Is Maintaining Thyroid-Stimulating Hormone Effective in Patients Undergoing Thyroid Lobectomy for Low-Risk Differentiated Thyroid Cancer? A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
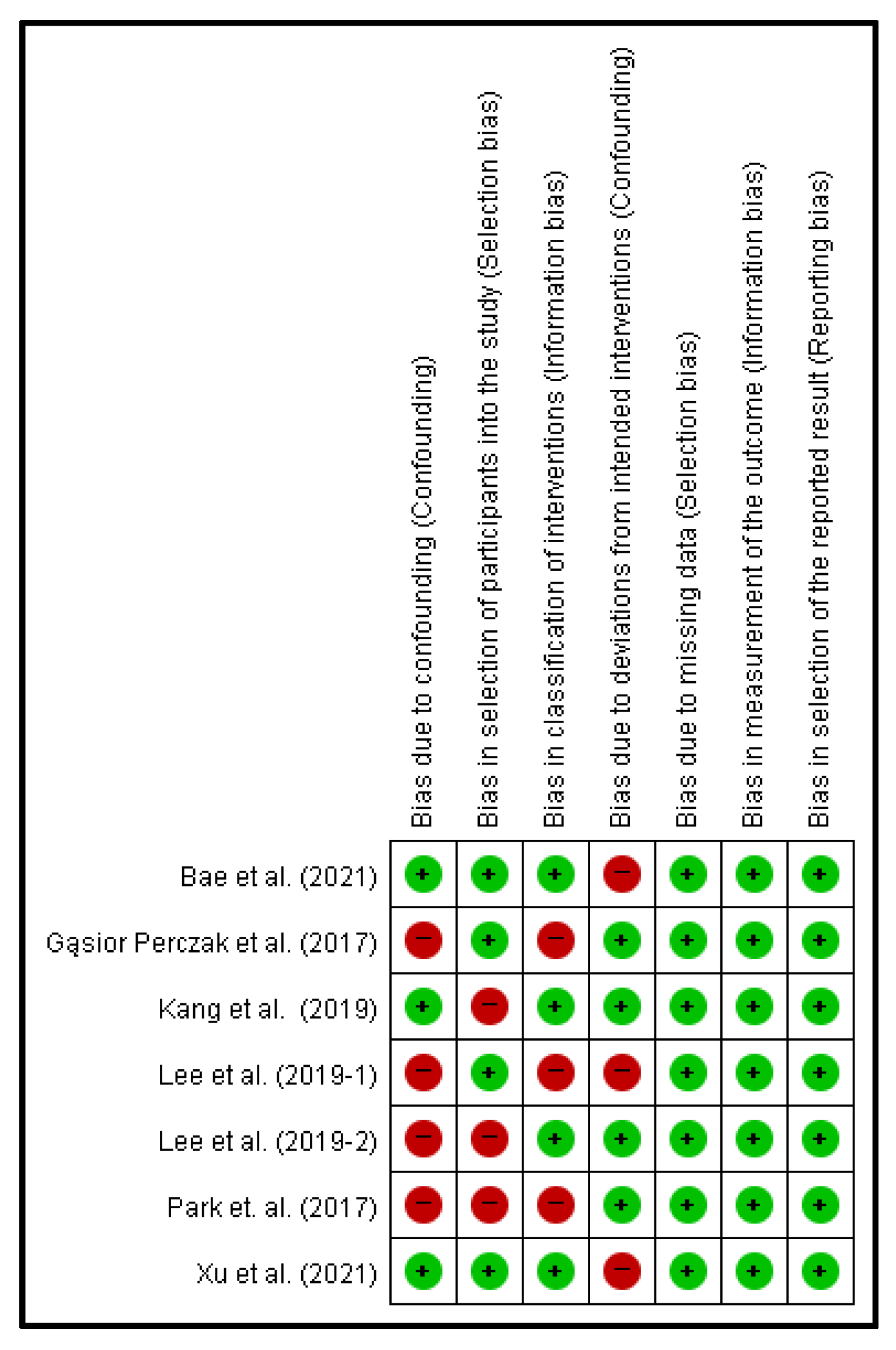
2.4. Risk of Bias Analysis
2.5. Quantitative Data Analysis
2.6. Assessment of Study Heterogeneity
3. Results
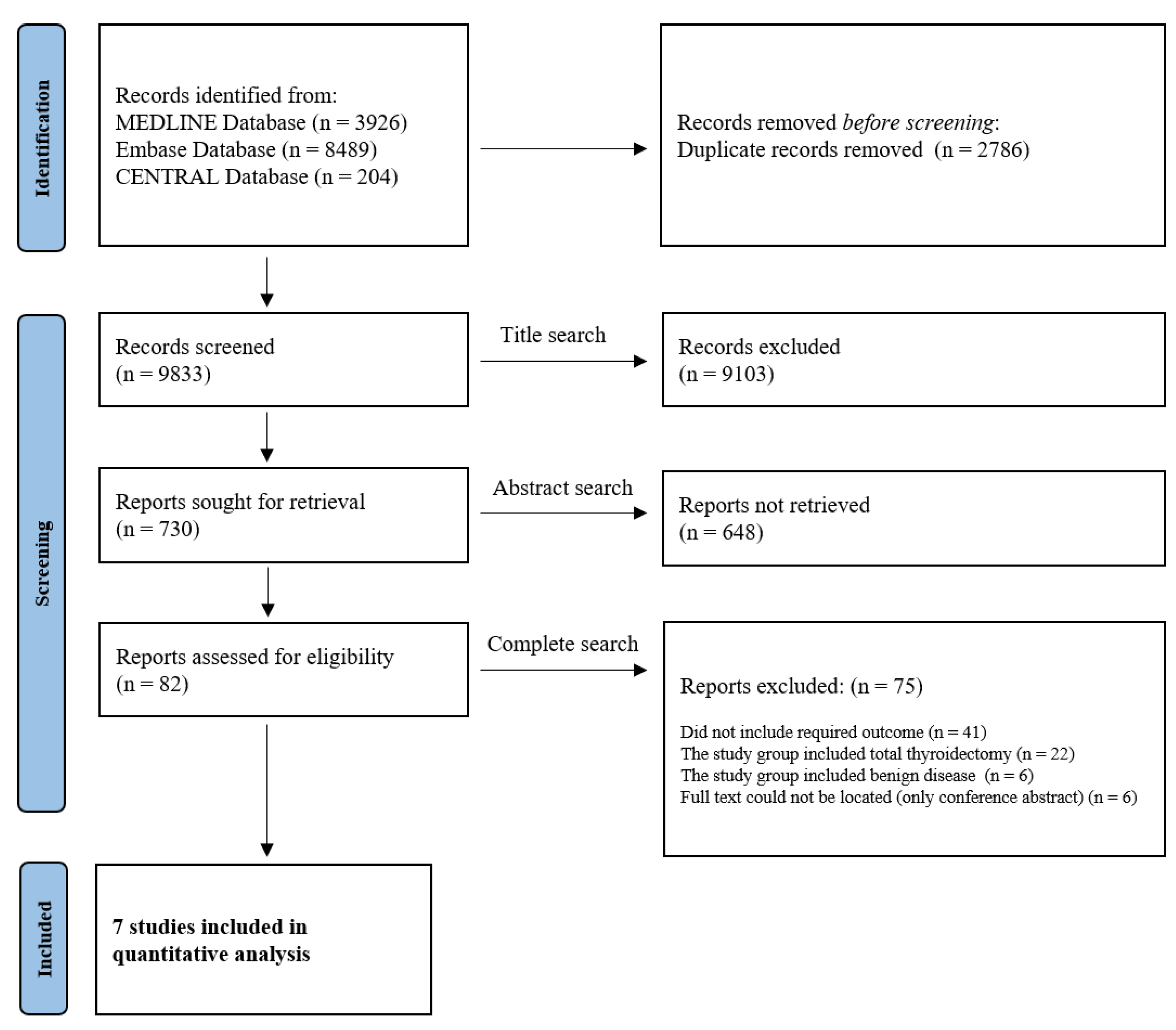
3.1. Study Selection
3.2. Recurrence Rate in Patients Who Received TSH Maintenance Less Than 2 mU/L after Thyroid Lobectomy for Low-Risk DTC
3.3. Association between TSH Maintenance Less Than 2 mU/L and the Recurrence Rate in Patients with Thyroid Lobectomy for Low-Risk DTC
3.4. Clinical Outcomes Related to TSH Levels after Thyroid Lobectomy for Low-Risk DTC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Fahiminiya, S.; de Kock, L.; Foulkes, W.D. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016, 375, 2306–2307. [Google Scholar] [CrossRef] [PubMed]
- Lamartina, L.; Grani, G.; Arvat, E.; Nervo, A.; Zatelli, M.C.; Rossi, R.; Puxeddu, E.; Morelli, S.; Torlontano, M.; Massa, M.; et al. 8th edition of the AJCC/TNM staging system of thyroid cancer: What to expect (ITCO#2). Endocr. Relat. Cancer 2018, 25, L7–L11. [Google Scholar] [CrossRef] [PubMed]
- Lamartina, L.; Handkiewicz-Junak, D. Follow-up of low risk thyroid cancer patients: Can we stop follow-up after 5 years of complete remission? Eur. J. Endocrinol. 2020, 182, D1–D16. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, R.M.; Tala, H.; Shah, J.; Leboeuf, R.; Ghossein, R.; Gonen, M.; Brokhin, M.; Omry, G.; Fagin, J.A.; Shaha, A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: Using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 2010, 20, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Freudenthal, B.; Williams, G.R. Thyroid Stimulating Hormone Suppression in the Long-term Follow-up of Differentiated Thyroid Cancer. Clin. Oncol. 2017, 29, 325–328. [Google Scholar] [CrossRef]
