Looking for a Simplified Diagnostic Model to Identify Potentially Lethal Cases of Prostate Cancer at Initial Diagnosis: An ImGO Pilot Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Setting and Design
2.2. Study Objectives
2.3. Study Procedures
2.3.1. Histological Analysis
2.3.2. Exploratory NGS Analysis
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Histological Review
3.2.1. Gleason Score Review
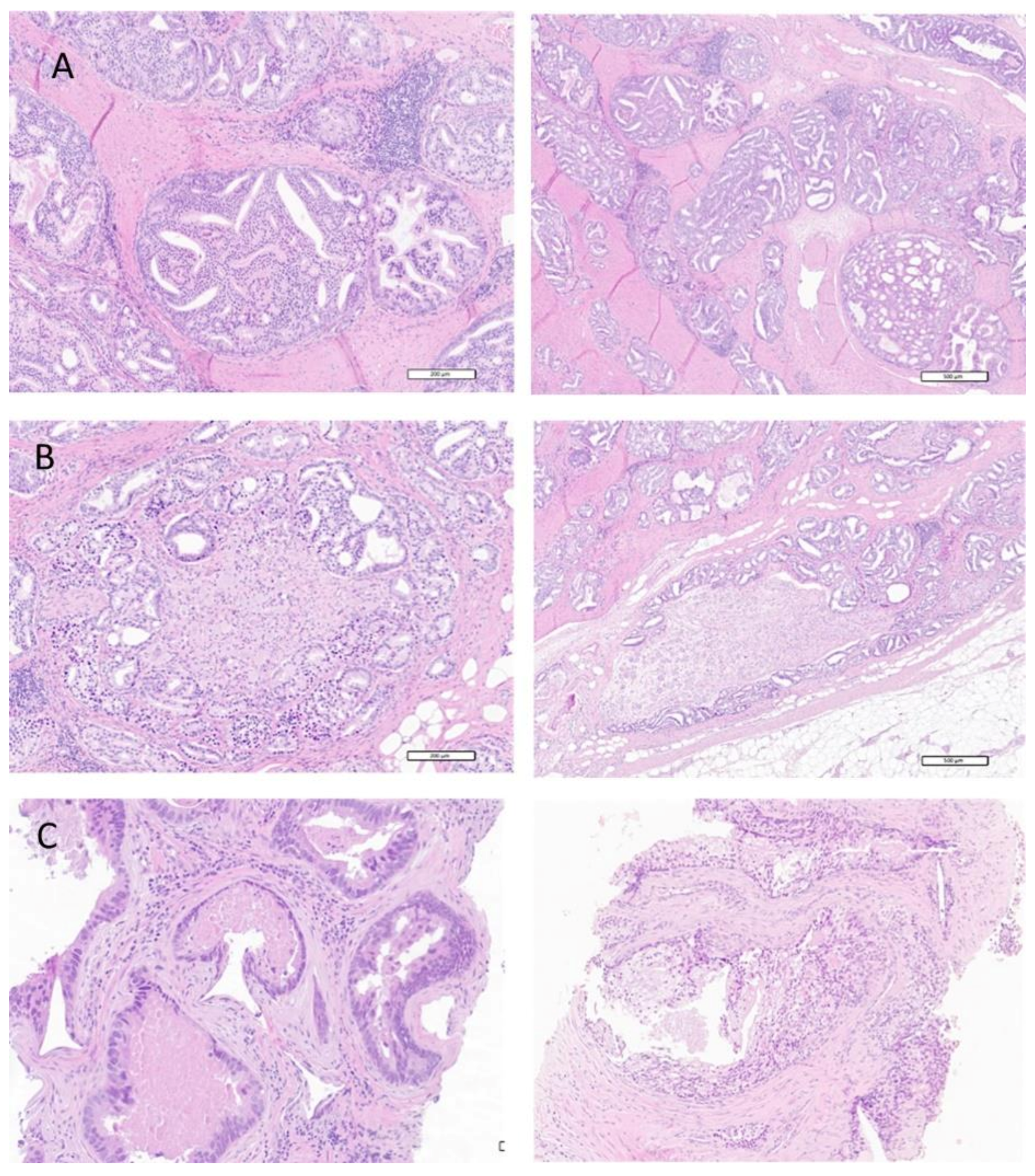
3.2.2. Prevalence of Cribriform Pattern and Intraductal Disease
3.3. Secondary Analyses
3.3.1. Patterns of Treatment and Clinical Outcomes
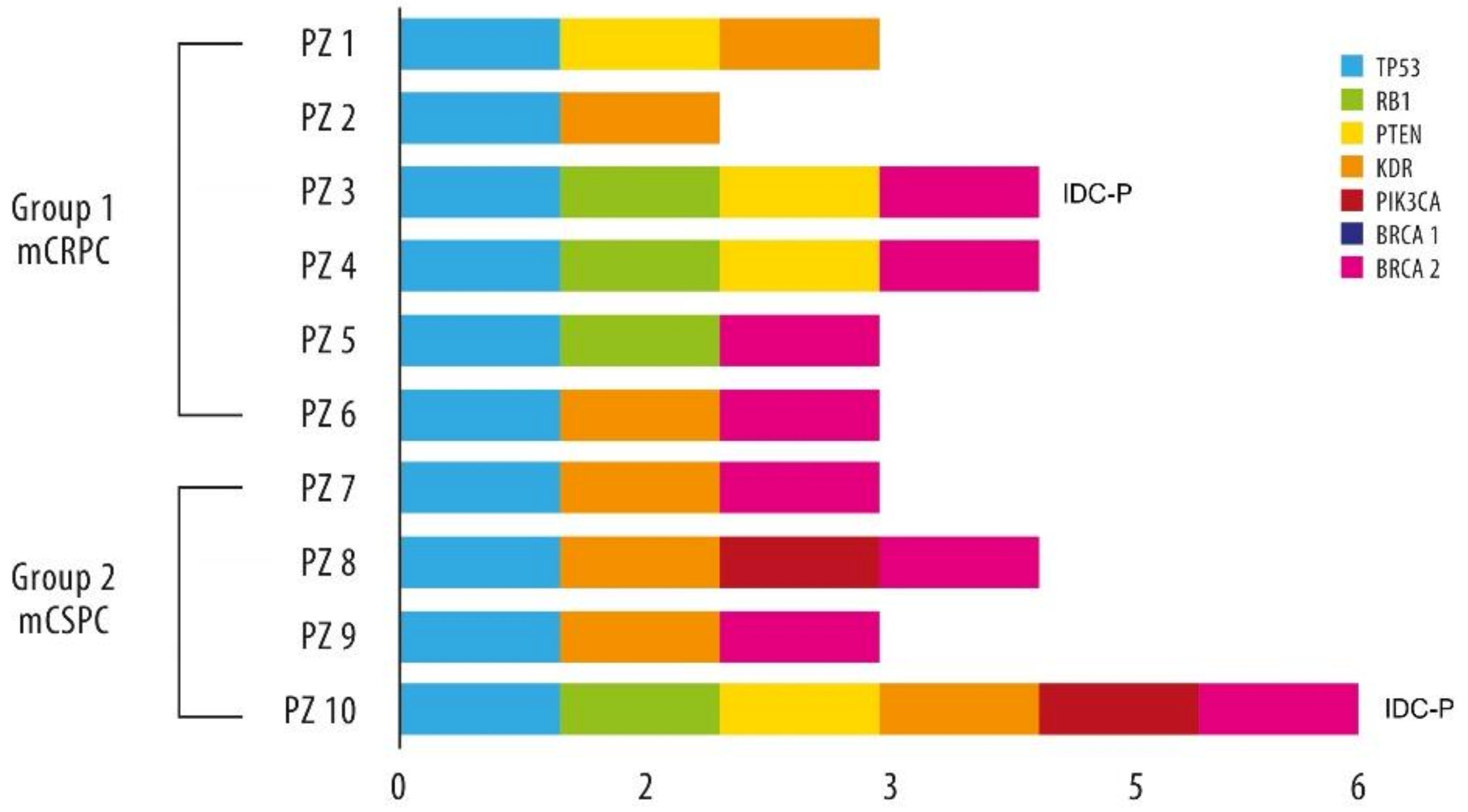
3.3.2. Exploratory NGS Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tolkach, Y.; Kristiansen, G. The heterogeneity of prostate cancer: A practical approach. Pathobiology 2018, 85, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhao, D.; Spring, D.J.; DePinho, R.A. Genetics and biology of prostate cancer. Genes Dev. 2018, 32, 1105–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cucchiara, V.; Cooperberg, M.R.; Dall’Era, M.; Lin, D.W.; Montorsi, F.; Schalken, J.A.; Evans, C.P. Genomic markers in prostate cancer decision making. Eur. Urol. 2018, 73, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Laajala, T.D.; Gerke, T.; Tyekucheva, S.; Costello, J.C. Modeling genetic heterogeneity of drug response and resistance in cancer. Curr. Opin. Syst. Biol. 2019, 17, 8–14. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- VanderWeele, D.J.; Antonarakis, E.S.; Carducci, M.A.; Dreicer, R.; Fizazi, K.; Gillessen, S.; Higano, C.S.; Morgans, A.K.; Petrylak, D.P.; Sweeney, C.J.; et al. Metastatic hormone-sensitive prostate cancer: Clinical decision making in a rapidly evolving landscape of life-prolonging therapy. J. Clin. Oncol. 2019, 37, 2961–2967. [Google Scholar] [CrossRef]
