Simple Summary
The kinetics of SARS-CoV-2 spike-protein antibodies and the cellular immune landscape following vaccination in patients with hematologic neoplasms are poorly understood. The aim of our prospective and longitudinal study, which included 398 adults, was to compare day 35 and day 120 anti-spike-IgG antibody and day 120 SARS-CoV-2-specific T-cell responses in patients with hematologic malignancies to a reference cohort. Although day 35 seroconversion in controls (98%) was higher compared to patients with myeloid (82%) and lymphoid (48%) neoplasms, substantial increases in day 120 seroconversion were seen in both the myeloid (97%) and lymphoid (66%) cohorts. Remarkably, spike-specific CD4+- and CD8+-cells in the lymphoid (71%/31%) and control (74%/42%) cohorts were comparable. We provide strong evidence of vaccine-elicited immunogenicity in most patients with hematologic malignancies. Both kinetics of seroconversion and cellular responses are crucial to determine which patients with hematologic malignancies will generate immunity. The findings have implications on public health policy regarding recommendations for SARS-CoV-2 booster doses.
Abstract
Purpose: To assess humoral responses longitudinally and cellular immunogenicity following SARS-CoV-2-vaccination in patients with hematologic and oncologic malignancies receiving checkpoint-inhibitors. Methods: This prospective multicenter trial of the East-German-Study-Group-for-Hematology-and-Oncology, enrolled 398 adults in a two (patients; n = 262) to one (controls; n = 136) ratio. Pre-vaccination, day 35 (d35), and day 120 (d120) blood samples were analyzed for anti-spike antibodies and d120 IL-2+IFNγ+TNFα+-CD4+- and CD8+-cells. Laboratories were blinded for patients and controls. Results: Patients belonged to the myeloid (n = 131), lymphoid (n = 104), and checkpoint-inhibitor (n = 17) cohorts. While d35 seroconversion was higher in controls (98%) compared to patients (68%) (p < 0.001), d120 seroconversion improved across all patient cohorts [checkpoint-inhibitors (81% to 100%), myeloid (82% to 97%), lymphoid (48% to 66%)]. CD4+- and CovCD8+-cells in the lymphoid (71%/31%) and control (74%/42%) cohorts were comparable but fewer in the myeloid cohort (53%, p = 0.003 /24%, p = 0.03). In patients with hematologic malignancies, no correlation between d120 humoral and cellular responses was found. A sizeable fraction of lymphoid patients demonstrated T-cell responses without detectable spike-specific-IgGs. Conclusions: Evidence of vaccine-elicited humoral and/or cellular immunogenicity in most patients is provided. Both humoral and cellular responses are crucial to determine which patients will generate/maintain immunity. The findings have implications on public health policy regarding recommendations for SARS-CoV-2 booster doses.
1. Introduction
Published data indicate that mortality from SARS-CoV-2 infections in patients with cancer is mainly associated with general risk factors such as older age and comorbidities [1,2]. Patients with hematologic malignancies have a particular high risk of COVID-19 related death with ~40% mortality rate [3,4]. COVID-19 vaccines have been developed and deployed with remarkable speed [5,6,7,8]. However, patients with malignancy were excluded from pivotal vaccination trials. Recent data indicate that active cancer therapy receiving patients with solid tumors develop adequate antibody responses to vaccination (although the magnitude of these responses is diminished relative to control cohorts) [9,10]. Depending on the type and activity of the disease, there is accumulating evidence that humoral immunity up to 42 days after the second dose of SARS-CoV-2 vaccines is impaired in patients with hematologic malignancies, especially if they were treated with B-cell depleting therapies such as anti-CD20 antibodies [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. These data raised concerns about the efficacy of vaccines in generating humoral immunity in patients with hematologic malignancies, particularly in those with lymphoid neoplasms. However, little is known to the durability of vaccine-elicited antibody responses in patients with hematologic neoplasms. Although neutralizing antibodies are important in vaccine-induced protection as evidenced by the correlations of antibody responses and clinical outcome of COVID-19 infections [27,28,29], growing evidence points towards an equally important role for T-cells [30,31,32,33]. Circulating SARS-CoV-2-specific CD8+ and CD4+ T-cells were identified in ~70% and 100% of COVID-19 convalescent patients, respectively [34]. These T-cell responses were shown to be associated with improved survival after infections even in patients with hematologic neoplasms [35,36]. Similarly, early polyfunctional spike protein-specific T-cell responses were described after COVID-19 vaccination [37,38,39,40]. Indeed, stable and functional CD8+-T-cell responses could be mobilized one week after prime vaccination with BNT162b2 when circulating CD4+-T-cells and neutralizing antibodies were still weakly detectable [40]. mRNA-based vaccination generated robust CD4+- and CD8+-T-cell responses could be generated, despite poor antibody responses, in patients with multiple sclerosis on anti-CD20 antibody therapy after mRNA-based vaccination [41]. Liebers, et al. detected spike protein-specific T-cells 17 days after the second dose of vaccine using an IFNγ ELISPOT in 29/50 (58%) lymphoma patients who had received anti-CD20 treatments [24]. Using an intracellular cytokine assay for IFNγ, TNFα and IL2, Harrington, et al. reported polyfunctional CD8+- and CD4+-T-cells of 35% and 75%, respectively, in 21 patients with BCR-ABL1-negative myeloproliferative neoplasms (MPN) after a median of 21 days after a single dose of BNT162b2 vaccine [15].
Despite the relatively small sample sizes, the lack of a predefined sample collection, and the lack of large reference cohorts, these initial results suggest that patients with hematologic malignancies with insufficient humoral responses might still benefit from vaccinations through cellular responses, considering that effective T-cell responses are essential for SARS-CoV-2 clearance [42]. Indeed, a significant portion of antigen-specific T-cell responses may be missed if only IFNγ–related readouts are used and IL-2 is not taken into consideration [43]. Further, little is known about the longevity of vaccine-elicited cellular immunogenicity in large cohorts of patients with hematologic neoplasms.
In order to investigate the kinetics of IgG responses and relationship to specific T-cell responses, days 35 and 120 vaccine-induced humoral and day 120 cellular responses in patients with hematologic and oncologic malignancies receiving checkpoint inhibitors (PD-L1-inhibitors) were compared to a reference cohort.