- Ahn, S.H.; Kim, W.S. The Effect of Prophylactic Central Neck Dissection During Hemithyroidectomy on Locoregional Recurrence in Patients With Papillary Thyroid Carcinoma: A Meta-Analysis. Clin. Exp. Otorhinolaryngol. 2020, 13, 194. [Google Scholar] [CrossRef]
- Mazzaferri, E.L.; Young, R.L.; Oertel, J.E.; Kemmerer, W.T.; Page, C.P. Papillary thyroid carcinoma: The impact of therapy in 576 patients. Medicine 1977, 56, 171–196. [Google Scholar] [CrossRef]
- Cooper, D.S.; Specker, B.; Ho, M.; Sperling, M.; Ladenson, P.W.; Ross, D.S.; Ain, K.B.; Bigos, S.T.; Brierley, J.D.; Haugen, B.R.; et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: Results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid 1998, 8, 737–744. [Google Scholar] [CrossRef]
- Kamel, N.; Gullu, S.; Dagci Ilgin, S.; Corapcioglu, D.; Tonyukuk Cesur, V.; Uysal, A.R.; Baskal, N.; Erdogan, G. Degree of thyrotropin suppression in differentiated thyroid cancer without recurrence or metastases. Thyroid 1999, 9, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Sugitani, I.; Fujimoto, Y. Does postoperative thyrotropin suppression therapy truly decrease recurrence in papillary thyroid carcinoma? A randomized controlled trial. J. Clin. Endocrinol. Metab. 2010, 95, 4576–4583. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, J.; Kukulska, A.; Oczko-Wojciechowska, M.; Kotecka-Blicharz, A.; Drosik-Rutowicz, K.; Haras-Gil, M.; Jarzab, B.; Handkiewicz-Junak, D. Early Diagnosis of Low-Risk Papillary Thyroid Cancer Results Rather in Overtreatment Than a Better Survival. Front. Endocrinol. 2020, 11, 571421. [Google Scholar] [CrossRef] [PubMed]
