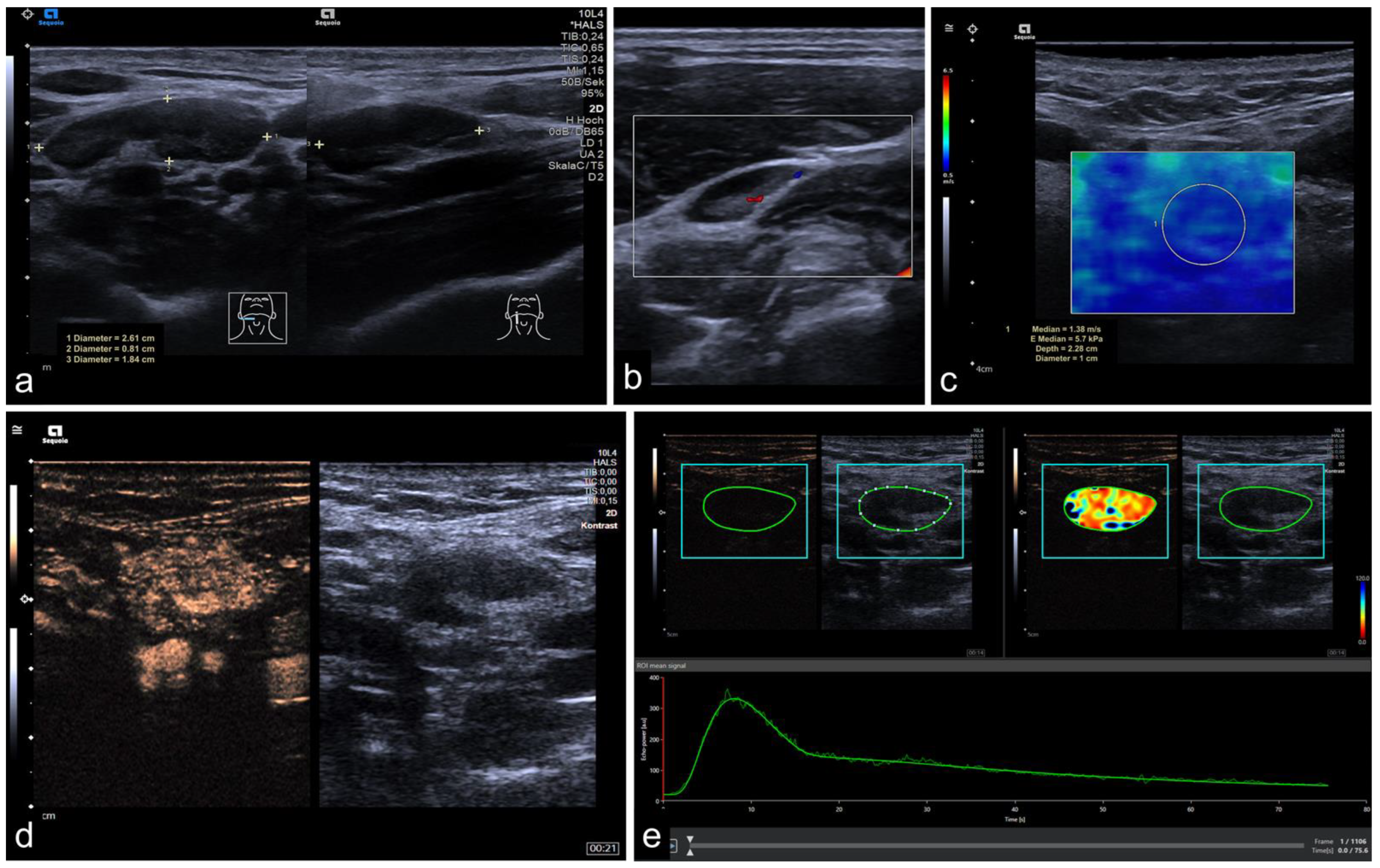
2.2. Imaging Protocol
2.3. Perfusion Analysis
2.4. Statistical Analysis
3. Results
3.1. Study Cohort
3.2. General Cohort
3.3. Subgroup Solbiati Index > 2
3.4. Infracentimetric CLN
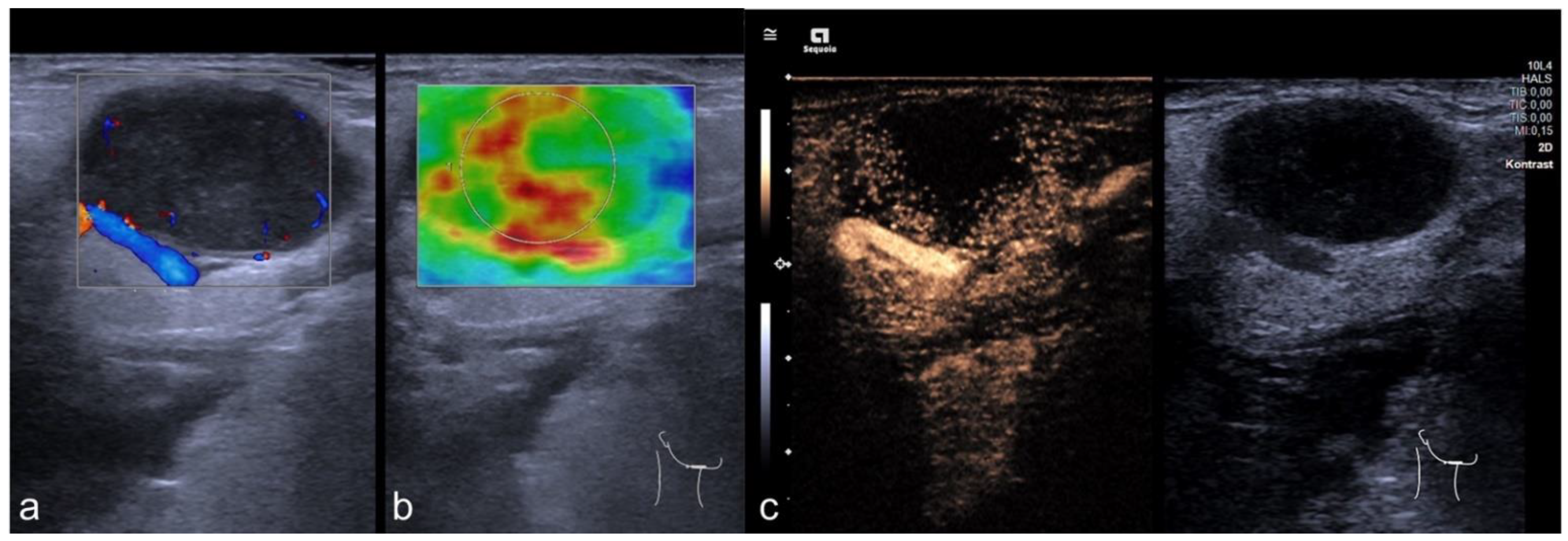
3.5. CEUS
3.6. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaddey, H.L.; Riegel, A.M. Unexplained Lymphadenopathy: Evaluation and Differential Diagnosis. Am. Fam. Physician 2016, 94, 896–903. [Google Scholar] [PubMed]
- Lopez, F.; Rodrigo, J.P.; Silver, C.E.; Haigentz, M., Jr.; Bishop, J.A.; Strojan, P.; Hartl, D.M.; Bradley, P.J.; Mendenhall, W.M.; Suarez, C.; et al. Cervical lymph node metastases from remote primary tumor sites. Head Neck 2016, 38 (Suppl. 1), E2374–E2385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galer, C.E.; Kies, M.S. Evaluation and management of the unknown primary carcinoma of the head and neck. J. Natl. Compr. Canc. Netw. 2008, 6, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Savage, S.A.; Wotherspoon, H.A.; Fitzsimons, E.J.; MacKenzie, K. Cervical lymphadenopathy resulting in a diagnosis of lymphoma. Scott. Med. J. 2008, 53, 13–16. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef] [Green Version]
- Castelijns, J.A.; van den Brekel, M.W. Detection of lymph node metastases in the neck: Radiologic criteria. AJNR Am. J. Neuroradiol. 2001, 22, 3–4. [Google Scholar]
- Wunschel, M.; Neumeier, M.; Utpatel, K.; Reichert, T.E.; Ettl, T.; Spanier, G. Staging more important than grading? Evaluation of malignancy grading, depth of invasion, and resection margins in oral squamous cell carcinoma. Clin. Oral Investig. 2021, 25, 1169–1182. [Google Scholar] [CrossRef]
- Weinstock, M.S.; Patel, N.A.; Smith, L.P. Pediatric Cervical Lymphadenopathy. Pediatr. Rev. 2018, 39, 433–443. [Google Scholar] [CrossRef]