2. Materials and Methods
2.1. Study Design
ImV-HOng (OSHO#98) is a longitudinal, prospective, multicenter, non-interventional study which compared day 35 (d35) and day 120 (d120) vaccine-elicited spike protein-specific humoral and d120 T-cell responses between patients and controls. The trial was conducted from 17 March 2021 to 6 December 2021 across seven centers of the East-German-Study-Group-for-Hematology-and-Oncology (OSHO). The trial was approved by the Ethical Review Boards and registered at the Paul-Ehrlich Institute (NIS-584) and Deutsches Register Klinischer Studien (DRKS00027372). The study received a grant from the German Leukemia and Lymphoma Foundation.
2.2. Study Cohorts
The study population comprised adult individuals willing to receive a SARS-CoV-2 vaccination. Participants were enrolled per random sampling after written informed consent into two cohorts at a 1 (controls) to 2 (patients) ratio. The control group included individuals without active cancer in the last five years. In addition to patients with myeloid and lymphoid neoplasms, patients with solid tumors receiving PD-L1 inhibition were also eligible for enrollment in the patient cohort in order to assess the impact of PD-L1 inhibition on immune response after vaccination. The inclusion and exclusion criteria are shown in Table 1.

Table 1.
Inclusion and exclusion criteria.
2.3. Outcomes
The primary outcome was d35 SARS-CoV-2 spike-specific antibody concentrations in patients compared to controls following the first vaccination dose. The WHO launched the first International Standard for anti-SARS-CoV-2 immunoglobulin (IgG), wherein the neat sample was assigned to contain 1000 binding antibody units (BAU)/mL [44,45], and BAU/mL were subsequently converted to U/mL (U/mL = 0.972 × BAU/mL). Key secondary outcomes were d120 spike-specific humoral and T-cell responses in patients compared to controls. Baseline patient-, disease-, vaccination-, and laboratory-characteristics and any potential associations with vaccine-elicited responses were explored.
2.4. Procedures
Blood samples were drawn up to three weeks prior to vaccination, on d35 (±7), and d120 (±14) after the first vaccination dose. The pseudonymized samples were serially analyzed for SARS-CoV-2 spike-specific-IgGs in the Central Laboratory of the University Hospital Halle (Saale). T-cell responses were analyzed at the Special Hematology Laboratory, Rostock University Medical Center. Laboratories were blinded for patient and control groups.
2.5. Laboratory Measurements
2.5.1. Measurement of SARS-CoV-2 Spike Protein Antibodies
The quantitative determination of IgG antibodies to the SARS-CoV-2 spike protein was carried out using the Roche Elecsys® Anti-SARS-CoV-2 S assay (Roche Diagnostics International Ltd., Rotkreuz, Switzerland). The assay is based on a recombinant protein representing the receptor binding domain of the spike antigen in a double-antigen sandwich assay format, with a high specificity and sensitivity [46]. Antibody titers were measured on a Roche Cobas e 801 analyzer integrated in a fully automated Roche Cobas 8000 platform. A concentration of IgG SARS-CoV-2 spike protein antibodies of >0.8 U/mL is considered positive.
2.5.2. SARS-CoV-2 Spike-Specific T-Cell Response
Heparinized whole blood was either left unstimulated (negative control), stimulated with 0.5 µg/mL Staphylococcus enterotoxin B (SEB, positive control) or stimulated using 0.6 nmol of (approximately 1 µg) wild-type spike protein of SARS-CoV2 peptides (SARS-CoV2 Prot_S Complete, REF: 130-127-953, Miltenyi Biotec [MB], Bergisch Gladbach, Germany) per ml blood for 4 h at 37 °C in the presence of Breveldin A. After incubation, bulk lysis, surface and intracellular staining were performed according to EuroFlow guidelines [47].
The panel comprised the following antibodies IL-2:BV421 (clone: MQ1-17H12, Biolegend, San Diego, CA, USA), CD45RA:VioGreen (clone: REA1047), CCR7:FITC (clone: REA546), IFNγ:PE (clone: 45-15), CD4:PE-Vio615 (clone: REA623), CD8:PE-Vio770 (clone: REA734), TNFa-APC (clone: REA656), and CD3:APC-Vio770 (clone: REA613) that were purchased from MB, unless stated otherwise. A median 2,660,024 nucleated cells per sample were acquired on Becton Dickinsion (FACS Lyric) or MB (MACS Quant) flow cytometers. Primary data were analyzed in Infinicyt (v2.0.4b, Cytognos SL, Salamanca, Spain). Gating was in line with recommended standards for ICS assays [48,49].
Raw event numbers and frequencies per population were exported and analyzed using R (v4.1.1). Normalized percentages of SEB-activated and spike-specific T-cells were calculated by subtracting the respective frequencies of the negative control measured for the same sample and expressed as percentage of total CD4+ and CD8+ T-cells of the sample [37,38]. A cohort of 14 not vaccinated and self-reportedly non-infected controls was used to calculate the limit of detection as follows: the z-score for each control sample was calculated per parameter. Samples with a z-score above two were considered as outlier for that parameter and removed (one outlier per parameter was detected). The limit of detection (LOD) was calculated as mean +2SD. All samples above the LOD [0.00459% for CD4 + IL-2 + IFNγ + TNFα + (CovCD4) and 0.00287% for CD8 + IL-2 + IFNγ + TNFα + (CovCD8) T-cells] were considered positive.
2.6. Statistical Analysis
Sample size was calculated based on published data to the immune response after 30 μg BNT162b2 (Comirnaty ©Biontech/Pfizer) vaccine [37]. Assuming a standard deviation of 0.9 for the logarithm of geometric mean concentrations, enrollment of 236 and 118 evaluable patients and controls respectively would provide 80% power (alpha error, 5%) to detect a significant difference in d35 seroconversions between patients and controls.