- Nixon, I.J.; Ganly, I.; Patel, S.G.; Palmer, F.L.; Whitcher, M.M.; Tuttle, R.M.; Shaha, A.; Shah, J.P. Thyroid lobectomy for treatment of well differentiated intrathyroid malignancy. Surgery 2012, 151, 571–579. [Google Scholar] [CrossRef]
- Haigh, P.I.; Urbach, D.R.; Rotstein, L.E. Extent of thyroidectomy is not a major determinant of survival in low- or high-risk papillary thyroid cancer. Ann. Surg. Oncol. 2005, 12, 81–89. [Google Scholar] [CrossRef]
- Matsuzu, K.; Sugino, K.; Masudo, K.; Nagahama, M.; Kitagawa, W.; Shibuya, H.; Ohkuwa, K.; Uruno, T.; Suzuki, A.; Magoshi, S.; et al. Thyroid lobectomy for papillary thyroid cancer: Long-term follow-up study of 1088 cases. World J. Surg. 2014, 38, 68–79. [Google Scholar] [CrossRef]
- Hartl, D.M.; Guerlain, J.; Breuskin, I.; Hadoux, J.; Baudin, E.; Al Ghuzlan, A.; Terroir-Cassou-Mounat, M.; Lamartina, L.; Leboulleux, S. Thyroid Lobectomy for Low to Intermediate Risk Differentiated Thyroid Cancer. Cancers 2020, 12, 3282. [Google Scholar] [CrossRef]
- Papaleontiou, M.; Chen, D.W.; Banerjee, M.; Reyes-Gastelum, D.; Hamilton, A.S.; Ward, K.C.; Haymart, M.R. Thyrotropin Suppression for Papillary Thyroid Cancer: A Physician Survey Study. Thyroid 2021, 31, 1383–1390. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Bae, M.R.; Nam, S.H.; Roh, J.L.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Thyroid stimulating hormone suppression and recurrence after thyroid lobectomy for papillary thyroid carcinoma. Endocrine 2021, 75, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Huang, Y.; Huang, H.; Zhang, X.; Qian, J.; Wang, X.; Xu, Z.; Liu, S.; Liu, J. Optimal Serum