A Bout of High-Intensity Interval Training (HIIT) in Children and Adolescents during Acute Cancer Treatment—A Pilot Feasibility Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
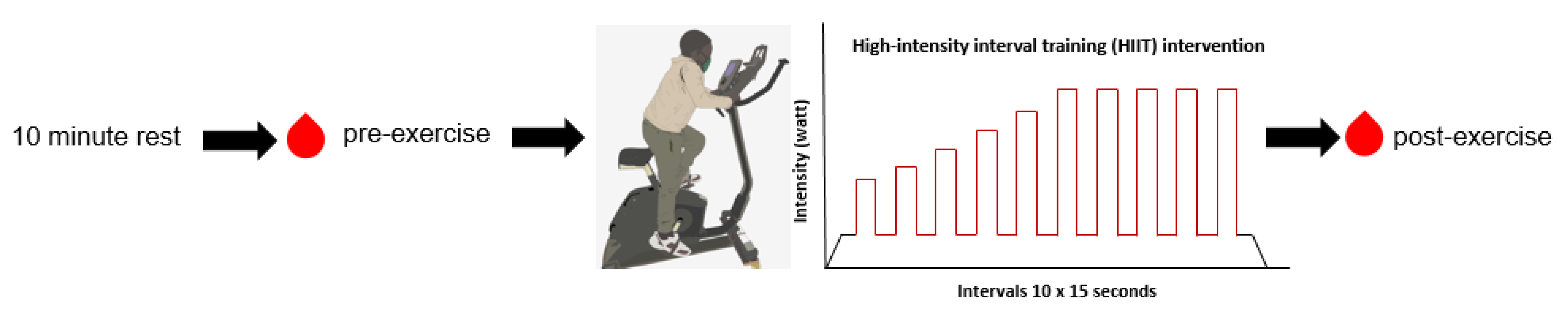
2.1. Study Design and Supervised HIIT Intervention
2.2. Participants
2.3. Procedures and Trial Endpoints
2.3.1. Feasibility and Safety
- The attending physician gives medical consent regarding the capability to participate (see also Section 2.2 exclusion criteria) and appropriate blood values to perform HIIT;
- The intervention is conducted in a room within the outpatient clinic with medical supervision during and after the intervention;
- Low-intensity warm-up, including mobilization of the joints;