Continuous covariates were summarized as medians and interquartile ranges (IQRs) and categorical parameters as absolute and relative frequencies. Humoral responses (i.e., anti-Spike IgG concentrations > 0.8 U/mL) on d35 and d120 were compared between patients and controls by evaluating the mean difference in concentrations using t-tests and reporting the 95% confidence interval (CI). Cellular responses on d120 (i.e., CovCD4+ and CovCD+ above the LOD) were similarly compared and expressed. Vaccine-elicited seroconversion and cellular response rates in patient cohorts (i.e., type of diagnosis; cancer therapy vs. none) were evaluated in subgroup analyses. Regression models were used to test the association of baseline characteristics with vaccine-induced humoral and cellular responses. Baseline patient-related factors included age [continuous variable, 5- and 10-years frequency-matching], gender, baseline B- and T-cell counts, pre-vaccination anti-spike-IgGs, and cohort category. Vaccine-related variables were type of vaccine, number of injections, and interval between injections (continuous variable; interval ≤35 vs. >35 days).
Secondary endpoint analyses were explorative. Statistical tests were two-tailed and p values < 0.05 were considered significant. Analyses were performed using IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY, USA: IBM Corp.
3. Results
3.1. Patient Characteristics
A total of 398 adults were enrolled [controls, n = 136; patients, n = 262]. Patients had myeloid (n = 135) and lymphoid (n = 108) neoplasms, and cancer under checkpoint inhibition (n = 19). A CONSORT-Flowchart of participants is shown in Figure 1. This analysis comprises 385 participants who actually received the first vaccination [patients n = 252 (96.2%); controls n = 133 (97.8%)]. Table 2 illustrates the characteristics of vaccinated participants. Patients in the myeloid cohort were most frequently diagnosed with BCR-ABL1-positive (n = 29) and negative myeloproliferative neoplasms (n = 57). Compared to controls, patients were older (p < 0.001). Prior to vaccination, 186 (76.2%) patients were on active cancer therapy. An allogeneic hematopoietic-cell-transplantation (HCT) was documented in 32 participants. The majority of participants (82.6%) received mRNA-based vaccines. A second dose was given to 230 (91.3%) patients and 107 (80.5%) controls after a median of 40 days for patients and 33 days for controls (p = 0.2). Reasons for only one injection were vaccination with the vector-based COVID-19 Vaccine Janssen by ©Johnson&Johnson (n = 21), a history of a SARS-CoV2 infection prior to vaccination (n = 17), and others (n = 10). Due to health authority guidelines, ~50% of participants received the second dose 42 days after the first. A history of a SARS-CoV2 infection prior to vaccination with a median of 7 months and a median pre-vaccination anti-spike-IgG concentration of 122 U/mL (IQR 23.9-480) was documented in 20 (5.2%) subjects. No antibodies were detected in one patient and one control. Anti-spike-IgGs prior to vaccination were detected in 11 participants (9 patients and 2 controls) with no history of a previous infection.
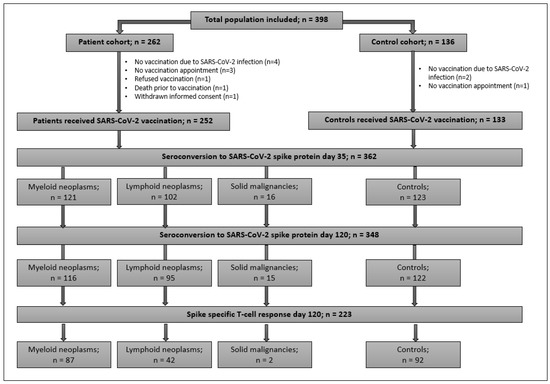
Figure 1.
CONSORT flowchart of study population.

Table 2.
Baseline characteristics of vaccinated study population.
3.2. SARS-CoV-2 Spike-Specific Humoral Response
3.2.1. Day 35 Spike-Specific Seroconversion
Though no difference in d35 anti-spike-IgG mean values between patients and controls (95% CI, −1438.5–559.8) were detected, anti-spike-IgGs >0.8 U/mL were measured in 121 (98%) controls and 162 (68%) patients (p < 0.001). Seroconversion rates in both the myeloid (82%) and lymphoid cohorts (48%) were lower compared to controls (p < 0.001) (Table 3; Figure 2). The same trends were seen when median titers were considered. Seroconversion occurred in 13 (81%) patients under checkpoint inhibitor therapy.

Table 3.
Humoral and T-cell response to vaccination in controls and patient cohorts.
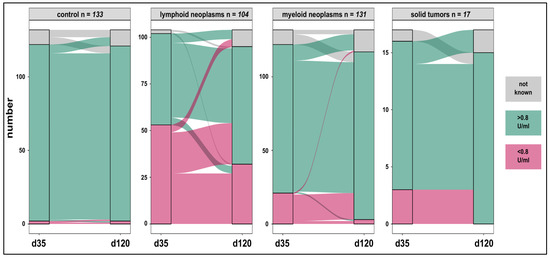
Figure 2.
Humoral anti-spike-specific responses in patients and controls on day 35 and day 120 after vaccination. Specific IgG responses were maintained at high rates in controls (98%) and increased in patients with oncologic malignancies on checkpoint inhibitors (81% to 100%), lymphoid (48% to 66%), and myeloid neoplasms (82% to 97%). A complete loss of the anti-spike IgG was rarely seen.
3.2.2. Day 120 Spike-Specific Seroconversion
The difference in d120 mean IgG values between patients and controls was not significant (95% CI, −639.4–889.1). However, mean values on d120 were significantly higher across all study participants compared to d35 with a mean difference of 477 U/mL (95% CI, 92.4–861.6). Seroconversion in controls was maintained (98%) and substantial increases in the myeloid (97%) and checkpoint inhibitor (100%) cohorts were seen. These response rates were higher compared to those seen in the lymphoid group (66%) (p < 0.001). Similarly, median IgG levels were highest in controls (1212 U/mL) and lowest in the lymphoid cohort (88.3 U/mL) (p < 0.001) (Table 3). Overall, 76% of controls and patients maintained humoral immunogenicity over time (Figure 2). An association between d35 and d120 anti-spike-IgGs in 333 paired samples [controls (n = 117); patients (n = 216)] was found (R2 = 0.34; p < 0.001). Seroconversion on d120, despite d35 IgGs < 0.8 U/mL was documented in 18/20 (90%) and 22/49 (45%) patients with myeloid and lymphoid neoplasms respectively. Five of six subjects who lost d35 response belonged to the lymphoid group. Anti-spike-IgGs > 0.8 U/mL on d120 were detected in 30/33 (91%) subjects including 14 patients who received only one vaccine injection.
