Components of the Lectin Pathway of Complement in Solid Tumour Cancers
Abstract
Simple Summary
Abstract
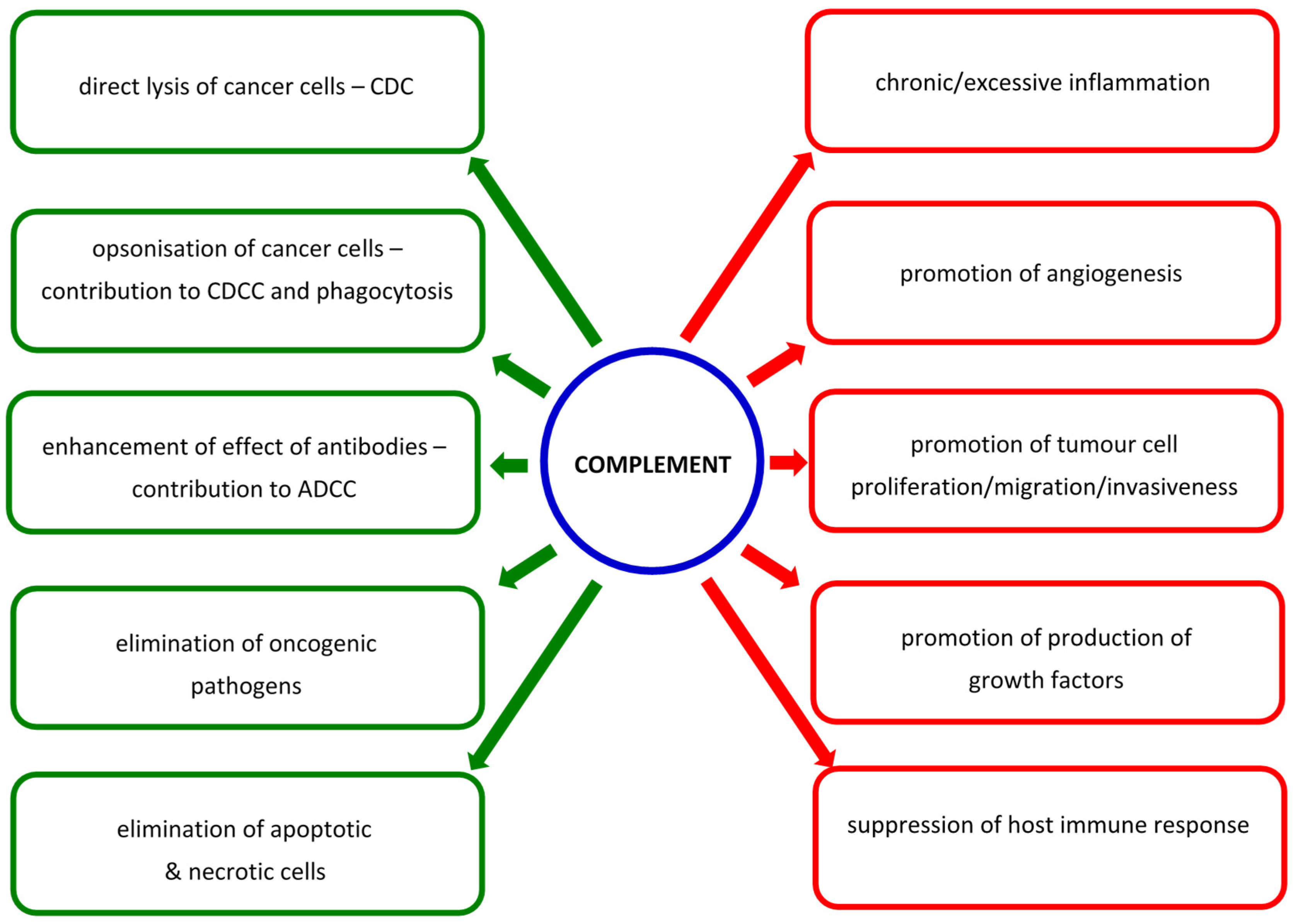
1. Introduction
2. Gynaecological Cancers
3. Lung Cancer
4. Alimentary Tract Cancers
5. Other Solid Tumours
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ehrnthaller, C.; Ignatius, A.; Gebhard, F.; Huber-Lang, M. New insights of an old defense system: Structure, function, and clinical relevance of the complement system. Mol. Med. 2011, 17, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Nesargikar, P.N.; Spiller, B.; Chavez, R. The complement system: History, pathways, cascade and inhibitors. Eur. J. Microbiol. Immunol. 2012, 2, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Roumenina, L.T.; Daugan, M.V.; Petitprez, F.; Sautes-Fridman, C.; Fridman, W.H. Context-dependent roles of complement in cancer. Nat. Rev. Cancer 2019, 19, 698–715. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, M.J.; Sughrue, M.E.; Kane, A.J.; Millis, S.A.; Parsa, A.T. Cancer and the complement cascade. Mol. Cancer Res. 2010, 8, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, S.; Hone, S.; Kirschfink, M. The complement system in cancer: Ambivalence between tumour destruction and promotion. Immunobiology 2017, 222, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Wang, X.-Y.; Li, R.-Y.; Jia, S.-C.; Sun, P.; Zhao, M. Recent progress in the understanding of complement activation and its role in tumor growth and anti-tumor therapy. Biomed. Pharmacother. 2017, 91, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Revel, M.; Daugan, M.V.; Sautes-Fridman, C.; Fridman, W.H.; Roumenina, L.T. Complement system: Promoter or suppressor of cancer progression? Antibodies 2020, 9, 57. [Google Scholar] [CrossRef]
- Cedzyński, M.; Świerzko, A.S. Components of the lectin pathway of complement in haematologic malignancies. Cancers 2020, 12, 1792. [Google Scholar] [CrossRef]
- Dobo, J.; Szakacs, D.; Oroszlan, G.; Kortvely, E.; Kiss, B.; Boros, E.; Szasz, R.; Zavodszky, P.; Gal, P.; Pal, G. MASP-3 is the exclusive pro-factor D activator in resting blood: The lectin and the alternative complement pathways are fundamentally linked. Sci. Rep. 2016, 6, 31877. [Google Scholar] [CrossRef]
- Hayashi, M.; Machida, T.; Ishida, Y.; Ogata, Y.; Omori, T.; Takasumi, M.; Endo, Y.; Suzuki, T.; Sekimata, M.; Homma, Y.; et al. Cutting edge: Role of MASP-3 in the physiological activation of factor D of the alternative complement pathway. J. Immunol. 2019, 203, 1411–1416. [Google Scholar] [CrossRef]
- Holers, M.V.; Borodovsky, A.; Scheinman, R.I.; Ho, N.; Ramos Ramirez, J.; Dobo, J.; Gal, P.; Lindenberger, J.; Hansen, A.G.; Desai, D.; et al. Key components of the complement lectin pathway are not only required for the development of inflammatory arthritis but also regulate the transcription of factor D. Front. Immunol. 2020, 11, 201. [Google Scholar] [CrossRef] [PubMed]
- Krarup, A.; Wallis, R.; Presanis, J.S.; Gal, P.; Sim, R.B. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS ONE 2007, 2, e623. [Google Scholar] [CrossRef] [PubMed]
- Krarup, A.; Gulla, K.C.; Gal, P.; Hajela, K.; Sim, R.B. The action of MBL-associated serine protease 1 (MASP1) on factor XIII and fibrinogen. Biochim. Biophys. Acta 2008, 1784, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Gulla, K.C.; Gupta, K.; Krarup, A.; Gal, P.; Schwaeble, W.J.; Sim, R.B.; O’Connor, C.D.; Hajela, K. Activation of mannan-binding lectin-associated serine proteases leads to generation of a fibrin clot. Immunology 2010, 129, 482–495. [Google Scholar] [CrossRef]