- Labrecque, M.P.; Coleman, I.M.; Brown, L.G.; True, L.D.; Kollath, L.; Lakely, B.; Nguyen, H.M.; Yang, Y.C.; da Costa, R.M.G.; Kaipainen, A.; et al. Molecular profiling stratifies diverse phenotypes of treatment-refractory metastatic castration-resistant prostate cancer. J. Clin. Investig. 2019, 129, 4492–4505. [Google Scholar] [CrossRef] [Green Version]
- Martin, N.E.; Mucci, L.A.; Loda, M.; Depinho, R.A. Prognostic determinants in prostate cancer. Cancer J. 2011, 17, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Kristiansen, G. Markers of clinical utility in the differential diagnosis and prognosis of prostate cancer. Mod. Pathol. 2018, 31, 143–155. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell 2015, 163, 1011–1025. [Google Scholar] [CrossRef] [Green Version]
- Linch, M.; Goh, G.; Hiley, C.; Shanmugabavan, Y.; McGranahan, N.; Rowan, A.; Wong, Y.N.S.; King, H.; Furness, A.; Freeman, A.; et al. Intratumoural evolutionary landscape of high-risk prostate cancer: The PROGENY study of genomic and immune parameters. Ann. Oncol. 2017, 28, 2472–2480. [Google Scholar] [CrossRef]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef] [Green Version]
- Danneman, D.; Drevin, L.; Robinson, D.; Stattin, P.; Egevad, L. Gleason inflation 1998-2011: A registry study of 97,168 men: Trends in Gleason grading. BJU Int. 2015, 115, 248–255. [Google Scholar] [CrossRef]
- Albertsen, P.C.; Hanley, J.A.; Barrows, G.H.; Penson, D.F.; Kowalczyk, P.D.; Sanders, M.M.; Fine, J. Prostate cancer and the Will Rogers phenomenon. J. Natl. Cancer Inst. 2005, 97, 1248–1253. [Google Scholar] [CrossRef]
- Epstein, J.I.; Allsbrook, W.C., Jr.; Amin, M.B.; Egevad, L.L.; ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2005, 29, 1228–1242. [Google Scholar] [CrossRef] [Green Version]
- Montironi, R.; Zhou, M.; Magi-Galluzzi, C.; Epstein, J.I. Features and prognostic significance of intraductal carcinoma of the prostate. Eur. Urol. Oncol. 2018, 1, 21–28. [Google Scholar] [CrossRef]