- Schneider, U.; Grass, I.; Laudien, M.; Quetz, J.; Graefe, H.; Wollenberg, B.; Meyer, J.E. Comparison of Clinical Examination and Various Imaging Modalities in the Diagnosis of Head and Neck Cancer. Int. Arch. Otorhinolaryngol. 2021, 25, e179–e184. [Google Scholar] [CrossRef]
- Dudea, S.M.; Lenghel, M.; Botar-Jid, C.; Vasilescu, D.; Duma, M. Ultrasonography of superficial lymph nodes: Benign vs. malignant. Med. Ultrason. 2012, 14, 294–306. [Google Scholar]
- Solbiati, L.; Rizzatto, G. Ultrasound of Superficial Structures: High Frequencies, Doppler and Interventional Procedures; Churchill Livingstone: Edinburgh, Scotland, 1995. [Google Scholar]
- Sidhu, P.S. Multiparametric Ultrasound (MPUS) Imaging: Terminology Describing the Many Aspects of Ultrasonography. Ultraschall Med. 2015, 36, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Greis, C. Quantitative evaluation of microvascular blood flow by contrast-enhanced ultrasound (CEUS). Clin. Hemorheol. Microcirc. 2011, 49, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics 2017, 37, 855–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.; Miao, L.Y.; Cui, L.G.; Sun, P.F.; Qian, L.X. Value of Shear Wave Elastography Versus Contrast-Enhanced Sonography for Differentiating Benign and Malignant Superficial Lymphadenopathy Unexplained by Conventional Sonography. J. Ultrasound Med. 2017, 36, 189–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Mori, S.; Sakamoto, M.; Takahashi, S.; Kodama, T. Mouse model of lymph node metastasis via afferent lymphatic vessels for development of imaging modalities. PLoS ONE 2013, 8, e55797. [Google Scholar] [CrossRef] [Green Version]
- Kilic, A.; Er, H.C. Virtual touch tissue imaging quantification shear wave elastography for determining benign versus malignant cervical lymph nodes: A comparison with conventional ultrasound. Diagn. Interv. Radiol. 2019, 25, 114–121. [Google Scholar] [CrossRef]