- Participants perform the intervention in a seated position to avoid tangling of the central venous catheter with the bike’s handlebar;
- Follow-up observation of every participant for 30 min after finishing the intervention;
- At least two individuals in the room during the intervention in case of an emergency;
- Close observation of the participant, including objective signs of exertion (e.g., flushed cheeks, paleness, intense breathing, quality of coordination and movement).
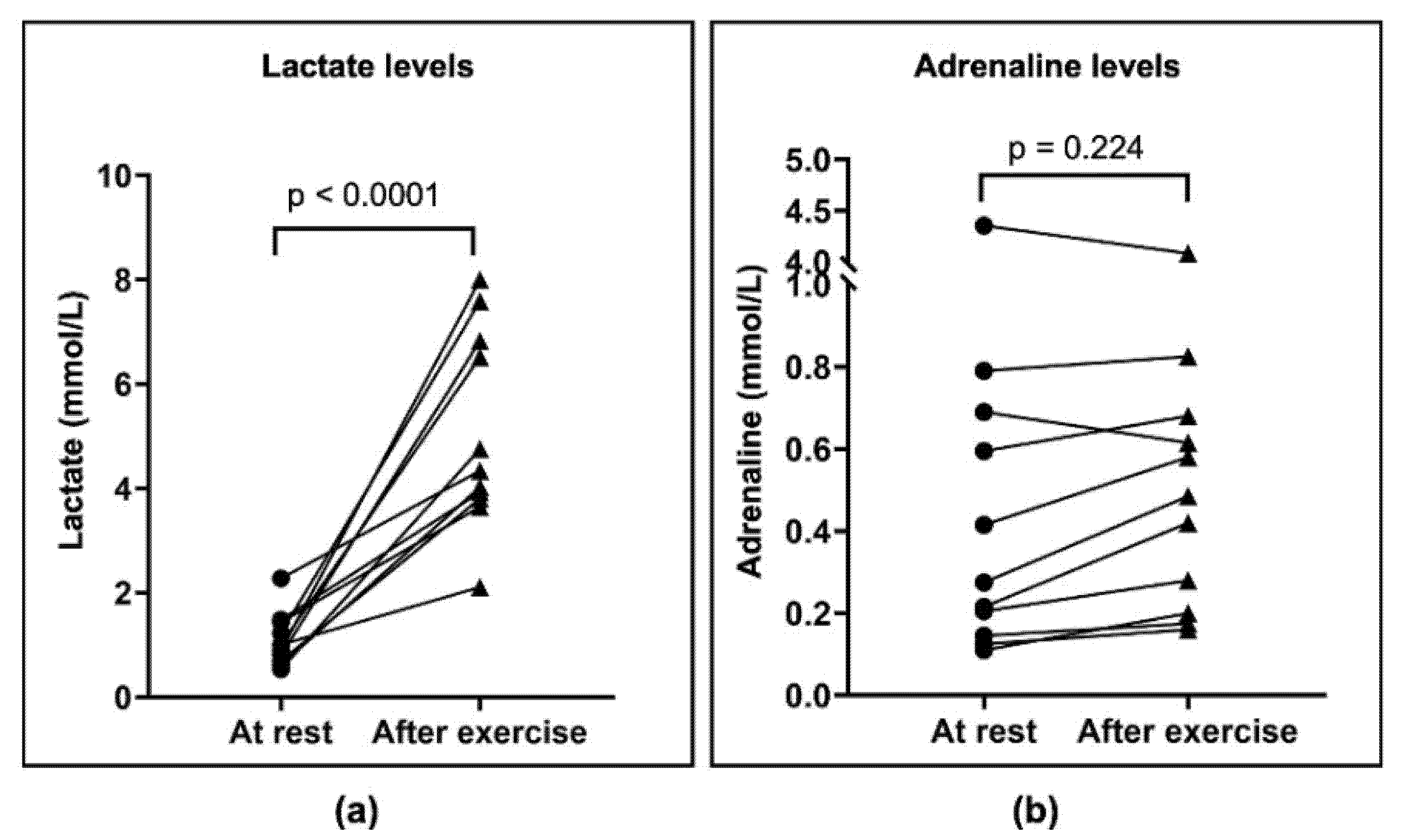
2.3.2. Lactate and Adrenaline Concentrations
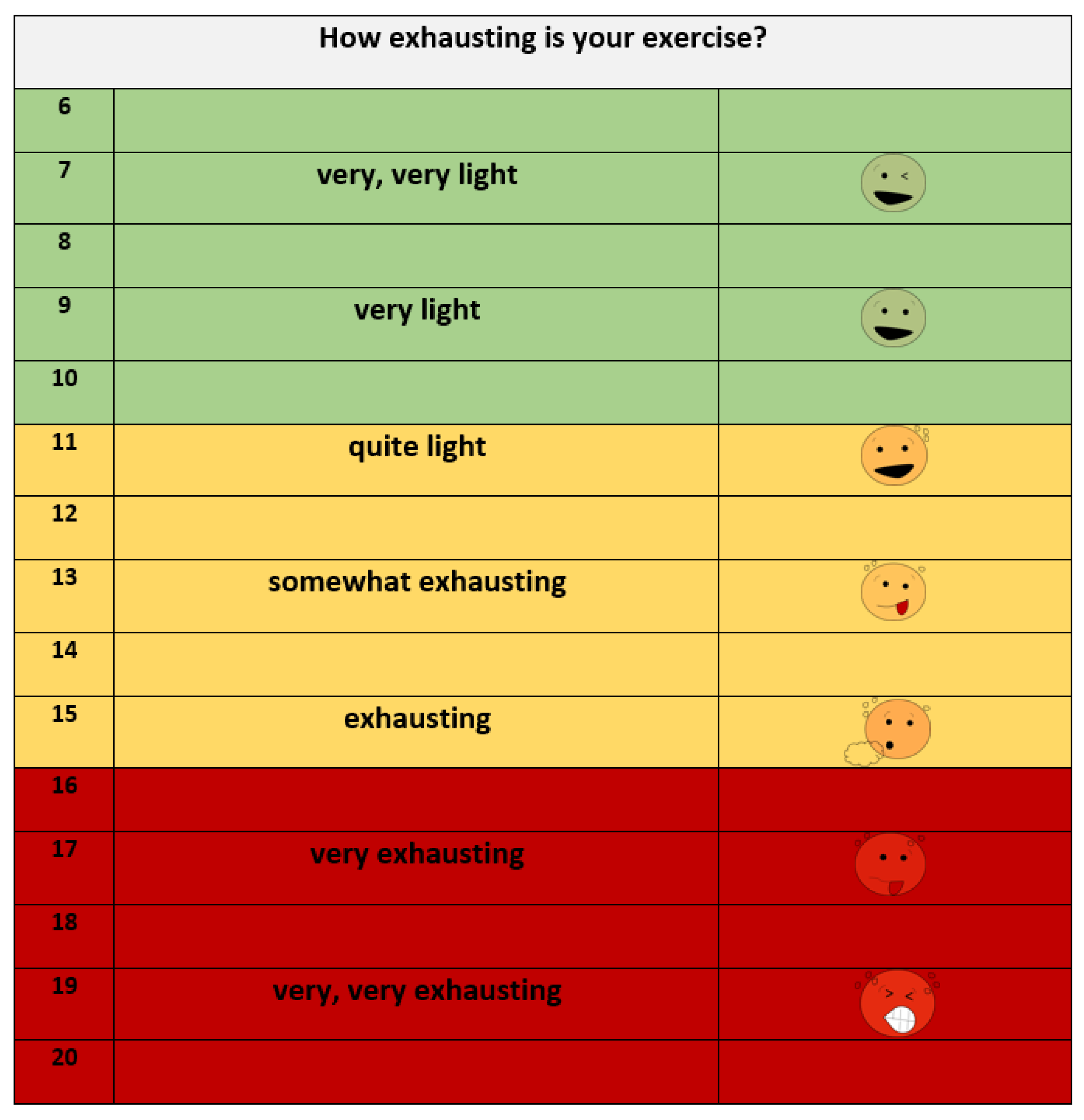
2.3.3. Objective and Subjective Exhaustion Parameter
2.3.4. Physical Activity Pre-Diagnosis
2.4. Statistical Analysis
3. Results
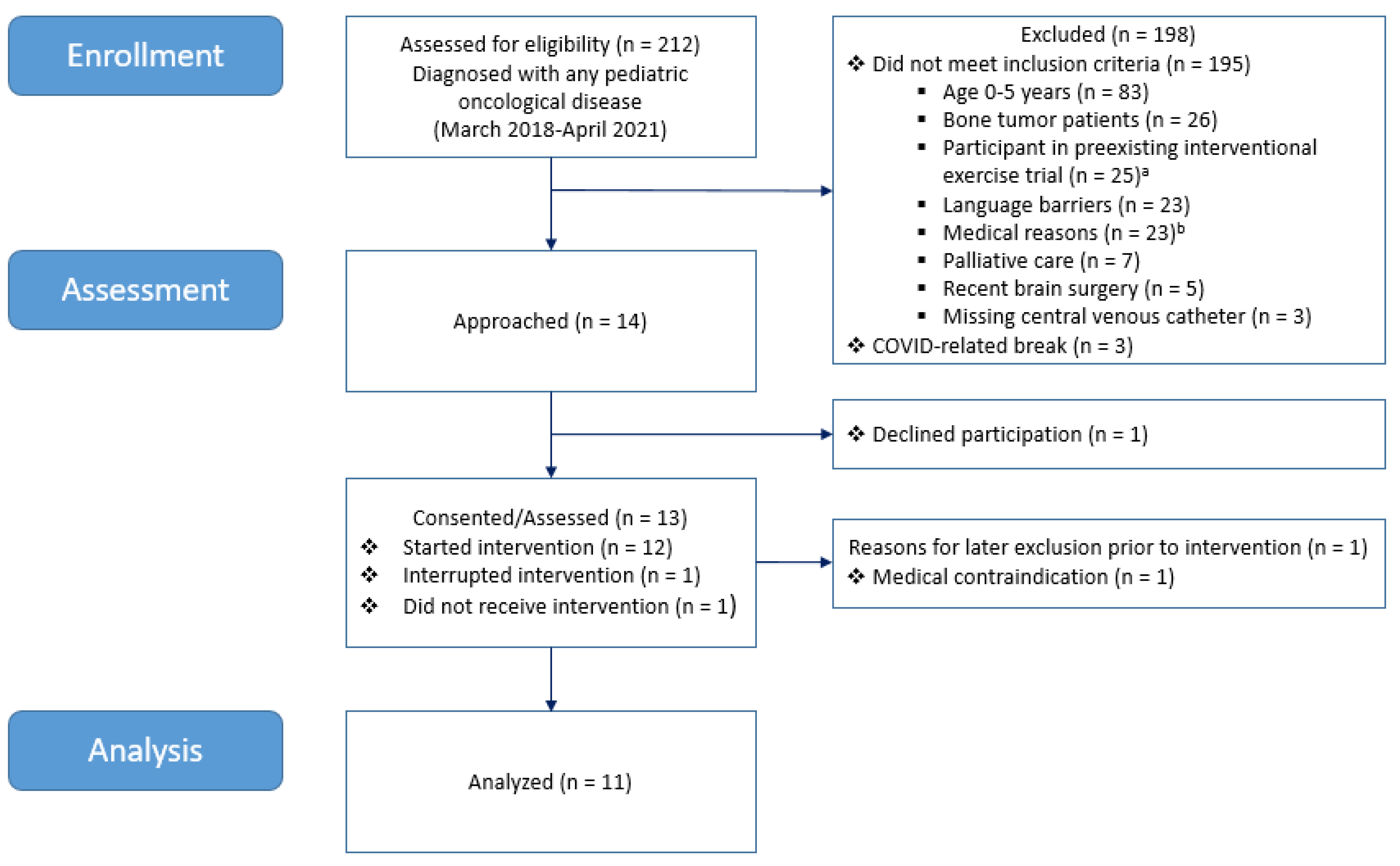