3.3. Day 120 SARS-CoV-2 Spike-Specific T-Cell Response
CovCD4 and/or CovCD8 were detected in 155/223 (69.5%) subjects [controls: 81.5%; patients 61% (p = 0.02)] with CovCD4 being more frequent than CovCD8 responses (p < 0.001) (Table 3, Figure 3). The differences in mean values of CovCD4 (95% CI, −0.00–0.02) and CovCD8 (95% CI, −0.00–0.02) between patients and controls were not significant. CovCD4 cells were more frequently detected in the control (74%) and lymphoid (71%) cohorts compared to the myeloid cohort (53%, p = 0.003 vs. controls), mirrored by a similar trend for CovCD8 cells (myeloid 28%, controls 42%, p = 0.03, Table 3). For controls, significant, but relatively weak pair-wise correlations were seen between d120 IgG responses, CovCD4, and CovCD8 cells (Figure 3). Such a correlation could not be detected in the myeloid or lymphoid cohorts. A sizeable fraction of patients in the lymphoid cohort demonstrated CovCD4 and/or CovCD8 responses without detectable spike-specific IgGs (Figure 3).
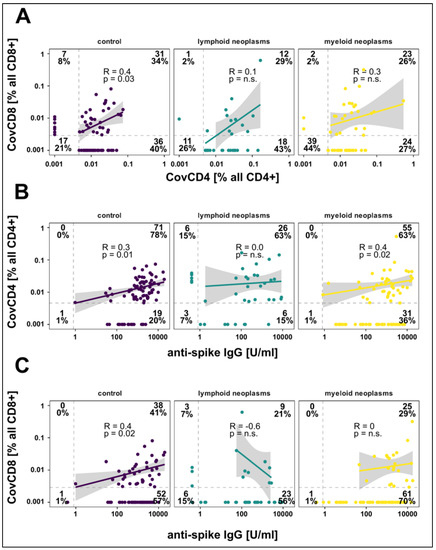
Figure 3.
Correlation between spike-specific CD4 + IL-2 + IFNγ + TNFα + (CovCD4) and CD8 + IL-2 + IFNγ + TNFα + (CovCD8) cell responses (A) and anti-spike IgG concentrations (B,C) on day 120 after vaccination. Results are shown for controls as well as for patients with lymphoid and myeloid neoplasms. Broken lines represent the limit of detection (LOD). Regression lines, Spearman correlation coefficients, and significance are calculated for double positive patients.
3.4. Predictors of Spike-Specific Immune Responses
Both patient- and vaccine-related factors were evaluated for a potential impact on humoral and cellular immune responses. Patient-related variables included age [continuous variable, 5- and 10-years frequency-matching], gender, baseline B- and T-cell counts, pre-vaccination anti-spike-IgGs, cohort category [controls vs. patients], and diagnosis [myeloid neoplasm vs. lymphoid neoplasm vs. solid tumor receiving PD-L1 inhibition]. Vaccine-related factors were the type of vaccine, number of injections (one versus two), and interval between injections [continuous variable; interval ≤35 vs. >35 days].
For the entire study cohort, with the exception of pre-vaccination anti-spike-IgGs (R2 = 0.2; p < 0.001), no relationship was found between d35 and d120 humoral responses and patient-related variables, including older age and vaccine-related factors. In the myeloid group, only pre-vaccination anti-spike-IgGs were associated with higher d35 and d120 humoral responses. The interval between injections had no significant impact on vaccine-induced humoral or cellular responses. Although statistically not significant (p = 0.05), an interval >35 days between injections tended to be associated with lower d35 but not d120 humoral responses in the lymphoid cohort only. In the myeloid and lymphoid cohorts, CovCD4 and CovCD8-cell responses were not associated with patient- or vaccine-related variables.
Due to the diversity of cancer therapies, the impact of treatment on vaccine-elicited responses was explored without regression models. For the myeloid cohort, d35 (83%) and d120 (97.4%) humoral as well as CovCD4 (53%) and CovCD8 (28%) responses were comparable in patients receiving tyrosine-kinase-inhibitors for BCR-ABL1-positive CML, JAK-inhibitors for myeloproliferative neoplasms, and other therapies to those on no treatment (p = 0.3). For the lymphoid group, d35 seroconversion was lowest in the B-cell depleting therapy (13%) or bruton-tyrosine-kinase-inhibitor (BTKi) (21.4%) groups compared to other (58%) or no (62.5%) treatment groups (p < 0.001). No patient on B-cell depleting therapy had detectable d120 anti-spike-IgGs compared to positive seroconversions in the BTKi [7/15; (47%)], other treatment [23/30; (77%)], and no therapy [33/38; (87%)] groups (p < 0.001). CovCD4 [30/42 (71.4%)] and CovCD8 [13/42 (31%)] were detected across all treatment categories.
Humoral responses on d35 were documented in 21/30 (70%) subjects with a history of HCT. On d120, 8/9 subjects with negative d35 antibodies seroconverted including 3/4 patients with a HCT-to-vaccination interval <12 months. CovCD4 (62.5%) and CovCD8 (29%) were measured after HCT.
4. Discussion
Despite the older age of patients compared to controls, sustainable and/or improvements in seroconversion rates and anti-splike-IgG concentrations over time were observed across all cohorts. As expected, d35 seroconversion was higher in controls (98%) compared to patients (68%) (p < 0.001). However, d120 seroconversion improved across all patient cohorts [oncologic malignancies under PD-L1 inhibitor therapies (81% to 100%), myeloid neoplasms (82% to 97%), lymphoid neoplasms (48% to 66%)]. Indeed, patients with myeloid and oncologic malignancies under PD-L1 inhibitor therapies had comparable seroconversion rates to the control group. The few cases with pre-vaccination anti-spike-IgGs without a known history of COVID-19 infection might represent asymptomatic infections or cross-reactive antibodies generated during previous infections with other coronaviral strains [50].