- Dobo, J.; Major, B.; Kekesi, K.A.; Szabo, I.; Megyeri, M.; Hajela, K.; Juhasz, G.; Zavodszky, P.; Gal, P. Cleavage of kininogen and subsequent bradykinin release by the complement component: Mannose-binding lectin-associated serine protease (MASP)-1. PLoS ONE 2011, 6, e20036. [Google Scholar] [CrossRef] [PubMed]
- Yongqing, T.; Drentin, N.; Duncan, R.C.; Wijeyewickrema, L.C.; Pike, R.N. Mannose-binding lectin serine proteases and associated proteins of the lectin pathway of complement: Two genes, five proteins and many functions? Biochim. Biophys. Acta 2012, 1824, 253–262. [Google Scholar] [CrossRef]
- Hess, K.; Ajjan, R.; Phoenix, F.; Dobó, J.; Gál, P.; Schroeder, V. Effects of MASP-1 of the complement system on activation of coagulation factors and plasma clot formation. PLoS ONE 2012, 7, e35690. [Google Scholar] [CrossRef]
- Kozarcanin, H.; Lood, C.; Munthe-Fog, L.; Sandholm, K.; Hamad, O.A.; Bengtsson, A.A.; Skjoedt, M.O.; Huber-Lang, M.; Garred, P.; Nilsson-Ekdahl, K.; et al. The lectin complement pathway serine proteases (MASPs) represent a possible crossroad between the coagulation and complement systems in thromboinflammation. J. Thromb. Haemost. 2016, 14, 531–545. [Google Scholar] [CrossRef]
- Jenny, L.; Noser, D.; Larsen, J.B.; Dobo, J.; Gal, P.; Pal, G.; Schroeder, V. MASP-1 of the complement system alters fibrinolytic behaviour of blood clots. Mol. Immunol. 2019, 114, 1–9. [Google Scholar] [CrossRef]
- Dobo, J.; Harmat, V.; Beinrohr, L.; Sebestyen, E.; Zavodszky, P.; Gal, P. MASP-1, a promiscuous complement protease: Structure of its catalytic region reveals the basis of its broad specificity. J. Immunol. 2009, 183, 1207–1214. [Google Scholar] [CrossRef]
- Megyeri, M.; Mako, V.; Beinrohr, L.; Doleschall, Z.; Prohaszka, Z.; Cervenak, L.; Zavodszky, P.; Gal, P. Complement protease MASP-1 activates human endothelial cells: PAR4 activation is a link between complement and endothelial function. J. Immunol. 2009, 183, 3409–3416. [Google Scholar] [CrossRef] [PubMed]
- Jani, P.K.; Kajdacsi, E.; Megyeri, M.; Dobo, J.; Doleschall, Z.; Futosi, K.; Timar, C.I.; Mocsai, A.; Mako, V.; Gal, P.; et al. MASP-1 induces a unique cytokine pattern in endothelial cells: A novel link between complement system and neutrophil granulocytes. PLoS ONE 2014, 9, e87104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schwaner, E.; Nemeth, Z.; Jani, P.K.; Kajdacsi, E.; Debreczeni, M.L.; Doleschall, Z.; Dobo, J.; Gal, P.; Rigo, J.; Andras, K.; et al. Transcriptome analysis of inflammation-related gene expression in endothelial cells activated by complement MASP-1. Sci. Rep. 2017, 7, 10462. [Google Scholar] [CrossRef] [PubMed]
- Debreczeni, M.L.; Nemeth, Z.; Kajdacsi, E.; Schwaner, E.; Mako, V.; Masszi, A.; Doleschall, Z.; Rigo, J.; Walter, F.R.; Deli, M.A.; et al. MASP-1 increases endothelial permeability. Front. Immunol. 2019, 10, 991. [Google Scholar] [CrossRef]
- Swierzko, A.S.; Florczak, K.; Cedzynski, M.; Szemraj, J.; Wydra, D.; Bak-Romaniszyn, L.; Emerich, J.; Sułowska, Z. Mannan-binding lectin (MBL) in women with tumours of the reproductive system. Cancer Immunol. Immunother. 2007, 56, 959–971. [Google Scholar] [CrossRef]
- Swierzko, A.S.; Szala, A.; Sawicki, S.; Szemraj, J.; Sniadecki, M.; Sokolowska, A.; Kaluzynski, A.; Wydra, D.; Cedzynski, M. Mannose-Binding Lectin (MBL) and MBL-associated serine protease-2 (MASP-2) in women with malignant and benign ovarian tumours. Cancer Immunol. Immunother. 2014, 63, 1129–1140. [Google Scholar] [CrossRef]