Thyrotropin Level for Patients with Papillary Thyroid Carcinoma After Lobectomy. Thyroid 2021, 32, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Gasior-Perczak, D.; Palyga, I.; Szymonek, M.; Kowalik, A.; Walczyk, A.; Kopczynski, J.; Lizis-Kolus, K.; Sluszniak, A.; Sluszniak, J.; Lopatynski, T.; et al. Delayed risk stratification system in pT1aN0/Nx DTC patients treated without radioactive iodine. Endocr. Connect. 2017, 6, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.G.; Kim, Y.A.; Choi, J.E.; Lee, S.J.; Kang, S.H. Usefulness of 1-year of thyroid stimulating hormone suppression on additional levothyroxine in patients who underwent hemithyroidectomy with papillary thyroid microcarcinoma. Gland Surg. 2019, 8, 636–643. [Google Scholar] [CrossRef]
- Lee, Y.M.; Jeon, M.J.; Kim, W.W.; Sung, T.Y.; Chung, K.W.; Shong, Y.K.; Hong, S.J. Optimal Thyrotropin Suppression Therapy in Low-Risk Thyroid Cancer Patients after Lobectomy. J. Clin. Med. 2019, 8, 1279. [Google Scholar] [CrossRef]
- Lee, M.C.; Kim, M.J.; Choi, H.S.; Cho, S.W.; Lee, G.H.; Park, Y.J.; Park, D.J. Postoperative Thyroid-Stimulating Hormone Levels Did Not Affect Recurrence after Thyroid Lobectomy in Patients with Papillary Thyroid Cancer. Endocrinol. Metab. 2019, 34, 150–157. [Google Scholar] [CrossRef]
- Park, S.; Kim, W.G.; Han, M.; Jeon, M.J.; Kwon, H.; Kim, M.; Sung, T.Y.; Kim, T.Y.; Kim, W.B.; Hong, S.J.; et al. Thyrotropin Suppressive Therapy for Low-Risk Small Thyroid Cancer: A Propensity Score-Matched Cohort Study. Thyroid 2017, 27, 1164–1170. [Google Scholar] [CrossRef]
- The South Korean National Cancer Statistics 2018. Available online: https://ncc.re.kr/cancerStatsList.ncc?searchKey=total&searchValue=&pageNum=1 (accessed on 12 March 2022).