- Cohen, R.J.; Wheeler, T.M.; Bonkhoff, H.; Rubin, M.A. A proposal on the identification, histologic reporting, and implications of intraductal prostatic carcinoma. Arch. Pathol. Lab. Med. 2007, 131, 1103–1109. [Google Scholar] [CrossRef]
- Kweldam, C.F.; van der Kwast, T.; van Leenders, G.J. On cribriform prostate cancer. Transl. Androl. Urol. 2018, 7, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Risbridger, G.P.; Taylor, R.A.; Clouston, D.; Sliwinski, A.; Thorne, H.; Hunter, S.; Li, J.; Mitchell, G.; Murphy, D.; Frydenberg, M.; et al. Patient-derived xenografts reveal that intraductal carcinoma of the prostate is a prominent pathology in BRCA2 mutation carriers with prostate cancer and correlates with poor prognosis. Eur. Urol. 2015, 67, 496–503. [Google Scholar] [CrossRef]
- Junejo, N.N.; AlKhateeb, S.S. BRCA2 gene mutation and prostate cancer risk: Comprehensive review and update. SMJ 2020, 41, 9–17. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.M.; Montgomery, B.; Taplin, M.E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, N.A.; Zibelman, M.; Lindsay, T.; Feldman, R.A.; Saul, M.; Gatalica, Z.; Korn, W.M.; Heath, E.I. An emerging landscape for canonical and actionable molecular alterations in primary and metastatic prostate cancer. Mol. Cancer Ther. 2020, 19, 1373–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyquist, M.D.; Corella, A.; Coleman, I.; De Sarkar, N.; Kaipainen, A.; Ha, G.; Gulati, R.; Ang, L.; Chatterjee, P.; Lucas, J.; et al. Combined TP53 and RB1 loss promotes prostate cancer resistance to a spectrum of therapeutics and confers vulnerability to replication stress. Cell Rep. 2020, 31, 107669. [Google Scholar] [CrossRef] [PubMed]
- Quigley, D.A.; Dang, H.X.; Zhao, S.G.; Lloyd, P.; Aggarwal, R.; Alumkal, J.J.; Foye, A.; Kothari, V.; Perry, M.D.; Bailey, A.M.; et al. Genomic hallmarks and structural variation in metastatic prostate cancer. Cell 2018, 174, 758–769.e9. [Google Scholar] [CrossRef] [Green Version]