- Nevadunsky, N.S.; Korneeva, I.; Caputo, T.; Witkin, S.S. Mannose-binding lectin codon 54 genetic polymorphism and vaginal protein levels in women with gynecologic malignancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 163, 216–218. [Google Scholar] [CrossRef]
- Szala, A.; Sawicki, S.; Swierzko, A.S.; Szemraj, J.; Sniadecki, M.; Michalski, M.; Kaluzynski, A.; Lukasiewicz, J.; Maciejewska, A.; Wydra, D.; et al. Ficolin-2 and ficolin-3 in women with malignant and benign ovarian tumours. Cancer Immunol. Immunother. 2013, 62, 1411–1419. [Google Scholar] [CrossRef]
- Suryawanshi, S.; Huang, X.; Elishaev, E.; Budiu, R.A.; Zhang, L.; Kim, S.H.; Donnellan, N.; Mantia-Smaldone, G.; Ma, T.; Tseng, G.; et al. Complement pathway is frequently altered in endometriosis and endometriosis-associated ovarian cancer. Clin. Cancer Res. 2014, 20, 6163–6174. [Google Scholar] [CrossRef]
- Jang, H.; Jun, Y.; Kim, S.; Jung, Y.; Jo Park, B.; Lee, J.; Kim, J.; Lee, S.; Kim, J. FCN3 functions as a tumor suppressor of lung adenocarcinoma through induction of endoplasmic reticulum stress. Cell Death Dis. 2021, 12, 407. [Google Scholar] [CrossRef]
- Sipos, A.; Ujlaki, G.; Miko, E.; Maka, E.; Szabo, J.; Uray, K.; Krasznai, Z.; Bai, P. The role of microbiome in ovarian cancer: Mechanistic Insights into oncobiosis and to bacterial metabolite signaling. Mol. Med. 2021, 27, 33. [Google Scholar] [CrossRef]
- Alizadehmohajer, N.; Shojaeifar, S.; Nedaeinia, R.; Esparvarinha, M.; Mohammadi, F.; Ferns, G.A.; Ghayour-Mobarhan, M.; Manian, M.; Balouchi, A. Association between the microbiota and women’s cancers—Cause or consequences? Biomed. Pharmacother. 2020, 127, 110203. [Google Scholar] [CrossRef]
- Borella, F.; Carosso, A.R.; Cosma, S.; Preti, M.; Collemi, G.; Cassoni, P.; Bertero, L.; Benedetto, C. Gut microbiota and gynecological cancers: A summary of pathogenic mechanisms and future directions. ACS Infect. Dis. 2021, 7, 987–1009. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Tian, T.; Wei, Z.; Shih, N.; Feldman, F.D.; Alwine, J.C.; Coukos, G.; Robertson, E.S. The ovarian cancer oncobiome. Oncotarget 2017, 22, 36225–36245. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Lu, X.; Yang, X.; Xu, N. Association of MBL2 exon 1 polymorphisms with high-risk papillomavirus infection and cervical cancers: A meta-analysis. Arch. Gynecol. Obstet. 2016, 294, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Siamakpour-Reihani, S.; Patterson Cobb, L.; Jiang, C.; Zhang, D.; Previs, R.A.; Owzar, K.; Nixon, A.B.; Alvarez Secord, A. Differential expression of immune related genes in high-grade ovarian serous carcinoma. Gynecol. Oncol. 2020, 156, 662–668. [Google Scholar] [CrossRef]
- Sahar, T.; Nigam, A.; Anjum, S.; Gupta, N.; Wajid, S. Secretome profiling and computational biology of human leiomyoma samples unravel molecular signatures with potential for diagnostic and therapeutic interventions. Reprod. Sci. 2021, 28, 2672–2684. [Google Scholar] [CrossRef]
- Kaur, D.; Arora, C.; Raghava, G.P.S. Pattern recognition receptor based prognostic biomarkers for predicting survival of uterine corpus endometrial cancer patients. Mol. Diagn. Ther. 2021, 25, 629–646. [Google Scholar] [CrossRef]
- Kong, L.; Wang, J.; Cheng, J.; Zang, C.; Chen, F.; Wang, W.; Zhao, H.; Wang, Y.; Wang, D. Comprehensive identification of the human secretome as potential indicators in treatment outcome of HPV-positive and -negative cervical cancer patients. Gynecol. Obstet. Investig. 2020, 85, 405–415. [Google Scholar] [CrossRef]