Another key finding was the remarkable and largely seroconversion-independent d120 SARS-CoV-2–specific CD4+TNFa+IFNγ+IL-2+- and CD8+TNFa+IFNγ+IL-2+-cells across all cohorts, particularly in the lymphoid group with the lowest seroconversion rate. Indeed, the cellular response in patients with lymphoid neoplasms with detectable CovCD4+- in 71% and CovCD8+-cells in 31% of cases was comparable to that measured in the control group (CovCD4+- 74% and CovCD8+-cells 42%). A sizeable fraction of lymphoid patients demonstrated T-cell responses without detectable spike-specific-IgGs. Overall, CD4+ T-cell responses outnumbered CD8+ responses in our study. This is in line with what has been observed in immunocompetent individuals [34,51].
To our knowledge, this work is the first to describe the kinetics of SARS-CoV-2 vaccine-induced humoral and cellular responses over time. The impaired early (d35) seroconversion in patients with hematologic malignancies is in line with previous publications [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. However, the majority of patients demonstrate sustained and/or improved humoral and/or cellular responses if measured later (d120). These immune responses were seen irrespective of the type of vaccine or interval between injections. In a recent longitudinal study, antibodies against the SARS-CoV-2 spike antigen and specific memory cell responses were detected in 96% and 63% of health care workers four- and eight-months post infection [52,53].
In line with the literature, our data imply that the previously reported “early“ T-cell responses [15,24,34,35,36,37,38,39,40,41] are likely to persist for several months in patients with hematologic malignancies after vaccination similar to what has been observed in immunocompetent individuals after COVID-19 infections [30,31,32,53].
Taken together, our results underscore the need for large-scale follow-up data to establish standardized post-vaccination time-windows for humoral and cellular response assessments to identify “true vaccination failures” in cancer patients.
However, the routine applicability of tests to measure humoral and cellular immune responses remains challenging. Although several assays for anti-SARS-CoV-2-IgG are commercially available, current assays generate discrepant results. In fact, we are still far from the identification of optimal thresholds for IgG-positivity as a surrogate for neutralization capacity and neutralizing antibodies (NAbs) which confer protection [29,54,55,56,57]. Further, correlations between NAbs and clinical efficacy against infections are weak and likely rely on the population tested [58,59].
The issue is even more complicated regarding cellular response assays. Generally, they are not readily available and mainly used for research purpose. There is often a preponderance of using IFNγ–related readouts to assess T-cell responses [24,60]. However, data suggest that polyfunctional T-cells have higher protective efficacy after vaccination compared to IFNγ monofunctional T-cells [61]. The true percentages of patients developing polyfunctional vaccine-induced CD4+TNFa+IFNγ+IL-2+- and CD8+TNFa+IFNγ+IL-2+-cells might be incorrectly assessed if IL-2 is not considered [43].
Although cellular responses are promising indicators of immunity, our data do not suggest that those with a response compared to those without such a response are more likely to be protected. Yet, even if infections cannot be prevented, it is still possible that T-cell responses are sufficient to ensure mild courses of COVID-19 disease. Thus, studies are necessary to evaluate the degree of cellular-induced clinical protection. Further, SARS-CoV-2 variants such as Omicron (B.1.1.529) with their antibody escape highlight the importance of addressing whether T-cell recognition is also affected.
One limitation is that we did not measure NAbs with virus neutralization assays which are considered to be the gold standard. Yet, we used an anti-SARS-CoV-2 IgG assay with cutoffs for reasonable prediction of NAb [62]. After enrollment started, health authorities in some federal states in Germany changed the interval between injections from 21 to 42 days. The potential impact on d35 response evaluation was discussed with the statistician and accounted for by including the interval as a vaccine-related variable in the regression model. As ~50% of participants across all cohorts received the second injection 42 days later, comparison between groups was feasible. Finally, despite the relatively large number of participants, data of secondary outcomes remain explorative and need to be confirmed in larger trials.
In summary, our longitudinal study describes the nature of SARS-CoV-2 vaccine-induced humoral and cellular immune landscape in patients with hematologic on oncologic malignancies under PD-L1 inhibition.
5. Conclusions
We provide strong empirical evidence of early and late SARS-CoV-2 vaccine-elicited immunogenicity in patients with hematologic neoplasms and oncologic patients receiving checkpoint inhibitors. Even with blunted and heterogeneous antibody responses, T-cell priming seems to be largely intact. This study provides key information and fills knowledge gaps with respect to T-cell responses in vulnerable persons. Both the kinetics of anti-SARS-CoV-2 antibodies over time and cellular responses are crucial to determine which patients will generate and maintain immunity after vaccination.
The findings have implications on clinical decision-making for designing vaccine strategies given the current timing and recommendations for SARS-CoV-2 booster doses. It will be important in the future to determine whether the residual humoral immunity and sustained T-cell responses, retain the ability to respond to emerging SARS-CoV-2 variants. Larger studies with clinical outcomes are needed.
Author Contributions
H.K.A.-A., S.J., S.S. and N.J. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. S.J. and S.S. contributed equally as first authors. Conceptualization, H.K.A.-A., M.B., N.J. and S.S.; methodology, H.K.A.-A., M.B., S.B., B.L.-K., N.J. and S.S.; formal analysis, H.K.A.-A., S.B., R.E., N.J., S.J. and S.S.; investigation, H.K.A.-A., T.B., S.B., R.E., N.J., S.J., F.B.K., B.L.-K., J.M., B.P., G.P.-K., S.S., M.S. and C.S.; resources, H.K.A.-A., T.B., S.B., R.E., N.J., F.B.K., B.L.-K., J.M., B.P., G.P.-K., S.S., M.S. and C.S.; data curation, H.K.A.-A., S.B., R.E., N.J., S.J., F.B.K., B.L.-K., N.N., S.S. and C.Z.; writing—original draft preparation, H.K.A.-A., S.B., R.E., S.J., N.J., F.B.K., B.L.-K. and S.S.; writing—review and editing, all authors; visualization, H.K.A.-A., S.B., R.E. and N.J.; supervision, H.K.A.-A., S.B. and B.L.-K.; project administration, H.K.A.-A., S.B., R.E., S.J., C.J., F.B.K., B.L.-K., N.N., S.S. and C.Z.; funding acquisition, H.K.A.-A. and C.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Medical Faculty of the Martin-Luther University Halle-Wittenberg and the University Medical School Rostock. This work was supported by a grant from the German Leukemia and Lymphoma Foundation (Stiftung Deutsche Leukämie- & Lymphom-Hilfe), grant number 32105069.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of the MARTIN-LUTHER-UNIVERSITY HALLE-WITTENBERG (protocol code 2021-026; date of approval: 17 March 2021), the Ethics Committee of the SÄCHSISCHE LANDESÄRZTEKAMMER (protocol code EK-BR-45/21-1; date of approval: 20 April 2021), and the Ethics Committee of the ÄRZTEKAMMER SACHSEN-ANHALT (protocol code 37/21; date of approval: 31 May 2021),” for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The trial was registered at Deutsches Register Klinischer Studien (DRKS00027372) and the Paul-Ehrlich Institute (NIS-584).