- Tam, S.; Boonsripitayanon, M.; Amit, M.; Fellman, B.M.; Li, Y.; Busaidy, N.L.; Cabanillas, M.E.; Dadu, R.; Sherman, S.; Waguespack, S.G.; et al. Survival in Differentiated Thyroid Cancer: Comparing the AJCC Cancer Staging Seventh and Eighth Editions. Thyroid 2018, 28, 1301–1310. [Google Scholar] [CrossRef]
- Choi, Y.S.; Hong, Y.T.; Yi, J.W. Initial Experience With Robotic Modified Radical Neck Dissection Using the da Vinci Xi System Through the Bilateral Axillo-Breast Approach. Clin. Exp. Otorhinolaryngol. 2021, 14, 137–144. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Saito, E.; Abe, Y.; Homma, M.; Muraki, T. Presence of TSH receptor in thyroid neoplasms. J. Clin. Endocrinol. Metab. 1976, 42, 395–398. [Google Scholar] [CrossRef]
- Rossi, R.L.; Cady, B.; Silverman, M.L.; Wool, M.S.; ReMine, S.G.; Hodge, M.B.; Salzman, F.A. Surgically incurable well-differentiated thyroid carcinoma. Prognostic factors and results of therapy. Arch. Surg. 1988, 123, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferri, E.L.; Jhiang, S.M. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am. J. Med. 1994, 97, 418–428. [Google Scholar] [CrossRef]
- Pujol, P.; Daures, J.P.; Nsakala, N.; Baldet, L.; Bringer, J.; Jaffiol, C. Degree of thyrotropin suppression as a prognostic determinant in differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 1996, 81, 4318–4323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McGriff, N.J.; Csako, G.; Gourgiotis, L.; Lori, C.G.; Pucino, F.; Sarlis, N.J. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann. Med. 2002, 34, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Hovens, G.C.; Stokkel, M.P.; Kievit, J.; Corssmit, E.P.; Pereira, A.M.; Romijn, J.A.; Smit, J.W. Associations of serum thyrotropin concentrations with recurrence and death in differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2007, 92, 2610–2615. [Google Scholar] [CrossRef]
- Lamartina, L.; Montesano, T.; Falcone, R.; Biffoni, M.; Grani, G.; Maranghi, M.; Ciotti, L.; Giacomelli, L.; Ramundo, V.; Lomonaco, C.; et al. Is It Worth Suppressing Tsh in Low- and Intermediate-Risk Papillary Thyroid Cancer Patients before the First Disease Assessment? Endocr. Pract. 2019, 25, 165–169. [Google Scholar] [CrossRef]
- James, B.C.; Timsina, L.; Graham, R.; Angelos, P.; Haggstrom, D.A. Changes in total thyroidectomy versus thyroid lobectomy for papillary thyroid cancer during the past 15 years. Surgery 2019, 166, 41–47. [Google Scholar] [CrossRef]
- Park, Y.M.; Lee, D.Y.; Oh, K.H.; Cho, J.G.; Baek, S.K.; Kwon, S.Y.; Jung, K.Y.; Woo, J.S. Clinical implications of pathologic factors after thyroid lobectomy in patients with papillary thyroid carcinoma. Oral Oncol. 2017, 75, 1–5. [Google Scholar] [CrossRef]