- Maestri, C.A.; Nisihara, R.; Mendes, H.W.; Jensenius, J.; Thiel, S.; Messias-Reason, I.; de Carvalho, N.S. MASP-1 and MASP-2 serum levels are associated with worse prognostic in cervical cancer progression. Front. Immunol. 2018, 9, 2742. [Google Scholar] [CrossRef]
- Andersen, J.D.; Boylan, K.L.M.; Xue, F.S.; Anderson, L.B.; Witthuhn, B.A.; Markowski, T.W.; Higgins, L.A.; Skubitz, A.P.N. Identification of candidate biomarkers in ovarian cancer serum by depletion of highly abundant proteins and differential in-gel electrophoresis. Electrophoresis 2010, 31, 559–610. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Ortiz, G.; Ciudad, C.J.; Pina, P.; Vazquez, K.; Hidalgo, A.; Alatorre, B.; Garcia, J.A.; Salamanca, F.; Peralta-Rodriguez, R.; Rangel, A.; et al. Gene identification by cDNA arrays in HPV-positive cervical cancer. Arch. Med. Res. 2005, 36, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hao, Y.; Kamilijiang, M.; Hasimu, A.; Yuan, J.; Wu, G.; Reyimu, H.; Kadeer, N.; Abudula, A. Potential predictive plasma biomarkers for cervical cancer by 2D-DIGE proteomics and Ingenuity Pathway Analysis. Tumor Biol. 2015, 36, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Saldova, R.; Wormald, M.R.; Dwek, R.A.; Rudd, P.M. Glycosylation changes on serum glycoproteins in ovarian cancer may contribute to disease pathogenesis. Dis. Markers. 2008, 25, 219–232. [Google Scholar] [CrossRef]
- Zhou, M.; Kong, Y.; Wang, Z.; Li, W.; Chen, S.; Wang, L.; Wang, C.; Zhang, Q. LC-MS/MS-based quantitative proteomics analysis of different stages of non-small-cell lung cancer. BioMed Res. Int. 2021, 2021, 5561569. [Google Scholar] [CrossRef]
- Kang, J.U.; Koo, S.H.; Kwon, K.C.; Park, J.W.; Kim, J.M. Identification of novel candidate target genes, including EPHB3, MASP1 and SST at 3q26.2-q29 in squamous cell carcinoma of the lung. BMC Cancer 2009, 9, 237. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, E.-D.; Ma, S.; Bai, W.-Q.; Yin, Y.-J.; Shi, G.-L. The clinical significance of serum MASP-2 and IDH1 in the early diagnosis of non-small cell lung cancer. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef]
- Wang, F.-Y.; Tahara, T.; Arisawa, T.; Shibata, T.; Yamashita, H.; Nakamura, M.; Yoshioka, D.; Okubo, M.; Maruyama, N.; Kamano, T.; et al. Mannan-binding lectin (MBL) polymorphism and gastric cancer risk in Japanese population. Dig. Dis. Sci. 2008, 53, 2904–2908. [Google Scholar] [CrossRef]
- Baccarelli, A.; Hou, L.; Chen, J.; Lissowska, J.; El-Omar, E.M.; Grillo, P.; Giacomini, S.M.; Yaeger, M.; Bernig, T.; Zatonski, W.; et al. Mannose-binding lectin-2 genetic variation and stomach cancer risk. Int. J. Cancer 2006, 119, 1970–1975. [Google Scholar] [CrossRef]
- Scudiero, O.; Nardone, G.; Omodei, D.; Tatangelo, F.; Vitale, D.F.; Salvatore, F.; Castaldo, G. A mannose-binding lectin-defective haplotype is a risk factor for gastric cancer. Clin. Chem. 2006, 52, 1625–1626. [Google Scholar] [CrossRef]
- Su, C.; Lin, Y.; Cai, L.; Niu, J. Association between mannose-binding lectin variants, haplotypes and risk of hepatocellular carcinoma: A case-control study. Sci. Rep. 2016, 6, 321467. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-Y.; Dong, J.-F.; Chen, Z.-Q.; Ding, G.-S.; Fu, Z.-R. MiR-942-3p promotes the proliferation and invasion of hepatocellular carcinoma cells by targeting MBL2. Cancer Control 2019, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Awan, F.M.; Naz, A.; Obaid, A.; Ali, A.; Ahmad, J.; Anjum, S.; Janjua, H.A. Identification of circulating biomarker candidates for hepatocellular carcinoma (HCC): An integrated prioritization approach. PLoS ONE 2015, 10, e0138913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, H. Decreased expression of COLEC10 predicts poor overall survival in patients with hepatocellular carcinoma. Cancer Manag. Res. 2018, 10, 2369–2375. [Google Scholar] [CrossRef]