Acknowledgments
The authors are grateful to Andreas Wienke for sample size calculation and design of statistical methods and to the work of the clinical, laboratory, and administrative personnel without whom this study would not have been possible. The authors thank all study participants.
Conflicts of Interest
A-A.H.K.: Consulting: Novartis, BMS, Takeda, Pfizer, Abbvie; Honoraria: Novartis, BMS, Takeda, Pfizer, Abbvie; Research Funding: Novartis, BMS, Incyte; B.S.: Consulting: Roche, Honoraria Roche, AbbVie, Novartis, AstraZeneca, Amgen, Janssen, Research Funding: Janssen, Miltenyi Biotec, Roche, Genentech, AbbVie; J.C.: Honoraria Novartis, Amgen, Janssen, AbbVie Research Funding: Janssen, Miltenyi Biotec, Roche, Centogene; All other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Lee, L.Y.W.; Cazier, J.-B.; Angelis, V.; Arnold, R.; Bisht, V.; Campton, N.A.; Chackathayil, J.; Cheng, V.W.T.; Curley, H.M.; Fittall, M.W.T.; et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 2020, 395, 1919–1926. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, S.-H.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- García-Suárez, J.; de la Cruz, J.; Cedillo, Á.; Llamas, P.; Duarte, R.; Jiménez-Yuste, V.; Hernández-Rivas, J.A.; Gil-Manso, R.; Kwon, M.; Sánchez-Godoy, P.; et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: Lessons from a large population-based registry study. J. Hematol. Oncol. 2020, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Xhaard, A.; Xhaard, C.; D’Aveni, M.; Salvator, H.; Chabi, M.-L.; Berceanu, A.; Coman, T.; Beguin, Y.; Chalandon, Y.; Poiré, X.; et al. Risk factors for a severe form of COVID-19 after allogeneic haematopoietic stem cell transplantation: A Société Francophone de Greffe de Moelle et de Thérapie cellulaire (SFGM-TC) multicentre cohort study. Br. J. Haematol. 2021, 192, e121–e124. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Oosting, S.F.; van der Veldt, A.A.M.; GeurtsvanKessel, C.H.; Fehrmann, R.S.N.; van Binnendijk, R.S.; Dingemans, A.-M.C.; Smit, E.F.; Hiltermann, T.J.N.; den Hartog, G.; Jalving, M.; et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: A prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021, 22, 1681–1691. [Google Scholar] [CrossRef]
- Shroff, R.T.; Chalasani, P.; Wei, R.; Pennington, D.; Quirk, G.; Schoenle, M.V.; Peyton, K.L.; Uhrlaub, J.L.; Ripperger, T.J.; Jergović, M.; et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat. Med. 2021, 27, 2002–2011. [Google Scholar] [CrossRef]
- Diefenbach, C.; Caro, J.; Koide, A.; Grossbard, M.; Goldberg, J.D.; Raphael, B.; Hymes, K.; Moskovits, T.; Kreditor, M.; Kaminetzky, D.; et al. Impaired Humoral Immunity to SARS-CoV-2 Vaccination in Non-Hodgkin Lymphoma and CLL Patients. medRxiv 2021. [Google Scholar] [CrossRef]
- Herishanu, Y.; Avivi, I.; Aharon, A.; Shefer, G.; Levi, S.; Bronstein, Y.; Morales, M.; Ziv, T.; Arbel, Y.S.; Scarfò, L.; et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021, 137, 3165–3173. [Google Scholar] [CrossRef] [PubMed]
- Addeo, A.; Shah, P.K.; Bordry, N.; Hudson, R.D.; Albracht, B.; Di Marco, M.; Kaklamani, V.; Dietrich, P.-Y.; Taylor, B.S.; Simand, P.-F.; et al. Immunogenicity of SARS-CoV-2 messenger RNA Vaccines in Patients with Cancer. Cancer Cell. Cancer Cell 2021, 39, 1091–1098.e2. [Google Scholar] [CrossRef]
- Pimpinelli, F.; Marchesi, F.; Piaggio, G.; Giannarelli, D.; Papa, E.; Falcucci, P.; Pontone, M.; Di Martino, S.; Laquintana, V.; La Malfa, A.; et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: Preliminary data from a single institution. J. Hematol. Oncol. 2021, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Harrington, P.; de Lavallade, H.; Doores, K.J.; O’Reilly, A.; Seow, J.; Graham, C.; Lechmere, T.; Radia, D.; Dillon, R.; Shanmugharaj, Y.; et al. Single dose of BNT162b2 mRNA vaccine against SARS-CoV-2 induces high frequency of neutralising antibody and polyfunctional T-cell responses in patients with myeloproliferative neoplasms. Leukemia 2021, 35, 3573–3577. [Google Scholar] [CrossRef]
- Harrington, P.; Doores, K.J.; Radia, D.; O’Reilly, A.; Lam, H.P.J.; Seow, J.; Graham, C.; Lechmere, T.; McLornan, D.; Dillon, R.; et al. Single dose of BNT162b2 mRNA vaccine against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) induces neutralising antibody and polyfunctional T-cell responses in patients with chronic myeloid leukaemia. Br. J. Haematol. 2021, 194, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Oekelen, O.V.; Gleason, C.R.; Agte, S.; Srivastava, K.; Beach, K.F.; Aleman, A.; Kappes, K.; Mouhieddine, T.H.; Wang, B.; Chari, A.; et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell 2021, 39, 1028–1030. [Google Scholar] [CrossRef] [PubMed]
- Maneikis, K.; Šablauskas, K.; Ringelevičiūtė, U.; Vaitekėnaitė, V.; Čekauskienė, R.; Kryžauskaitė, L.; Naumovas, D.; Banys, V.; Pečeliūnas, V.; Beinortas, T.; et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: A national prospective cohort study. Lancet Haematol. 2021, 8, e583–e592. [Google Scholar] [CrossRef]
- Malard, F.; Gaugler, B.; Gozlan, J.; Bouquet, L.; Fofana, D.; Siblany, L.; Eshagh, D.; Adotevi, O.; Laheurte, C.; Ricard, L.; et al. Weak immunogenicity of SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Cancer J. 2021, 11, 142. [Google Scholar] [CrossRef]