3.1. Patient Recruitment and Characteristics
3.2. Endpoints
3.2.1. Feasibility and Safety
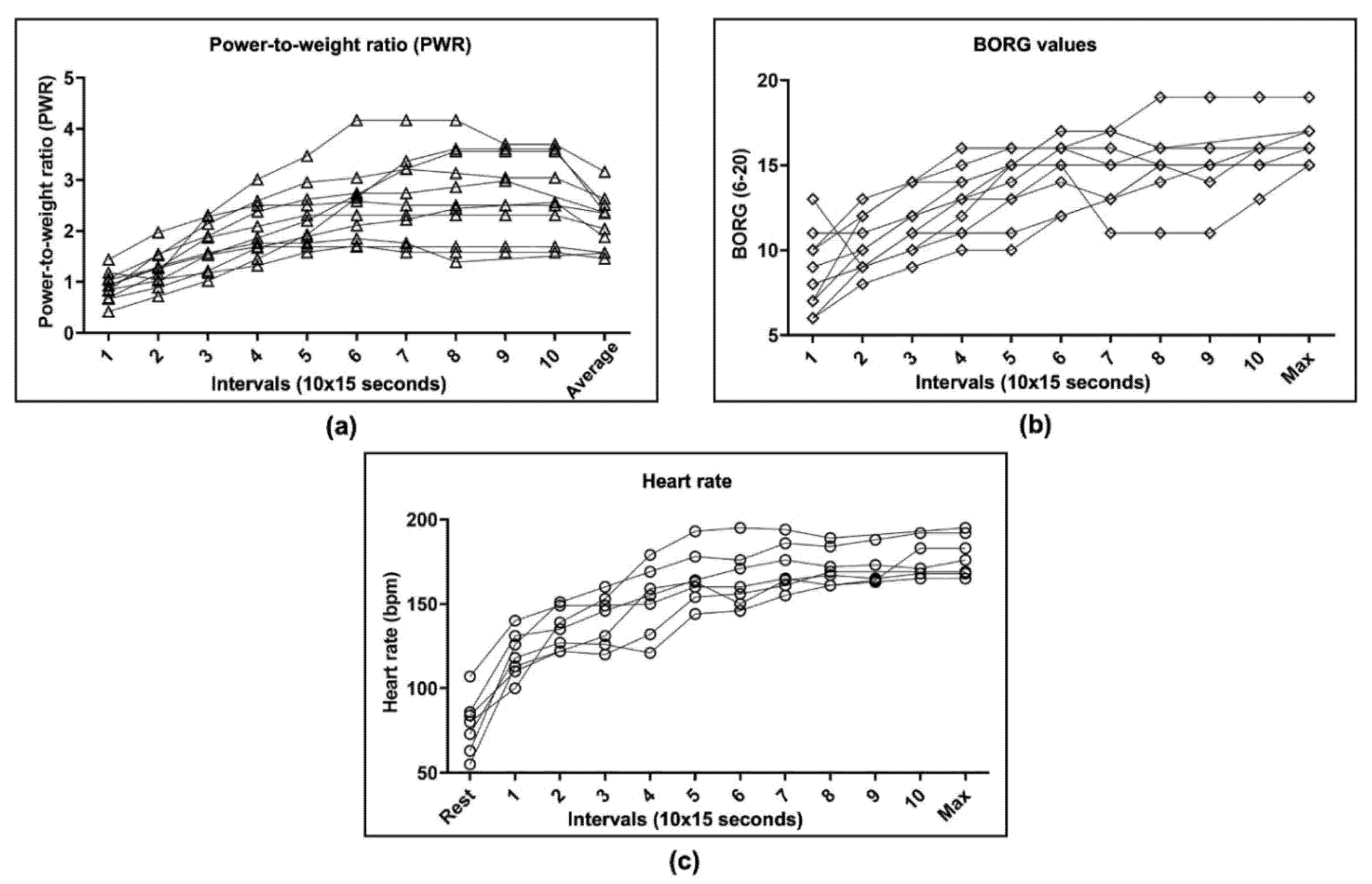
3.2.2. Physical Parameters
3.2.3. Physical Activity Pre-Diagnosis
4. Discussion
5. Conclusions and Perspective
- The adaption of exercise tests specifically for childhood cancer patients is needed to define standardized workloads for training protocols.
- The development of individually adapted exercise protocols (low-intensity exercise training) for patients with multiple impairments and health restrictions due to their underlying disease and cancer treatment.
- The application of repeated HIIT interventions and analysis of physiological parameters during the entire course of chemotherapy treatment.