- Chami, L.; Giron, A.; Ezziane, M.; Leblond, V.; Charlotte, F.; Pellot-Barakat, C.; Lucidarme, O. Quantitative and Qualitative Approach for Shear Wave Elastography in Superficial Lymph Nodes. Ultrasound Med. Biol. 2021, 47, 2117–2127. [Google Scholar] [CrossRef]
- Arain, A.A.; Rajput, M.S.A.; Ansari, S.A.; Mahmood, Z.; Ahmad, A.N.; Dogar, M.R.; Suahil, A. Occult Nodal Metastasis in Oral Cavity Cancers. Cureus 2020, 12, e11640. [Google Scholar] [CrossRef]
- Finegersh, A.; Moss, W.J.; Saddawi-Konefka, R.; Faraji, F.; Coffey, C.S.; Califano, J.A.; Brumund, K.T.; Orosco, R.K. Meta-analysis of risk of occult lymph node metastasis in the irradiated, clinically N0 neck. Head Neck 2020, 42, 2355–2363. [Google Scholar] [CrossRef]
- Sharbel, D.D.; Abkemeier, M.; Groves, M.W.; Albergotti, W.G.; Byrd, J.K.; Reyes-Gelves, C. Occult Metastasis in Laryngeal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Ann. Otol. Rhinol. Laryngol. 2021, 130, 67–77. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, X.; Qiao, Y.; Huang, S.; Kou, Y. Occult lymph node metastasis in the contralateral neck of oropharyngeal squamous cell carcinoma: A meta-analysis and literature review. Eur. Arch. Otorhinolaryngol. 2022, 279, 2157–2166. [Google Scholar] [CrossRef] [PubMed]
- Ganeshalingam, S.; Koh, D.M. Nodal staging. Cancer Imaging 2009, 9, 104–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, Y.; Liu, S.; Li, A.C.; Pan, X.B.; Liang, Z.G.; Cheng, W.Q.; Zhu, X.D. A Pilot Study: N-Staging Assessment of Shear Wave Elastrography in Small Cervical Lymph Nodes for Nasopharyngeal Carcinoma. Front. Oncol. 2020, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Pauzie, A.; Gavid, M.; Dumollard, J.M.; Timoshenko, A.; Peoc’h, M.; Prades, J.M. Infracentimetric cervical lymph node metastasis in head and neck squamous cell carcinoma: Incidence and prognostic value. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016, 133, 307–311. [Google Scholar] [CrossRef]