- Monin, L.; Laing, A.G.; Muñoz-Ruiz, M.; McKenzie, D.R.; del Molino del Barrio, I.; Alaguthurai, T.; Domingo-Vila, C.; Hayday, T.S.; Graham, C.; Seow, J.; et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: Interim analysis of a prospective observational study. Lancet Oncol. 2021, 22, 765–778. [Google Scholar] [CrossRef]
- Le Bourgeois, A.; Coste-Burel, M.; Guillaume, T.; Peterlin, P.; Garnier, A.; Béné, M.C.; Chevallier, P. Safety and Antibody Response After 1 and 2 Doses of BNT162b2 mRNA Vaccine in Recipients of Allogeneic Hematopoietic Stem Cell Transplant. JAMA Netw. Open 2021, 4, e2126344. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.; Panopoulou, A.; Shea, R.L.; Tsui, M.; Saso, R.; Sud, A.; West, S.; Smith, K.; Barwood, J.; Kaczmarek, E.; et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021, 8, e389–e392. [Google Scholar] [CrossRef]
- Ghione, P.; Gu, J.J.; Attwood, K.; Torka, P.; Goel, S.; Sundaram, S.; Mavis, C.; Johnson, M.; Thomas, R.; McWhite, K.; et al. Impaired humoral responses to COVID-19 vaccination in patients with lymphoma receiving B-cell–directed therapies. Blood 2021, 138, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Liebers, N.; Speer, C.; Benning, L.; Bruch, P.-M.; Kraemer, I.; Meissner, J.; Schnitzler, P.; Kräusslich, H.-G.; Dreger, P.; Mueller-Tidow, C.; et al. Humoral and cellular responses after COVID-19 vaccination in anti-CD20 treated lymphoma patients. Blood 2021, 139, 142–147. [Google Scholar] [CrossRef]
- Roeker, L.E.; Knorr, D.A.; Thompson, M.C.; Nivar, M.; Lebowitz, S.; Peters, N.; Deonarine, I., Jr.; Momotaj, S.; Sharan, S.; Chanlatte, V.; et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia 2021, 35, 2703–2705. [Google Scholar] [CrossRef]
- Terpos, E.; Trougakos, I.P.; Gavriatopoulou, M.; Papassotiriou, I.; Sklirou, A.D.; Ntanasis-Stathopoulos, I.; Papanagnou, E.-D.; Fotiou, D.; Kastritis, E.; Dimopoulos, M.A. Low neutralizing antibody responses against SARS-CoV-2 in elderly myeloma patients after the first BNT162b2 vaccine dose. Blood 2021, 137, 3674–3676. [Google Scholar] [CrossRef]
- Lucas, C.; Klein, J.; Sundaram, M.E.; Liu, F.; Wong, P.; Silva, J.; Mao, T.; Oh, J.E.; Mohanty, S.; Huang, J.; et al. Delayed production of neutralizing antibodies correlates with fatal COVID-19. Nat. Med. 2021, 27, 1309. [Google Scholar] [CrossRef]
- Dispinseri, S.; Secchi, M.; Pirillo, M.F.; Tolazzi, M.; Borghi, M.; Brigatti, C.; De Angelis, M.L.; Baratella, M.; Bazzigaluppi, E.; Venturi, G.; et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat. Commun. 2021, 12, 2670. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Dawen Yu, E.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Stralin, K.; Gorin, J.-B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiolet, S.; et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 2020, 183, 158–168.e14. [Google Scholar] [CrossRef] [PubMed]
- Rydyznski Moderbacher, C.; Ramirez, S.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369, eabc8511. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Rydyznski Moderbacher, C.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020, 181, 1489–1501.e1415. [Google Scholar] [CrossRef] [PubMed]
- Bange, E.M.; Han, N.A.; Wileyto, P.; Kim, J.Y.; Gouma, S.; Robinson, J.; Greenplate, A.R.; Hwee, M.A.; Porterfield, F.; Owoyemi, O.; et al. CD8 + T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat. Med. 2021, 27, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Sattler, A.; Angermair, S.; Stockmann, H.; Heim, K.M.; Khadzhynov, D.; Treskatsch, S.; Halleck, F.; Kreis, M.E.; Kotsch, K. SARS-CoV-2-specific T cell responses and correlations with COVID-19 patient predisposition. J. Clin. Investig. 2020, 130, 6477–6489. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021, 27, 1530–1535. [Google Scholar] [CrossRef]
- Swanson, P.A.; Padilla, M.; Hoyland, W.; McGlinchey, K.; Fields, P.A.; Bibi, S.; Faust, S.N.; McDermott, A.B.; Lambe, T.; Pollard, A.J.; et al. AZD1222/ChAdOx1 nCoV-19 vaccination induces a polyfunctional spike protein-specific Th1 response with a diverse TCR repertoire. Sci. Transl. Med. 2021, 13, eabj7211. [Google Scholar] [CrossRef]
- Oberhardt, V.; Luxenburger, H.; Kemming, J.; Schulien, I.; Ciminski, K.; Giese, S.; Csernalabics, B.; Lang-Meli, J.; Janowska, I.; Staniek, J.; et al. Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine. Nature 2021, 597, 268–273. [Google Scholar] [CrossRef]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Knezevic, I.; Mattiuzzo, G.; Page, M.; Minor, P.; Griffiths, E.; Nuebling, M.; Moorthy, V. WHO International Standard for evaluation of the antibody response to COVID-19 vaccines: Call for urgent action by the scientific community. Lancet Microbe 2021, 3, e235–e240. [Google Scholar] [CrossRef]
- WHO International Institute for Biological Standards and Control. First WHO International Standard Anti-SARS-CoV-2 Immunoglobulin (Human). Version 2.0. NIBSC Code: 20/136. Counter = 1213. Available online: https://www.nibsc.org/products/brm_product_catalogue/detail_page.aspx?catid=20/136 (accessed on 17 December 2020).