- The initiation of multicenter studies to generate a greater sample size and increase informative value.
- The analysis of metabolite concentration changes in exercise-conditioned serum to detect relevant exercise and cancer-related metabolites in children.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyu, H.H.; Stein, C.; Pinto, C.B.; Rakovac, I.; Weber, M.W.; Purnat, T.D.; Amuah, J.; Glenn, S.D.; Cercy, K.; Biryukov, S.; et al. Causes of death among children aged 5–14 years in the WHO European Region: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Child Adolesc. Health 2018, 2, 321–337. [Google Scholar] [CrossRef]
- GLOBOCAN; World Health Organization. Cancer Today. Available online: https://gco.iarc.fr/today/home (accessed on 23 November 2021).
- Clinical Oncology Society of Australia (COSA). Available online: https://www.cosa.org.au/media/332488/cosa-position-statement-v4-web-final.pdf (accessed on 23 November 2021).
- Patel, A.V.; Friedenreich, C.M.; Moore, S.C.; Hayes, S.C.; Silver, J.K.; Campbell, K.L.; Winters-Stone, K.; Gerber, L.H.; George, S.M.; Fulton, J.E.; et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med. Sci. Sports Exerc. 2019, 51, 2391–2402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AWMF—Das Portal der wissenschaftlichen Medizin. Available online: https://www.awmf.org/uploads/tx_szleitlinien/025-036l_S2k_Bewegungsfoerderung-Bewegungstherapie-in-der-p%C3%A4diatrischen_Onkologie_2021-10.pdf (accessed on 23 November 2021).