- Bai, K.-H.; He, S.-Y.; Shu, L.-L.; Wang, W.-D.; Lin, S.-Y.; Zhang, Q.-Y.; Li, L.; Cheng, L.; Dai, Y.-J. Identification of cancer stem cell characteristics in liver hepatocellular carcinoma by WGCNA analysis of transcriptome stemness index. Cancer Med. 2020, 9, 4290–4298. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Hu, Y.; Ding, Q.; Yu, Y.; Wang, F.; Luo, F.; Zhang, X.-L. Serum ficolin-2 concentrations are significantly changed in patients with hepatitis B virus infection and liver diseases. Virol. Sin. 2015, 30, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-L.; Luo, F.-L.; Fu, J.-L.; Chen, T.-L.; Wu, S.-M.; Zhou, Y.-D.; Zhang, X.-L. Early increased ficolin-2 concentrations are associated with severity of liver inflammation and efficacy of anti-viral therapy in chronic hepatitis C patients. Scand. J. Immunol. 2013, 77, 144–150. [Google Scholar] [CrossRef]
- Liu, J.; Ali, M.A.M.; Shi, Y.; Zhao, Y.; Luo, F.; Yu, J.; Xiang, T.; Tang, J.; Li, D.; Hu, Q.; et al. Specifically binding of L-ficolin to N-glycans of HCV envelope glycoproteins E1 and E2 leads to complement activation. Cell. Mol. Immunol. 2009, 6, 235–244. [Google Scholar] [CrossRef]
- Hamed, M.R.; Brown, R.J.; Zothner, C.; Urbanowicz, R.A.; Mason, C.P.; Krarup, A.; McClure, C.P.; Irving, W.L.; Ball, J.K.; Harris, M.; et al. Recombinant human L-ficolin directly neutralizes hepatitis C virus entry. J. Innate Immun. 2014, 6, 676–684. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, Y.; Zhang, X.; Zhao, P.; Tao, W.; Zhong, J.; Li, Q.; Zhang, X.-L. Ficolin-2 inhibits hepatitis C virus infection, whereas apolipoprotein E3 mediates viral immune escape. J. Immunol. 2014, 193, 783–796. [Google Scholar] [CrossRef]
- Yang, G.; Liang, Y.; Zheng, T.; Song, R.; Wang, J.; Shi, H.; Sun, B.; Xie, C.; Li, Y.; Han, J.; et al. FCN2 inhibits epithelial-mesenchymal transition-induced metastasis of hepatocellular carcinoma via TGF-β/Smad signalling. Cancer Lett. 2016, 378, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.H.; Ren, B.; Keryanov, S.; Tseng, G.C.; Rao, U.N.M.; Monga, S.P.; Strom, S.; Demetris, A.J.; Nalesnik, M.; Yu, Y.P.; et al. Transcriptomic and genomic analysis of human hepatocellular carcinomas and hepatoblastomas. Hepatology 2006, 44, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Ferrin, G.; Ranchal, I.; Llamosa, C.; Rodriguez-Peralvarez, M.L.; Romero-Ruiz, A.; Aguilar-Melero, P.; Lopez-Cillero, P.; Briceno, J.; Muntane, J.; Montero-Alvarez, J.L.; et al. Identification of candidate biomarkers for hepatocellular carcinoma in plasma of HCV-infected cirrhotic patients by 2-D DIGE. Liver Int. 2014, 34, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Jalal, P.J.; King, B.J.; Saeed, A.; Adedeji, Y.; Mason, C.P.; Ball, J.K.; Irving, W.L.; McClure, C.P.; Tarr, A.W. Elevated serum activity of MBL and ficolin-2 as biomarkers for progression to hepatocellular carcinoma in chronic HCV infection. Virology 2019, 530, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Eurich, D.; Boas-Knoop, S.; Morawietz, L.; Neuhaus, R.; Somasundaram, R.; Ruehl, M.; Neumann, U.P.; Neuhaus, P.; Bahra, M.; Seehofer, D. Association of mannose-binding lectin-2 gene polymorphism with the development of hepatitis C-induced hepatocellular carcinoma. Liver Int. 2011, 31, 1006–1012. [Google Scholar] [CrossRef]