- Pourmand, G.; Salem, S.; Mehrsai, A.; Lotfi, M.; Amirzargar, M.A.; Mazdak, H.; Roshani, A.; Kheirollahi, A.; Kalantar, E.; Baradaran, N.; et al. The risk factors of prostate cancer: A multicentric case-control study in Iran. Asian Pac. J. Cancer Prev. 2007, 8, 422–428. [Google Scholar]
- Lotan, T.L.; Gumuskaya, B.; Rahimi, H.; Hicks, J.L.; Iwata, T.; Robinson, B.D.; Epstein, J.I.; De Marzo, A.M. Cytoplasmic PTEN protein loss distinguishes intraductal carcinoma of the prostate from high-grade prostatic intraepithelial neoplasia. Mod. Pathol. 2013, 26, 587–603. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.B.; Shore, K.T.; Yoon, J.; Mendrinos, S.; McKenney, J.K.; Tian, W. PTEN loss in prostatic adenocarcinoma correlates with specific adverse histologic features (intraductal carcinoma, cribriform Gleason pattern 4 and stromogenic carcinoma). Prostate 2019, 79, 1267–1273. [Google Scholar] [CrossRef]
- Abida, W.; Armenia, J.; Gopalan, A.; Brennan, R.; Walsh, M.; Barron, D.; Danila, D.; Rathkopf, D.; Morris, M.; Slovin, S.; et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis. Oncol. 2017, 2017, PO.17.00029. [Google Scholar] [CrossRef]
| Group 1: mCRPC (n = 50) | Group 2: mCSPC (n = 25) | Group 3: Control (n = 50) | Total (n = 125) | |
|---|---|---|---|---|
| Age (years), median (range) | 69 (55–84) | 67 (49–83) | 70 (52–84) | 69 (49–84) |
| PSA value at diagnosis (ng/mL), median (range) | 46 (1.7–419.0) | 843 (13.2–5800) | 7 (2.0–35.0) | 177 (1.67–5800) |
| Initial Gleason Score, n (%): | ||||
| 31 (62) | 18 (72) | 43 (86) | 92 (74) |
| 19 (38) | 7 (28) | 7 (14) | 33 (26) |
| Lymph node metastases, n (%): | ||||
| 17 (34) | 4 (16) | 48 (96) | 69 (55.2) |
| 33 (66) | 21 (84) | 3 (6) | 56 (44.8) |
| Bone metastases, n (%): | ||||
| 20 (40) | 2 (8) | 47 (94) | 69 (55) |
| 30 (60) | 23 (92) | 3 (6) | 56 (45) |
| Visceral metastases, n (%): | ||||
| 4 (8) | 4 (16) | 0 | 8 (6) |
| 1 (2) | 2 (8) | 0 | 3 (2) |
| 1 (2) | 2 (8) | 0 | 3 (2) |
| Gleason Score | Group 1: mCRPC (n = 50), n (%) | Group 2: mCSPC (n = 25), n (%) | Group 3: Control (n = 50), n (%) | |||