- Buffart, L.M.; Kalter, J.; Sweegers, M.G.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; May, A.M.; Galvão, D.A.; Chinapaw, M.J.; et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat. Rev. 2017, 52, 91–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, J.F.; Simonsen, C.; Hojman, P. Exercise Training in Cancer Control and Treatment. Compr. Physiol. 2018, 9, 165–205. [Google Scholar] [CrossRef]
- Großek, A.; Elter, T.; Oberste, M.; Wolf, F.; Joisten, N.; Hartig, P.; Walzik, D.; Rosenberger, F.; Kiesl, D.; Wahl, P.; et al. Feasibility and suitability of a graded exercise test in patients with aggressive hemato-oncological disease. Support. Care Cancer 2021, 29, 4859–4866. [Google Scholar] [CrossRef]
- Klika, R.J.; Stafford, L.H. Exercise Oncology: High-Intensity Interval Training for Cancer Survivors. ACSM’s Health Fit. J. 2021, 25, 44–53. [Google Scholar] [CrossRef]
- Moore, S.C.; Lee, I.-M.; Weiderpass, E.; Campbell, P.T.; Sampson, J.N.; Kitahara, C.M.; Keadle, S.K.; Arem, H.; de Gonzalez, A.B.; Hartge, P.; et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern. Med. 2016, 176, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.D.; Chen, W.; Feskanich, D.; Kroenke, C.; Colditz, G. Physical Activity and Survival After Breast Cancer Diagnosis. JAMA J. Am. Med. Assoc. 2005, 293, 2479–2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenfield, S.A.; Stampfer, M.J.; Giovannucci, E.; Chan, J. Physical Activity and Survival After Prostate Cancer Diagnosis in the Health Professionals Follow-Up Study. J. Clin. Oncol. 2011, 29, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Meyerhardt, J.A.; Giovannucci, E.L.; Holmes, M.D.; Chan, A.T.; Chan, J.A.; Colditz, G.; Fuchs, C.S. Physical Activity and Survival After Colorectal Cancer Diagnosis. J. Clin. Oncol. 2006, 24, 3527–3534. [Google Scholar] [CrossRef]
- Baldelli, G.; De Santi, M.; Gervasi, M.; Annibalini, G.; Sisti, D.; Højman, P.; Sestili, P.; Stocchi, V.; Barbieri, E.; Brandi, G. The effects of human sera conditioned by high-intensity exercise sessions and training on the tumorigenic potential of cancer cells. Clin. Transl. Oncol. 2020, 23, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Gatto, F.; Zerahn, B.; Nielsen, J.; Pedersen, B.K.; Hojman, P.; Gehl, J. Exercise-Mediated Lowering of Glutamine Availability Suppresses Tumor Growth and Attenuates Muscle Wasting. iScience 2020, 23, 100978. [Google Scholar] [CrossRef]
- Eime, R.M.; Young, J.A.; Harvey, J.T.; Charity, M.J.; Payne, W.R. A systematic review of the psychological and social benefits of participation in sport for children and adolescents: Informing development of a conceptual model of health through sport. Int. J. Behav. Nutr. Phys. Act. 2013, 10, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strong, W.B.; Malina, R.M.; Blimkie, C.J.; Daniels, S.R.; Dishman, R.K.; Gutin, B.; Hergenroeder, A.C.; Must, A.; Nixon, P.A.; Pivarnik, J.M.; et al. Evidence Based Physical Activity for School-age Youth. J. Pediatr. 2005, 146, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Baumann, F.T.; Bloch, W.; Beulertz, J. Clinical exercise interventions in pediatric oncology: A systematic review. Pediatr. Res. 2013, 74, 366–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rustler, V.; Hagerty, M.; Daeggelmann, J.; Marjerrison, S.; Bloch, W.; Baumann, F.T. Exercise interventions for patients with pediatric cancer during inpatient acute care: A systematic review of literature. Pediatr. Blood Cancer 2017, 64, e26567. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.S.; Valenzuela, P.L.; Rincon, C.; Takken, T.; Fiuza-Luces, C.; Santos-Lozano, A.; Lucia, A. Exercise training in childhood cancer: A systematic review and meta-analysis of randomized controlled trials. Cancer Treat. Rev. 2018, 70, 154–167. [Google Scholar] [CrossRef]