- Amit, M.; Yen, T.C.; Liao, C.T.; Binenbaum, Y.; Chaturvedi, P.; Agarwal, J.P.; Kowalski, L.P.; Ebrahimi, A.; Clark, J.R.; Cernea, C.R.; et al. Clinical nodal stage is a significant predictor of outcome in patients with oral cavity squamous cell carcinoma and pathologically negative neck metastases: Results of the international consortium for outcome research. Ann. Surg. Oncol. 2013, 20, 3575–3581. [Google Scholar] [CrossRef]
- Bialek, E.J.; Jakubowski, W.; Szczepanik, A.B.; Maryniak, R.K.; Prochorec-Sobieszek, M.; Bilski, R.; Szopinski, K.T. Vascular patterns in superficial lymphomatous lymph nodes: A detailed sonographic analysis. J. Ultrasound 2007, 10, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.L.; Ma, T.; Wang, Z.J.; Huang, L.; Liu, W.; Chen, M.; Sang, T.; Ren, X.G.; Tong, J.; Cao, C.L.; et al. The value of contrast-enhanced ultrasound for the diagnosis of metastatic cervical lymph nodes of papillary thyroid carcinoma: A systematic review and meta-analysis. J. Clin. Ultrasound 2022, 50, 60–69. [Google Scholar] [CrossRef]

| Patients | 101 |
| Female | 35/101 |
| Male | 66/101 |
| Mean age | 60.14 years (±16.9 years) |
| CLN | 104 |
| benign | 36/104 |
| malign | 68/104 |
| HNSCC | 30/104 |
| Lymphoma | 19/104 |
| Malignant melanoma | 9/104 |
| Adenocarcinoma (breast, salivary gland, lung) | 5/104 |
| Prostate cancer | 1/104 |
| Transitional cell carcinoma | 1/104 |
| Atypical fibroxanthoma | 1/104 |
| CLL | 1/104 |
| Renal cell cancer | 1/104 |
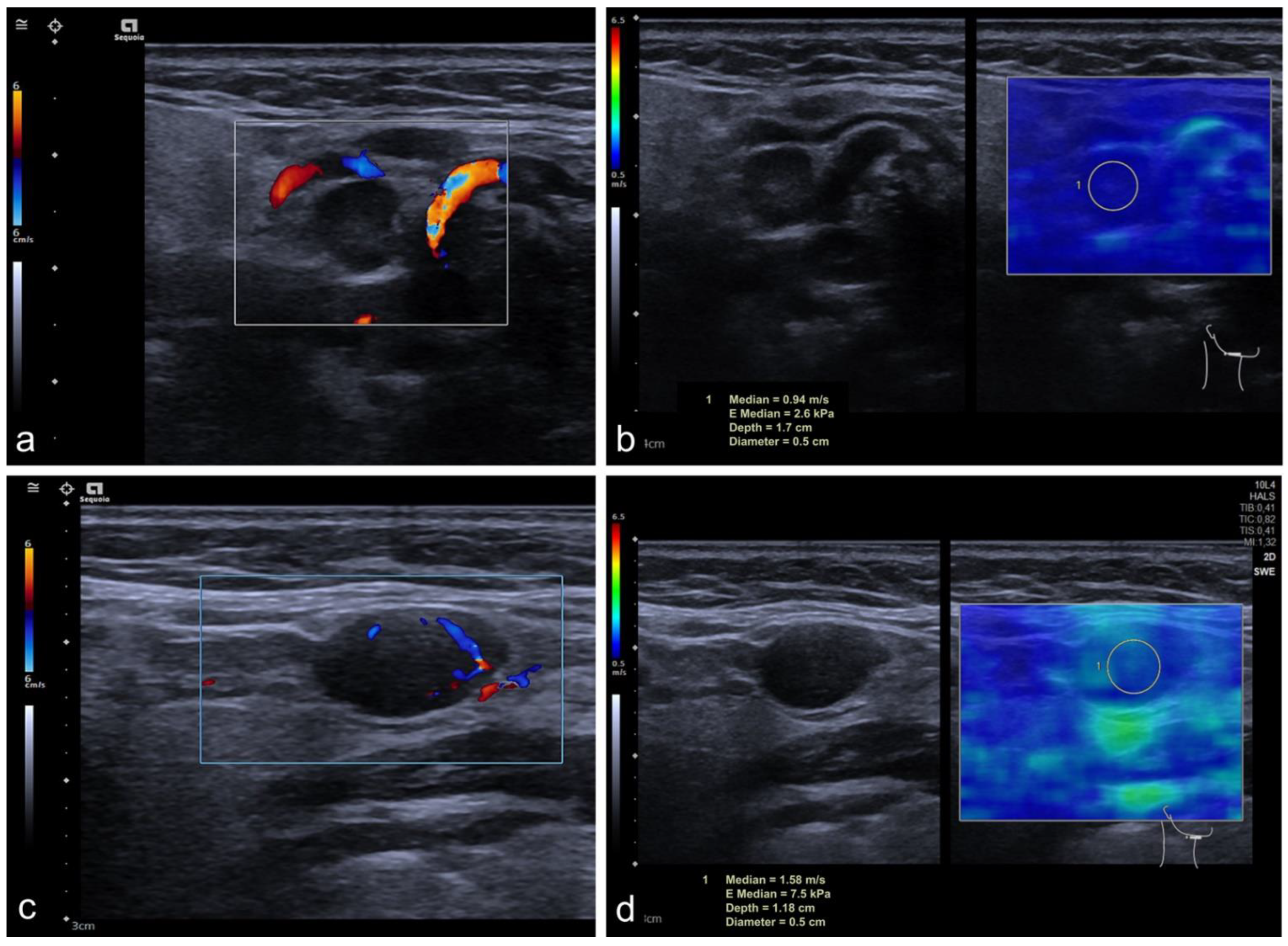
| SWE | 104 CLN |
| Benign | 36/104 |
| malignant | 68/104 |
| CEUS | 98 CLN |
| Benign | 34/98 |
| malignant | 64/98 |
| Metric Parameters | General Cohort | ||