- Kim, S.K.; Park, I.; Woo, J.W.; Lee, J.H.; Choe, J.H.; Kim, J.H.; Kim, J.S. Total thyroidectomy versus lobectomy in conventional papillary thyroid microcarcinoma: Analysis of 8676 patients at a single institution. Surgery 2017, 161, 485–492. [Google Scholar] [CrossRef]
- Chan, S.; Karamali, K.; Kolodziejczyk, A.; Oikonomou, G.; Watkinson, J.; Paleri, V.; Nixon, I.; Kim, D. Systematic Review of Recurrence Rate after Hemithyroidectomy for Low-Risk Well-Differentiated Thyroid Cancer. Eur. Thyroid J. 2020, 9, 73–84. [Google Scholar] [CrossRef]
- Ahn, D.; Lee, G.J.; Sohn, J.H.; Jeon, J.H. Oncological impact of hypothyroidism and levothyroxine supplementation following hemithyroidectomy in patients with papillary thyroid carcinoma. Head Neck 2020, 42, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, Y.M.; Lee, Y.H.; Hong, S.J.; Yoon, J.H. The prognostic value of serum thyroid-stimulating hormone level post-lobectomy in low- and intermediate-risk papillary thyroid carcinoma. J. Surg. Oncol. 2018, 118, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Xu, Y.; Bhandari, A.; Sindan, N.; Hirachan, S.; Yang, Q.; Guo, G.; Shen, Y. Serum TSH levels are associated with postoperative recurrence and lymph node metastasis of papillary thyroid carcinoma. Am. J. Transl. Res. 2021, 13, 6108–6116. [Google Scholar] [PubMed]
| Study | Study Design | Participant (n) | Age Mean (SD) | Gender (Male/ Female) (%) | ATA Risk | CND | Pathology | Follow Up Mean, Month (SD) | Outcome (Incidence of Recurrence) (%) | Comments | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No TSH Maintenance (≥2 mU/L) | TSH Maintenance (<2 mU/L) | ||||||||||
| Bae et al. [21] | Retrospective analysis | 134 | 235 | 49 (NR) | 89 (24.1%)/280 (75.9%) | low | Performed | PTC-NR | 72 (NR) | 4/134 (2.99%): 0/235 (0%) | Patients were indicated to receive T4 supplementation according to the 2015 ATA guidelines |
| Xu et al. [22] | Retrospective analysis | 189 | 757 | 42 (NR) (All participant) | 547 (23.8%)/1750 (76.2%) (All participant) | low | Performed depending on the surgeon’s preference | PTC-CV, FV | 70 (NR) | 10/189 (5.29%): 65/757 (8.59%) | TSH value is derived by calculating the mean value for course of follow-up. |
| Gąsior Perczak et al. [23] | Retrospective analysis | NA | 102 | 49.8 (13.6) | 8 (7.8%)/ 94 (92.2%) | low | Not performed | PTC-CV, FV | 60 (40.8) | 2/102 (1.96%) | TSH levels were within the recommended range for the patients (0.5–2 mU/L) |
| Kang et al. [24] | Retrospective analysis | 100 | 100 | 42.79 (9.60): 45.43 (8.90) | 13 (13%)/ 87 (87%): 14 (14%)/ 86 (86%) | low | Performed depending on the surgeon’s preference | PTC-NR | More than 60 (NR) | 2/100 (2%): 5/100 (5%) | Check TSH suppression by administering the same dose of LT4. TSH suppression performed only 1 year after surgery. |