|---|---|---|---|---|---|---|
| Initial | After Revision | Initial | After Revision | Initial | After Revision | |
| 3 + 3 | 0 | 1 (2) | 1 (4) | 1 (4) | 50 (100) | 43 (86) |
| 3 + 4 | 13 (26) | 7 (14) | 1 (4) | 1 (4) | 0 | 6 (12) |
| 4 + 3 | 9 (18) | 7 (14) | 2 (8) | 5 (20) | 0 | 1 (2) |
| 4 + 4 | 14 (28) | 21 (42) | 7 (28) | 8 (32) | 0 | 0 |
| 4 + 5 | 6 (12) | 11 (22) | 7 (28) | 6 (24) | 0 | 0 |
| 5 + 3 | 5 (10) | 0 | 2 (8) | 0 | 0 | 0 |
| 5 + 4 | 3 (6) | 3 (6) | 3 (12) | 3 (12) | 0 | 0 |
| 5 + 5 | 0 | 0 | 2 (8) | 1 (4) | 0 | 0 |
| Group 1: mCRPC (n = 50), n (%) | Group 2: mCSPC (n = 25), n (%) | Group 3: Control (n = 43), n (%) | Total (n = 118), n (%) | |
|---|---|---|---|---|
| Cribiform pattern: | ||||
| 28 (56) | 17 (68) | 0 | 45 (38) |
| 22 (44) | 8 (32) | 43 (100) | 80 (62) |
| Intraductal carcinoma: | ||||
| 1 (2) | 2 (8) | 0 | 3 (3) |
| 49 (98) | 23 (92) | 43 (100) | 115 (97) |
| Group 1: mCRPC (n = 28), n (%) | GROUP 2: mCSPC (n = 17), n (%) | Total (n = 45), n (%) | |
|---|---|---|---|
| Gleason score after revision: | |||
| 0 | 0 | 0 |
| 3 (11) | 1 (6) | 4 (9) |
| 3 (11) | 3 (17) | 6 (13) |
| 16 (56) | 7 (41) | 23 (50) |
| 3 (11) | 4 (24) | 7 (16) |
| 3 (11) | 1 (6) | 4 (9) |
| 0 | 1 (6) | 1 (3) |
| Intraductal carcinoma: | |||
| 1 (4) | 1 (6) | 2 (5) |
| 27 (96) | 16 (94) | 43 (95) |
| Age (years), median (range) | 68 (57–84) | 66 (49–78) | 67 (49–84) |
| PSA value at diagnosis, median (range) | 69 ng/mL (2–419) | 726 ng/mL (13–3500) | 300 ng/mL (1.7–3500) |
| Lymph node metastases: | |||
| 19 (68) | 14 (83) | 33 (73) |
| 9 (32) | 3 (17) | 12 (27) |
| bone metastases: | |||
| 15 (54) | 16 (94) | 31 (69) |
| 13 (46) | 1 (6) | 14 (31) |
| 4 (1–10) | 5 (1–21) | |
| Visceral metastases: | |||
| 2 (7) | 3 (18) | 5 (11) |
| 0 | 2 (12) | 2 (4) |
| 0 | 1 (6) | 1 (2) |
| Group 1: mCRPC (n = 50), n (%) | Group 2: mCSPC (n = 25), n (%) | Group 3: control (n = 43), n (%) | |
|---|---|---|---|
| Early docetaxel chemotherapy | 0 | 21 (84) | 0 |