- Riester, E.; Findeisen, P.; Hegel, J.K.; Kabesch, M.; Ambrosch, A.; Rank, C.M.; Pessl, F.; Laengin, T.; Niederhauser, C. Performance evaluation of the Roche Elecsys Anti-SARS-CoV-2 S immunoassay. J. Virol. Methods 2021, 297, 114271. [Google Scholar] [CrossRef]
- Kalina, T.; Flores-Montero, J.; van der Velden, V.H.J.; Martin-Ayuso, M.; Böttcher, S.; Ritgen, M.; Almeida, J.; Lhermitte, L.; Asnafi, V.; Mendonça, A.; et al. EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia 2012, 26, 1986–2010. [Google Scholar] [CrossRef] [Green Version]
- McNeil, L.K.; Price, L.; Britten, C.M.; Jaimes, M.; Maecker, H.; Odunsi, K.; Matsuzaki, J.; Staats, J.S.; Thorpe, J.; Yuan, J.; et al. A harmonized approach to intracellular cytokine staining gating: Results from an international multiconsortia proficiency panel conducted by the Cancer Immunotherapy Consortium (CIC/CRI). Cytom. Part A 2013, 83, 728–738. [Google Scholar] [CrossRef]
- Price, L.S.; Adamow, M.; Attig, S.; Fecci, P.; Norberg, P.; Reap, E.; Janetzki, S.; McNeil, L.K. Gating Harmonization Guidelines for Intracellular Cytokine Staining Validated in Second International Multiconsortia Proficiency Panel Conducted by Cancer Immunotherapy Consortium (CIC/CRI). Cytom. Part A 2021, 99, 107–116. [Google Scholar] [CrossRef]
- Bates, T.; Weinstein, J.; Farley, S.; Leier, H.; Messer, W.; Tafesse, F. Cross-reactivity of SARS-CoV structural protein antibodies against SARS-CoV-2. Cell Rep. 2021, 34, 108737. [Google Scholar] [CrossRef]
- Law, J.C.; Koh, W.H.; Budylowski, P.; Lin, J.; Yue, F.; Abe, K.T.; Rathod, B.; Girard, M.; Li, Z.; Rini, J.M.; et al. Systematic Examination of Antigen-Specific Recall T Cell Responses to SARS-CoV-2 versus Influenza Virus Reveals a Distinct Inflammatory Profile. J. Immunol. 2021, 206, 37–50. [Google Scholar] [CrossRef]
- Havervall, S.; Jernbom Falk, A.; Klingström, J.; Ng, H.; Greilert-Norin, N.; Gabrielsson, L.; Salomonsson, A.-C.; Isaksson, E.; Rudberg, A.-S.; Hellström, C.; et al. SARS-CoV-2 induces a durable and antigen specific humoral immunity after asymptomatic to mild COVID-19 infection. PLoS ONE 2022, 17, e0262169. [Google Scholar] [CrossRef] [PubMed]
- Havervall, S.; Ng, H.; Jernbom Falk, A.; Greilert-Norin, N.; Manberg, A.; Marking, U.; Laurén, I.; Gabrielsson, L.; Salomonsson, A.-C.; Aguilera, K.; et al. Robust humoral and cellular immune responses and low risk for reinfection at least 8 months following asymptomatic to mild COVID-19. J. Intern. Med. 2021, 291, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Shin, S.; Nam, M.; Hong, Y.J.; Roh, E.Y.; Park, K.U.; Song, E.Y. Performance evaluation of three automated quantitative immunoassays and their correlation with a surrogate virus neutralization test in coronavirus disease 19 patients and pre-pandemic controls. J. Clin. Lab. Anal. 2021, 35, e23921. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.G.; Ferreira, V.H.; Ku, T.; Ierullo, M.; Majchrzak-Kita, B.; Chaparro, C.; Selzner, N.; Schiff, J.; McDonald, M.; Tomlinson, G.; et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N. Engl. J. Med. 2021, 385, 1244–1246. [Google Scholar] [CrossRef]
- Dimeglio, C.; Herin, F.; Martin-Blondel, G.; Miedougé, M.; Izopet, J. Antibody titers and protection against a SARS-CoV-2 infection. J. Infect. 2021, 84, 248–288. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Oxford COVID Vaccine Trial Group. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Patel, E.U.; Bloch, E.M.; Clarke, W.; Hsieh, Y.H.; Boon, D.; Eby, Y.; Fernandez, R.E.; Baker, O.R.; Keruly, M.; Kirby, C.S.; et al. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. J. Clin. Microbiol. 2021, 59, e02257-20. [Google Scholar] [CrossRef]
- Benning, L.; Tollner, M.; Hidmark, A.; Schaier, M.; Nusshag, C.; Kaelbe, F.; Reichel, P.; Buylaert, M.; Grenz, J.; Ponath, G.; et al. Heterologous ChAdOx1 nCoV-19/BNT162b2 Prime-Boost Vaccination Induces Strong Humoral Responses among Health Care Workers. Vaccines 2021, 9, 857. [Google Scholar] [CrossRef]
- Salomé, B.; Horowitz, A. Impaired CD4 T-cell Response to SARS-CoV-2: Rationale for PD-1 Blockade in Patients with Cancer and COVID-19? Cancer Discov. 2021, 11, 1877–1878. [Google Scholar] [CrossRef]
- Lin, L.; Finak, G.; Ushey, K.; Seshadri, C.; Hawn, T.R.; Frahm, N.; Scriba, T.J.; Mahomed, H.; Hanekom, W.; Bart, P.-A.; et al. COMPASS identifies T-cell subsets correlated with clinical outcomes. Nat. Biotechnol. 2015, 33, 610–616. [Google Scholar] [CrossRef]
- Rus, K.; Korva, M.; Knap, N.; Zupanc, T.; Poljak, M. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J. Clin. Virol. 2021, 139, 104820. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).