- Braam, K.; Van Der Torre, P.; Takken, T.; Veening, M.; Broeder, E.V.D.-D.; Kaspers, G.J.L. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst. Rev. 2016, 3, CD008796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesting, S.; Weeber, P.; Schönfelder, M.; Renz, B.W.; Wackerhage, H.; Von Luettichau, I. Exercise as a Potential Intervention to Modulate Cancer Outcomes in Children and Adults? Front. Oncol. 2020, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Casado, A.; Martín-Ruiz, A.; Pérez, L.M.; Provencio, M.; Fiuza-Luces, C.; Lucia, A. Exercise and the Hallmarks of Cancer. Trends Cancer 2017, 3, 423–441. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; MacDonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Wackerhage, H.; Christensen, J.; Ilmer, M.; von Luettichau, I.; Renz, B.; Schönfelder, M. Cancer catecholamine conundrum. Trends Cancer 2021, 8, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Yang, Y.; Chen, C.; Li, L.; Li, J.; Wang, X.; Chu, Q.; Qiu, L.; Ba, Q.; Li, X.; et al. Environmental eustress modulates β-ARs/CCL2 axis to induce anti-tumor immunity and sensitize immunotherapy against liver cancer in mice. Nat. Commun. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.-X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; Li, H.; et al. Regulation of the Hippo-YAP Pathway by G-Protein-Coupled Receptor Signaling. Cell 2012, 150, 780–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2000, 37, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Eddolls, W.T.B.; McNarry, M.A.; Stratton, G.; Winn, C.O.N.; Mackintosh, K.A. High-Intensity Interval Training Interventions in Children and Adolescents: A Systematic Review. Sports Med. 2017, 47, 2363–2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perondi, M.B.; Gualano, B.; Artioli, G.G.; Painelli, V.D.S.; Filho, V.O.; Netto, G.; Muratt, M.; Roschel, H.; Pinto, A.L.D.S. Effects of a combined aerobic and strength training program in youth patients with acute lymphoblastic leukemia. J. Sports Sci. Med. 2012, 11, 387–392. [Google Scholar] [PubMed]
- Thabane, L.; Ma, J.; Chu, R.; Cheng, J.; Ismaila, A.; Rios, L.P.; Robson, R.; Thabane, M.; Giangregorio, L.; Goldsmith, C.H. A tutorial on pilot studies: The what, why and how. BMC Med. Res. Methodol. 2010, 10, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauß, G.; Beller, R.; Boos, J.; Däggelmann, J.; Stalf, H.; Wiskemann, J.; Götte, M. Adverse Events During Supervised Exercise Interventions in Pediatric Oncology—A Nationwide Survey. Front. Pediatr. 2021, 9, 682496. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 19 March 2020).
- Beulertz, J.; Prokop, A.; Ma, V.R.; Bloch, W.; Felsch, M.; Pd, F.T.B. Effects of a 6-Month, Group-Based, Therapeutic Exercise Program for Childhood Cancer Outpatients on Motor Performance, Level of Activity, and Quality of Life. Pediatr. Blood Cancer 2015, 63, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Stössel, S.; Neu, M.A.; Wingerter, A.; Bloch, W.; Zimmer, P.; Paret, C.; El Malki, K.; Baumann, F.T.; Russo, A.; Henninger, N.; et al. Benefits of Exercise Training for Children and Adolescents Undergoing Cancer Treatment: Results From the Randomized Controlled MUCKI Trial. Front. Pediatr. 2020, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Wallek, S.; Senn-Malashonak, A.; Vogt, L.; Schmidt, K.; Bader, P.; Banzer, W. Impact of the initial fitness level on the effects of a structured exercise therapy during pediatric stem cell transplantation. Pediatr. Blood Cancer 2017, 65, e26851. [Google Scholar] [CrossRef]
- Network ActiveOncoKids. Available online: https://www.activeoncokids.de/ (accessed on 23 November 2021).
- World Health Organization. Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240015128 (accessed on 23 November 2021).
- Finger, J.D.; Varnaccia, G.; Borrmann, A.; Lange, C.; Mensink, G. Physical activity among children and adolescents in Germany. Results of the cross-sectional KiGGS Wave 2 study and trends. J. Health Monit. 2018, 3, 23–30. [Google Scholar] [CrossRef]