- Gu, X.; Ji, Q.; Wang, H.; Jiang, M.; Yang, J.; Fang, M.; Wang, M.; Gao, C. Genetic variants of mannose-binding lectin 2 gene influence progression and prognosis of patients with hepatitis B virus infection in China. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 614–621. [Google Scholar] [CrossRef]
- Lin, Y.; Su, C.; Niu, J.; Guo, Z.; Cai, L. Impact of mannose-binding lectin polymorphism on the risk of hepatocellular carcinoma: A case-control study in Chinese Han population. J. Epidemiol. 2015, 25, 387–391. [Google Scholar] [CrossRef]
- Rong, Y.; Jin, D.; Hou, C.; Hu, J.; Wu, W.; Ni, X.; Wang, D.; Lou, W. Proteomics analysis of serum protein profiling in pancreatic cancer patients by DIGE: Up-regulation of mannose-binding lectin 2 and myosin light chain kinase 2. BMC Gastroenterol. 2010, 10, 68. [Google Scholar] [CrossRef]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J.I.; Shadaloey, S.A.; Wu, D.; Preiss, P.; Verma, N.; et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 2019, 574, 264–267. [Google Scholar] [CrossRef]
- Ytting, H.; Jensenius, J.C.; Christensen, I.J.; Thiel, S.; Nielsen, H.J. Increased activity of the mannan-binding lectin complement pathway in patients with colorectal cancer. Scand. J. Gastroenterol. 2004, 39, 674–679. [Google Scholar] [CrossRef]
- Ytting, H.; Christensen, I.J.; Jensenius, J.C.; Thiel, S.; Nielsen, H.J. Preoperative mannan-binding lectin pathway and prognosis in colorectal cancer. Cancer Immunol. Immunother. 2005, 54, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Ytting, H.; Christensen, I.J.; Steffensen, R.; Alsner, J.; Thiel, S.; Jensenius, J.C.; Hansen, U.; Nielsen, H.J. Mannan-binding lectin (MBL) and MBL-associated serine protease 2 (MASP-2) genotypes in colorectal cancer. Scand. J. Immunol. 2011, 73, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Bevier, M.; Huhn, S.; Sainz, J.; Lascorz, J.; Pardini, B.; Naccarati, A.; Vodickova, L.; Novotny, J.; Hemminki, K.; et al. Genetic variants in C-type lectin genes are associated with colorectal cancer susceptibility and clinical outcome. Int. J. Cancer 2013, 133, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, K.A.; Haznader, M.; Welsh, J.A.; Robles, A.I.; Ryan, B.M.; McClary, A.C.; Bowman, E.D.; Goodman, J.E.; Bernig, T.; Chanock, S.J.; et al. 3′UTR and functional secretor haplotypes in mannose-binding lectin 2 are associated with increased colon cancer risk in African Americans. Cancer Res. 2012, 72, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Sarvari, J.; Mojtahedi, Z.; Kuramitsu, Y.; Fattahi, M.R.; Ghaderi, A.; Nakamura, K.; Erfani, E. Comparative proteomics of sera from HCC patients with different origins. Hepat. Mon. 2014, 14, e13103. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Peng, H.; Wang, Y.; Xu, M.; Xie, X.; Peng, B.; Kuang, M. Screening for immune-potentiating antigens from hepatocellular carcinoma patients after radiofrequency ablation by serum proteomic analysis. BMC Cancer 2018, 18, 117. [Google Scholar] [CrossRef]
- Troldborg, A.; Hansen, A.; Hansen, S.W.K.; Jensenius, J.C.; Stengaard-Pedersen, K.; Thiel, S. Lectin complement pathway proteins in healthy individuals. Clin. Exp. Immunol. 2017, 188, 138–147. [Google Scholar] [CrossRef]
- Świerzko, A.S.; Michalski, M.; Sokołowska, A.; Nowicki, M.; Szala-Poździej, A.; Eppa, Ł.; Mitrus, I.; Szmigielska-Kapłon, A.; Sobczyk-Kruszelnicka, M.; Michalak, K.; et al. Associations of ficolins with haematological malignancies in patients receiving high-dose chemotherapy and autologous haematopoietic stem cell transplantations (auto-HSCT). Front. Immunol. 2020, 10, 3097. [Google Scholar] [CrossRef]