| Lee et al. [25] | Retrospective/prospective analysis | NA | 363 | 52.21 (9.88) | 67 (18.5%)/296 (81.5%) | low | Performed | PTC-NR | 67 (NR) | 1/363 (0.3%) | No description of the method in TSH maintenance. ATA 2015 guidelines are presented in the introduction. |
| Lee et al. [26] | Retrospective analysis | 863 | 665 | 47 (10) (All participant) | 177 (12%)/1351 (88%) (All participant) | low | Performed depending on the surgeon’s preference | PTC-CV, FV | 67.2 (NR) | 5/863 (0.58%): 16/665 (2.40%) | TSH value is derived by calculating the mean value for 5 years. |
| Park et al. [27] | Retrospective analysis (cohort study) | 233 | 233 | 47.60 (10.26): 47.23 (10.00) | 39 (16.7%)/ 194(83.3%): 31 (13.3%)/202 (86.7%) | low | Performed | PTC-CV, FV/ FTC | Median 103.2 (NR) | 6/233 (2.58%): 4/233 (1.72%) | Groups are classified based on TSH maintenance. |
| Study | Statistics for Each Study | Weight (%) | Event Rate and 95% CI | ||||
|---|---|---|---|---|---|---|---|
| Event Rate | Lower Limit | Upper Limit | Z-Value | p-Value | |||
| Bae et al. [21] | 0.002 | 0.000 | 0.033 | −4.348 | 0.000 | 6.49 |  |
| Xu et al. [22] | 0.086 | 0.068 | 0.108 | −18.232 | 0.000 | 19.66 | |
| Gąsior Perczak et al. [23] | 0.020 | 0.005 | 0.075 | −5.478 | 0.000 | 13.07 | |
| Kang et al. [24] | 0.050 | 0.021 | 0.115 | −6.417 | 0.000 | 16.41 | |
| Lee et al. [25] | 0.003 | 0.000 | 0.019 | −5.884 | 0.000 | 9.79 | |
| Lee et al. [26] | 0.024 | 0.015 | 0.039 | −14.632 | 0.000 | 18.75 | |
| Park et al. [27] | 0.017 | 0.006 | 0.045 | −8.025 | 0.000 | 15.82 | |
| Random | 0.023 | 0.010 | 0.052 | −8.595 | 0.000 | ||
| Heterogeneity: Tau2 = 0.962; df = 6 (p = 0.000); I2 = 87.433% | |||||||
| Study | Statistics for Each Study | Weight (%) | Odds Ratio and 95% CI | ||||
|---|---|---|---|---|---|---|---|
| Odds Ratio | Lower Limit | Upper Limit | Z-Value | p-Value | |||
| Bae et al. [21] | 0.062 | 0.003 | 1.153 | −1.865 | 0.062 | 7.64 |  |
| Xu et al. [22] | 1.618 | 0.847 | 3.338 | 1.485 | 0.138 | 29.91 | |
| Kang et al. [24] | 2.579 | 0.488 | 13.617 | 1.116 | 0.264 | 16.38 | |
| Lee et al. [26] | 4.231 | 1.542 | 11.608 | 2.801 | 0.005 | 24.98 | |
| Park et al. [27] | 0.661 | 0.184 | 2.373 | −0.635 | 0.525 | 21.09 | |
| Random | 1.449 | 0.582 | 3.607 | 0.796 | 0.426 | ||
| Heterogeneity: Tau2 = 0.602; df = 4 (p = 0.034); I2 = 61.538% | |||||||
| Study (Year) | Participant (n) | TSH Level (mU/L, Mean ± SD) | Clinical Outcome Related TSH Level | Comments | |
|---|---|---|---|---|---|
| No TSH Maintenance (≥2 mU/L) | TSH Maintenance (<2 mU/L) | ||||
| Bae et al. [21] | 134 | 235 | Serum TSH concentrations: proportions with TSH > 2 mU/L post-lobectomy 1 month: 77.0% 3–6 months: 82.3% 12 months: 66.7% 24 months: 59.9% | Preoperative TSH level (OR = 2.182, 95% CI, 1.301–3.659; p = 0.003) was the independent variable that predicted the need for TSH suppression. | |