|---|---|---|---|
| Benign (n = 36) | Malignant (n = 68) | p-value (n = 104) | |
| Long Axis Diameter (cm) | 1.47 ± 0.69 | 2.14 ± 1.06 | <0.001 |
| Short Axis Diameter (cm) | 0.74 ± 0.34 | 1.38 ± 0.62 | <0.001 |
| Solbiati Index * | 2.11 ± 0.81 | 1.60 ± 0.46 | <0.001 |
| Shear Wave Elastography (n = 104) | |||
| SWE (m/s) | 1.72 ± 0.69 | 2.63 ± 1.03 | <0.001 |
| Metric Parameters | Solbiati Index > 2 | ||
|---|---|---|---|
| Benign (n = 17) | Malignant (n = 10) | p-value (n = 27) | |
| Long Axis Diameter (cm) | 1.76 ± 0.81 | 2.63 ± 1.32 | > 0.05 |
| Short Axis Diameter (cm) | 0.67± 0.34 | 1.12 ± 0.62 | 0.031 |
| Solbiati Index * | - | - | - |
| Shear Wave Elastography (n = 27) | |||
| SWE (m/s) | 1.69 ± 0.57 | 2.45 ± 0.65 | 0.008 |
| Metric Parameters | Short Axis Diameter < 1 cm | ||
|---|---|---|---|
| Benign (n = 29) | Malignant (n = 20) | p-value (n = 49) | |
| Long Axis Diameter (cm) | 1.33 ± 0.51 | 1.33 ± 0.53 | > 0.05 |
| Short Axis Diameter (cm) | - | - | - |
| Solbiati Index * | 2.26 ± 0.81 | 1.88 ± 0.63 | > 0.05 |
| Shear Wave Elastography (n = 49) | |||
| SWE (m/s) | 1.69 ± 0.7 | 2.27 ± 0.88 | 0.025 |
| Tumor Entity | LAD (cm) | SAD (cm) | Solbiati Index * | SWE (m/s) | CEUS |
|---|---|---|---|---|---|
| Benign CLN (n = 36) | 1.47 ± 0.69 | 0.74 ± 0.34 | 2.11 ± 0.81 | 1.72 ± 0.69 | |
| HNSCC (n = 30) | p < 0.001 2.08 ± 0.8 | p < 0.001 1.31 ± 0.58 | p = 0.023 1.73 ± 0.54 | p < 0.001 2.59 ± 0.97 | p > 0.05 |
| Lymphoma (n = 19) | p < 0.001 2.41 ± 1.15 | p < 0.001 1.5 ± 0.59 | p = 0.008 1.6 ±0.33 | p < 0.001 2.78 ± 1.13 | p > 0.05 |
| Malignant melanoma (n = 9) | p = 0.038 2.09 ± 1.02 | p < 0.001 1.51 ± 0.65 | p = 0.001 1.36 ± 0.2 | p = 0.028 2.21 ± 0.62 | p > 0.05 |
| Adenocarcinoma (n = 5) | p = 0.36 1.15 ± 0.48 | p = 0.265 0.77 ± 0.17 | p = 0.069 1.48 ± 0.59 | p = 0.021 2.54 ± 0.64 | p > 0.05 |