- Frederiksen, K.; Krag, A.E.; Larsen, J.B.; Kiil, B.J.; Thiel, S.; Hvas, A.-M. Remote ischemic preconditioning does not influence lectin pathway protein levels in head and neck cancer patients undergoing surgery. PLoS ONE 2020, 15, e0230411. [Google Scholar] [CrossRef]
- Sokołowska, A.; Świerzko, A.S.; Gajek, G.; Gołos, A.; Michalski, M.; Nowicki, M.; Szala-Poździej, A.; Wolska-Washer, A.; Brzezińska, O.; Wierzbowska, A.; et al. Associations of ficolins and mannose-binding lectin with acute myeloid leukaemia in adults. Sci. Rep. 2020, 10, 10561. [Google Scholar] [CrossRef]
- Kitamura, F.; Miyata, T.; Uemura, N.; Uchihara, T.; Imai, K.; Hayashi, H.; Yamashita, Y.-I.; Matsusaki, K.; Ishimoto, T.; Baba, H. Proteomic analysis of malignant ascites from patients with pancreatic ductal adenocarcinoma. Anticancer Res. 2021, 41, 2895–2900. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Matta, A.; Shukla, N.K.; Deo, S.V.; Gupta, S.D.; Ralhan, R. Clinical significance of mannose-binding lectin-associated protease-2 expression in esophageal squamous cell carcinoma. Int. J. Cancer 2006, 118, 2930–2935. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Ma, B.Y.; Kawaski, N.; Okimura, K.; Baba, M.; Nakagawa, T.; Miwa, K.; Kawasaki, N.; Oka, S.; Kawasaki, T. Mannan-binding protein blocks the activation of metalloproteases meprin alpha and beta. J. Immunol. 2005, 175, 3177–3185. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, G.; Zhang, H.; Yu, P.; Xiang, X.; Lu, Y.; Dong, X.; Li, X. Expressions and clinical significance of mannose-binding lectin (MBL) and MBL-associated serine protease 2 (MASP-2) in patients with thyroid neoplasm. Chin.-Ger. J. Clin. Oncol. 2013, 12, P106–P108. [Google Scholar] [CrossRef]
- Fisch, U.P.; Zehnder, A.; Hirt, A.; Niggli, F.K.; Simon, A.; Ozsahin, H.; Schlapbach, L.J.; Ammann, R.A. Mannan-binding lectin (MBL) and MBL-associated serine protease 2 in children with cancer. Swiss Med. Wkly. 2011, 141, w13191. [Google Scholar] [CrossRef]
- Arellano-Garcia, M.E.; Li, R.; Liu, X.; Xie, Y.; Yan, X.; Loo, J.A.; Hu, S. Identification of a tetranectin as a potential biomarker for metastatic oral cancer. Int. J. Mol. Sci. 2010, 11, 3106–3121. [Google Scholar] [CrossRef]
- Reis, E.S.; Mastellos, D.C.; Ricklin, D.; Mantovani, A.; Lambris, J.D. Complement in cancer: Untangling an intricate relationship. Nat. Rev. Immunol. 2018, 18, 5–18. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Q.; Li, T.; Liao, Q.; Zhao, Y. Role of the complement system in the tumour microenvironment. Cancer Cell Int. 2019, 19, 300. [Google Scholar] [CrossRef]
- Okrój, M.; Potempa, J. Complement activation as a helping hand for inflammophilic pathogens and cancer. Front. Immunol. 2019, 9, 3125. [Google Scholar] [CrossRef]
- Sinha, A.; Singh, V.; Tandon, R.; Srivastava, L.M. Dichotomy of complement system: Tumorigenesis or destruction. Immunol. Lett. 2020, 223, 89–96. [Google Scholar] [CrossRef]
- Lu, P.; Ma, Y.; Wei, S.; Liang, X. The dual role of complement in cancers, from destroying tumors to promoting tumor development. Cytokine 2021, 143, 155522. [Google Scholar] [CrossRef] [PubMed]
- Bareke, H.; Akbuga, J. Complement system’s role in cancer and its therapeutic potential in ovarian cancer. Scand. J. Immunol. 2018, 88, e12672. [Google Scholar] [CrossRef] [PubMed]