- Mahon, A.D.; Marjerrison, A.D.; Lee, J.D.; Woodruff, M.E.; Hanna, L.E. Evaluating the Prediction of Maximal Heart Rate in Children and Adolescents. Res. Q. Exerc. Sport 2010, 81, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.A.; Clark, S.J.; Ng, J.; Castner, D.; Haqq, A.M.; Judelson, D.A. Hormonal and Metabolic Responses to Endurance Exercise in Children With Prader–Willi Syndrome and Non-Syndromic Obesity. Metabolism 2015, 64, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Saultier, P.; Vallet, C.; Sotteau, F.; Hamidou, Z.; Gentet, J.-C.; Barlogis, V.; Curtillet, C.; Verschuur, A.; Revon-Riviere, G.; Galambrun, C.; et al. A Randomized Trial of Physical Activity in Children and Adolescents with Cancer. Cancers 2021, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Gelbart, M.; Ziv-Baran, T.; Williams, C.A.; Yarom, Y.; Dubnov-Raz, G. Prediction of Maximal Heart Rate in Children and Adolescents. Clin. J. Sport Med. 2017, 27, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Moser, O.; Tschakert, G.; Mueller, A.; Groeschl, W.; Pieber, T.R.; Obermayer-Pietsch, B.; Koehler, G.; Hofmann, P. Effects of High-Intensity Interval Exercise versus Moderate Continuous Exercise on Glucose Homeostasis and Hormone Response in Patients with Type 1 Diabetes Mellitus Using Novel Ultra-Long-Acting Insulin. PLoS ONE 2015, 10, e0136489. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Study Group (Analyzed) n = 11 |
|---|---|
| Age (mean ± SD, median, [range], years) | |
| At diagnosis | 13.8 ± 3.4, 15 (7–18) |
| At intervention | 13.9 ± 3.6, 15 (7–18) |
| Age group (n) | |
| Children (6–11 years) a | 3 |
| Adolescents (12–17 years) | 6 |
| Young adults (>17 years) | 2 |
| Sex (% male) | |
| Male | 64 |
| Female | 36 |
| Time since Diagnosis (mean ± SD, median [range], days) | |
| 55 ± 11, 54 (34–74) | |
| Cancer type | |
| Leukemia | 2 |
| Lymphoma b | 6 |
| Other solid tumor c | 3 |
| Anticancer treatment received until examination | |
| Chemotherapy | 12 |
| Radiotherapy | 0 |
| Surgery d | 0 |
| Last application of chemotherapy before the intervention (days) e | 9 ± 6 (2–20) |
| Anthropometrical variables (mean ± SD, median [range]) | |
| Body weight (kg) | 61.6 ± 20.2, 59.0 (21.6–90.0) |
| BMI (kg/m2) | 20.7 ± 4.1, 20.6 (13.8–27.7) |
| Body surface (m2) | 1.69 ± 0.37, 1.72 (0.85–2.18) |
| Physiological parameters (at rest) (mean ± SD, median [range]) | |
| Heart rate (bpm) | 78 ± 13, 78 (55–107) |
| Blood pressure systolic (mmHg) | 117 ± 11, 111 (104–135) |
| Blood pressure diastolic (mmHg) | 80 ± 9, 80 (68–97) |
| Physical activity pre-diagnosis f (mean ± SD, days) | |
| Days with physical activity ≥ 60 min per day | 4 ± 3, 3 (0–7) |
| Days with moderate-to-vigorous intensity | 4 ± 3, 3 (0–7) |
| Feasibility Criteria | Objective |
|---|---|
| Recruitment | >50% of addressed participants |
| Acceptance | >50% finish the intervention (83%; 10/12) * |
| Practicability | Realizable for a specific group of participants with respect to strict inclusion criteria and an average time investment of 6 h per participant as estimated, and participants achieve the intended level of intensity (subjectively via BORG scale and objectively via lactate concentrations) |
| Data acquisition | Applicable data of all participants |
| Subjective data: | |
| Physical activity questionnaire (100%; 11/11) | |
| Rate of perceived exertion (BORG scale) (100%; 11/11) | |
| Objective data: | |
| Heart rate (64%; 7/11) | |
| Blood samples for lactate and adrenaline concentrations (100%; 11/11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kesting, S.; Weeber, P.; Schönfelder, M.; Pfluger, A.; Wackerhage, H.; von Luettichau, I. A Bout of High-Intensity Interval Training (HIIT) in Children and Adolescents during Acute Cancer Treatment—A Pilot Feasibility Study. Cancers 2022, 14, 1468. https://doi.org/10.3390/cancers14061468
Kesting S, Weeber P, Schönfelder M, Pfluger A, Wackerhage H, von Luettichau I. A Bout of High-Intensity Interval Training (HIIT) in Children and Adolescents during Acute Cancer Treatment—A Pilot Feasibility Study. Cancers. 2022; 14(6):1468. https://doi.org/10.3390/cancers14061468
Chicago/Turabian StyleKesting, Sabine, Peter Weeber, Martin Schönfelder, Anja Pfluger, Henning Wackerhage, and Irene von Luettichau. 2022. "A Bout of High-Intensity Interval Training (HIIT) in Children and Adolescents during Acute Cancer Treatment—A Pilot Feasibility Study" Cancers 14, no. 6: 1468. https://doi.org/10.3390/cancers14061468
APA StyleKesting, S., Weeber, P., Schönfelder, M., Pfluger, A., Wackerhage, H., & von Luettichau, I. (2022). A Bout of High-Intensity Interval Training (HIIT) in Children and Adolescents during Acute Cancer Treatment—A Pilot Feasibility Study. Cancers, 14(6), 1468. https://doi.org/10.3390/cancers14061468