| CLN metastasis of other origins (n = 5) | p = 0.15 2.56 ± 1.99 | p = 0.001 1.74 ± 0.88 | p = 0.018 1.39 ± 0.36 | p = 0.018 3.15 ± 1.74 | p > 0.05 |
| HNSCC | |||||
| Adenocarcinoma | p = 0.018 | p = 0.059 | p = 0.109 | p = 0.962 | p > 0.05 |
| Lymphoma | |||||
| Adenocarcinoma | p = 0.012 | p = 0.009 | p = 0.145 | p = 0.915 | p > 0.05 |
| CLN metastasis of other origins | p = 0.619 | p = 0.546 | p = 0.189 | p = 0.972 | p > 0.05 |
| Malignant Melanoma | |||||
| Adenocarcinoma | p = 0.039 | p = 0.004 | p = 0.549 | p = 0.423 | p > 0.05 |
| CLN metastasis of other origins | p = 0.947 | p = 0.593 | p = 0.641 | p = 0.257 | p > 0.05 |
| Adenocarcinoma | |||||
| CLN metastasis of other origins | p = 0.076 | p = 0.036 | p = 0.754 | p = 0.754 | p > 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lerchbaumer, M.H.; Wakonig, K.M.; Arens, P.; Dommerich, S.; Fischer, T. Quantitative Multiparametric Ultrasound (mpUS) in the Assessment of Inconclusive Cervical Lymph Nodes. Cancers 2022, 14, 1597. https://doi.org/10.3390/cancers14071597
Lerchbaumer MH, Wakonig KM, Arens P, Dommerich S, Fischer T. Quantitative Multiparametric Ultrasound (mpUS) in the Assessment of Inconclusive Cervical Lymph Nodes. Cancers. 2022; 14(7):1597. https://doi.org/10.3390/cancers14071597
Chicago/Turabian StyleLerchbaumer, Markus H., Katharina Margherita Wakonig, Philipp Arens, Steffen Dommerich, and Thomas Fischer. 2022. "Quantitative Multiparametric Ultrasound (mpUS) in the Assessment of Inconclusive Cervical Lymph Nodes" Cancers 14, no. 7: 1597. https://doi.org/10.3390/cancers14071597
APA StyleLerchbaumer, M. H., Wakonig, K. M., Arens, P., Dommerich, S., & Fischer, T. (2022). Quantitative Multiparametric Ultrasound (mpUS) in the Assessment of Inconclusive Cervical Lymph Nodes. Cancers, 14(7), 1597. https://doi.org/10.3390/cancers14071597