| Xu et al. [22] | 189 | 757 | TSH level ≤ 0.5 (n = 254) TSH level 0.5–2 (n = 503) TSH level 2–4 (n = 135) TSH level > 4 (n = 54) | 10-year RFS rate TSH level ≤ 0.5: 95.1% TSH level 0.5–2: 89.4% TSH level 2–4: 96.1% TSH level > 4: 91.2% Compare DFS and TSH level 0.5–2 and 2–4 p = 0.997 0.5–2 and >4 p = 0.487 | |
| Gąsior Perczak et al. [23] | NA | 102 | Only report TSH levels in recurrence patients (1.86) | NR | |
| Kang et al. [24] | 100 | 100 | Postoperative TSH > 10 patient’s number: No TSH maintenance: 25 (25%) TSH maintenance: 13 (13%) | 1-year TSH maintenance effect on postoperative TSH >10 p = 0.036 | |
| Lee et al. [25] | NA | 363 | Failure to cessation of TSH maintenance (0.90 ± 0.82) (n = 170) Success to cessation of TSH maintenance (0.96 ± 0.98) (n = 193) | NR | |
| Lee et al. [26] | 863 | 665 | TSH level ≥ 2 (n = 863) TSH level < 0.5 (n = 115) TSH level 0.5–1.9 (n = 550) Mean TSH levels: NR | Hazard ratio (95%CI) according to RFS and mean TSH Univariate: <0.5: 0.49 (0.14–1.64) 0.5–1.9: Ref. 2.0–4.4: 0.39 (0.12–1.24) ≥4.5: 0.36 (0.04–2.88) Multivariate: <0.5: 0.44 (0.12–1.61) 0.5–1.9: Ref. 2.0–4.4: 0.35 (0.11–1.13) ≥4.5: 0.31 (0.03–2.58) | |
| Park et al. [27] | 233 | 233 | Mean TSH levels: NR | Compare DFS and TSH level (TSH level ≥2 and <2) p = 0.85 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, H.-R.; Jeon, E.; Chang, J.W.; Kang, Y.E.; Song, K.; Kim, S.W.; Lim, D.M.; Ha, T.K.; Chung, K.-W.; Kim, H.-J.; et al. Is Maintaining Thyroid-Stimulating Hormone Effective in Patients Undergoing Thyroid Lobectomy for Low-Risk Differentiated Thyroid Cancer? A Systematic Review and Meta-Analysis. Cancers 2022, 14, 1470. https://doi.org/10.3390/cancers14061470
Won H-R, Jeon E, Chang JW, Kang YE, Song K, Kim SW, Lim DM, Ha TK, Chung K-W, Kim H-J, et al. Is Maintaining Thyroid-Stimulating Hormone Effective in Patients Undergoing Thyroid Lobectomy for Low-Risk Differentiated Thyroid Cancer? A Systematic Review and Meta-Analysis. Cancers. 2022; 14(6):1470. https://doi.org/10.3390/cancers14061470
Chicago/Turabian StyleWon, Ho-Ryun, Eonju Jeon, Jae Won Chang, Yea Eun Kang, Kunho Song, Sun Wook Kim, Dong Mee Lim, Tae Kwun Ha, Ki-Wook Chung, Hyo-Jeong Kim, and et al. 2022. "Is Maintaining Thyroid-Stimulating Hormone Effective in Patients Undergoing Thyroid Lobectomy for Low-Risk Differentiated Thyroid Cancer? A Systematic Review and Meta-Analysis" Cancers 14, no. 6: 1470. https://doi.org/10.3390/cancers14061470
APA StyleWon, H.-R., Jeon, E., Chang, J. W., Kang, Y. E., Song, K., Kim, S. W., Lim, D. M., Ha, T. K., Chung, K.-W., Kim, H.-J., Park, Y. J., & Koo, B. S. (2022). Is Maintaining Thyroid-Stimulating Hormone Effective in Patients Undergoing Thyroid Lobectomy for Low-Risk Differentiated Thyroid Cancer? A Systematic Review and Meta-Analysis. Cancers, 14(6), 1470. https://doi.org/10.3390/cancers14061470