- Kourtzelis, I.; Rafail, S. The dual role of complement in cancer and its implication in anti-tumor therapy. Ann. Transl. Med. 2016, 4, 265. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Kharghan, V. The role of the complement system in cancer. J. Clin. Investig. 2017, 127, 780–789. [Google Scholar] [CrossRef]
- O’Brien, R.M.; Cannon, A.; Reynolds, J.V.; Lusaght, J.; Lynam-Lennon, N. Complement in tumourigenesis and the response to cancer therapy. Cancers 2021, 13, 1209. [Google Scholar] [CrossRef]
- Storm, L.; Christensen, I.J.; Jensenius, J.C.; Nielsen, H.J.; Thiel, S.; Danish Study Group on Early Detection of Colorectal Cancer. Evaluation of complement proteins as screening markers for colorectal cancer. Cancer Immunol. Immunother. 2015, 64, 41–50. [Google Scholar] [CrossRef]
- Ytting, H.; Christensen, I.J.; Thiel, S.; Jensenius, J.C.; Nielsen, H.J. Serum mannan-binding lectin-associated serine protease 2 levels in colorectal cancer: Relation to recurrence and mortality. Clin. Cancer Res. 2005, 11, 1441–1446. [Google Scholar] [CrossRef][Green Version]
- Ytting, H.; Christensen, I.J.; Thiel, S.; Jensenius, J.C.; Nielsen, H.J. Pre- and postoperative levels in serum of mannan-binding lectin associated serine protease-2—A prognostic marker in colorectal cancer. Hum. Immunol. 2008, 69, 414–420. [Google Scholar] [CrossRef]
- Wang, J.; Gao, F.; Mo, F.; Hong, X.; Wang, H.; Zheng, S.; Lin, B. identification of CHI3L1 and MASP2 as a biomarker pair for liver cancer through integrative secretome and transcriptome analysis. Proteom. Clin. Appl. 2009, 3, 541–551. [Google Scholar] [CrossRef]
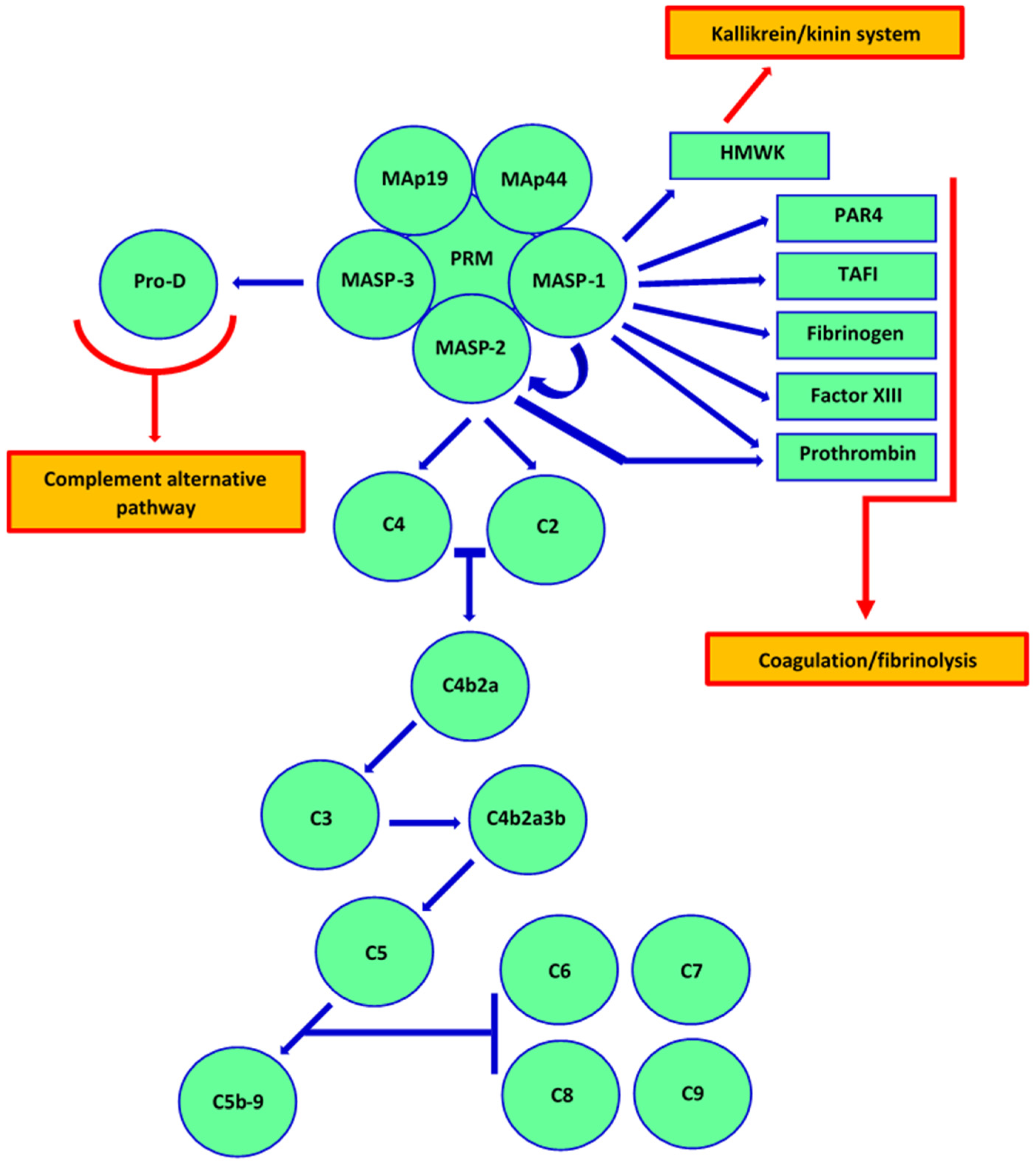
| Molecule | Disease (Association) | References |
|---|---|---|
| Mannose-binding lectin (MBL) | Pancreatic cancer (elevated protein expression; proteomic analysis of serum) | [68] |
| Collectin-10 (CL-10) | Hepatocellular carcinoma (decreased mRNA/protein expression) | [54] |
| Colorectal cancer (low concentration in serum) | [96] | |
| Collectin-11 (CL-11) | Lung adenocarcinoma (serum/urine concentration ratio) | [45] |
| Ficolin-1 | Uterine corpus endometrial carcinoma (high FCN1 gene expression) | [38] |
| Colorectal cancer (high concentration in serum) | [96] | |
| Ficolin-2 | Uterine cervical cancer (high mRNA/protein expression) | [42,43] |
| Oral cancer (elevated protein expression/proteomic analysis of serum) | [86] | |
| Ficolin-3 | Ovarian cancer (elevated expression/proteomic analysis of serum) | [41] |
| Lung adenocarcinoma (decreased FCN3 gene expression} | [30] | |
| MASP-2 | Colorectal cancer (high concentration in serum) | [71,97,98] |
| Non-small lung cancer (high concentration in serum) | [47] | |
| Hepatocellular carcinoma (higher expression: transcriptome/secretome analysis; higher concentration in serum) | [99] | |
| MAp44 | Colorectal cancer (low concentration in serum) | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cedzyński, M.; Świerzko, A.S. Components of the Lectin Pathway of Complement in Solid Tumour Cancers. Cancers 2022, 14, 1543. https://doi.org/10.3390/cancers14061543
Cedzyński M, Świerzko AS. Components of the Lectin Pathway of Complement in Solid Tumour Cancers. Cancers. 2022; 14(6):1543. https://doi.org/10.3390/cancers14061543
Chicago/Turabian StyleCedzyński, Maciej, and Anna S. Świerzko. 2022. "Components of the Lectin Pathway of Complement in Solid Tumour Cancers" Cancers 14, no. 6: 1543. https://doi.org/10.3390/cancers14061543
APA StyleCedzyński, M., & Świerzko, A. S. (2022). Components of the Lectin Pathway of Complement in Solid Tumour Cancers. Cancers, 14(6), 1543. https://doi.org/10.3390/cancers14061543