| mCRPC first-line treatment: | |||
| 42 (84) | 14 (56) | 0 (0) |
| 8 (16) | 11 (44) | 43 (100) |
| mCRPC second-line treatment: | |||
| 20 (40) | 8 (32) | 0 |
| 30 (60) | 17 (68) | 43 (100) |
| mCRPC third-line treatment: | |||
| 8 (16) | 2 (8) | 0 |
| 42 (84) | 23 (92) | 43 (100) |
| mCRPC other treatment: | |||
| 4 (8) | 0 | 0 |
| 46 (92) | 25 (100) | 43 (100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macrini, S.; Francesconi, S.; Caprera, C.; Lancia, D.; Corsi, M.; Gunnellini, M.; Rocchi, A.; Pireddu, A.; Marziani, F.; Mosillo, C.; et al. Looking for a Simplified Diagnostic Model to Identify Potentially Lethal Cases of Prostate Cancer at Initial Diagnosis: An ImGO Pilot Study. Cancers 2022, 14, 1542. https://doi.org/10.3390/cancers14061542
Macrini S, Francesconi S, Caprera C, Lancia D, Corsi M, Gunnellini M, Rocchi A, Pireddu A, Marziani F, Mosillo C, et al. Looking for a Simplified Diagnostic Model to Identify Potentially Lethal Cases of Prostate Cancer at Initial Diagnosis: An ImGO Pilot Study. Cancers. 2022; 14(6):1542. https://doi.org/10.3390/cancers14061542
Chicago/Turabian StyleMacrini, Serena, Simona Francesconi, Cecilia Caprera, Daniela Lancia, Matteo Corsi, Marco Gunnellini, Andrea Rocchi, Anjuta Pireddu, Fiovo Marziani, Claudia Mosillo, and et al. 2022. "Looking for a Simplified Diagnostic Model to Identify Potentially Lethal Cases of Prostate Cancer at Initial Diagnosis: An ImGO Pilot Study" Cancers 14, no. 6: 1542. https://doi.org/10.3390/cancers14061542
APA StyleMacrini, S., Francesconi, S., Caprera, C., Lancia, D., Corsi, M., Gunnellini, M., Rocchi, A., Pireddu, A., Marziani, F., Mosillo, C., Calandrella, M. L., Caserta, C., Giannarelli, D., Guida, A., Ascani, S., & Bracarda, S. (2022). Looking for a Simplified Diagnostic Model to Identify Potentially Lethal Cases of Prostate Cancer at Initial Diagnosis: An ImGO Pilot Study. Cancers, 14(6), 1542. https://doi.org/10.3390/cancers14061542






