Functional Ex Vivo Tissue-Based Chemotherapy Sensitivity Testing for Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Derived Xenografts
2.2. Primary Breast Cancer (BC) Specimens
2.3. Metastatic Breast Cancer Biopsies
2.4. Tissue Slicing and Drug Treatment Ex Vivo
2.5. Staining Protocols
2.6. Image Acquisition and Analysis
2.7. Defining Ex-Vivo Sensitivity Test Results
2.8. Statistics
3. Results
3.1. Ex Vivo Cisplatin Drug Screening on Tissue Slices Reflects an In Vivo Cisplatin Response in PDXs
3.2. Cisplatin Sensitivity on Surgical Resection Material
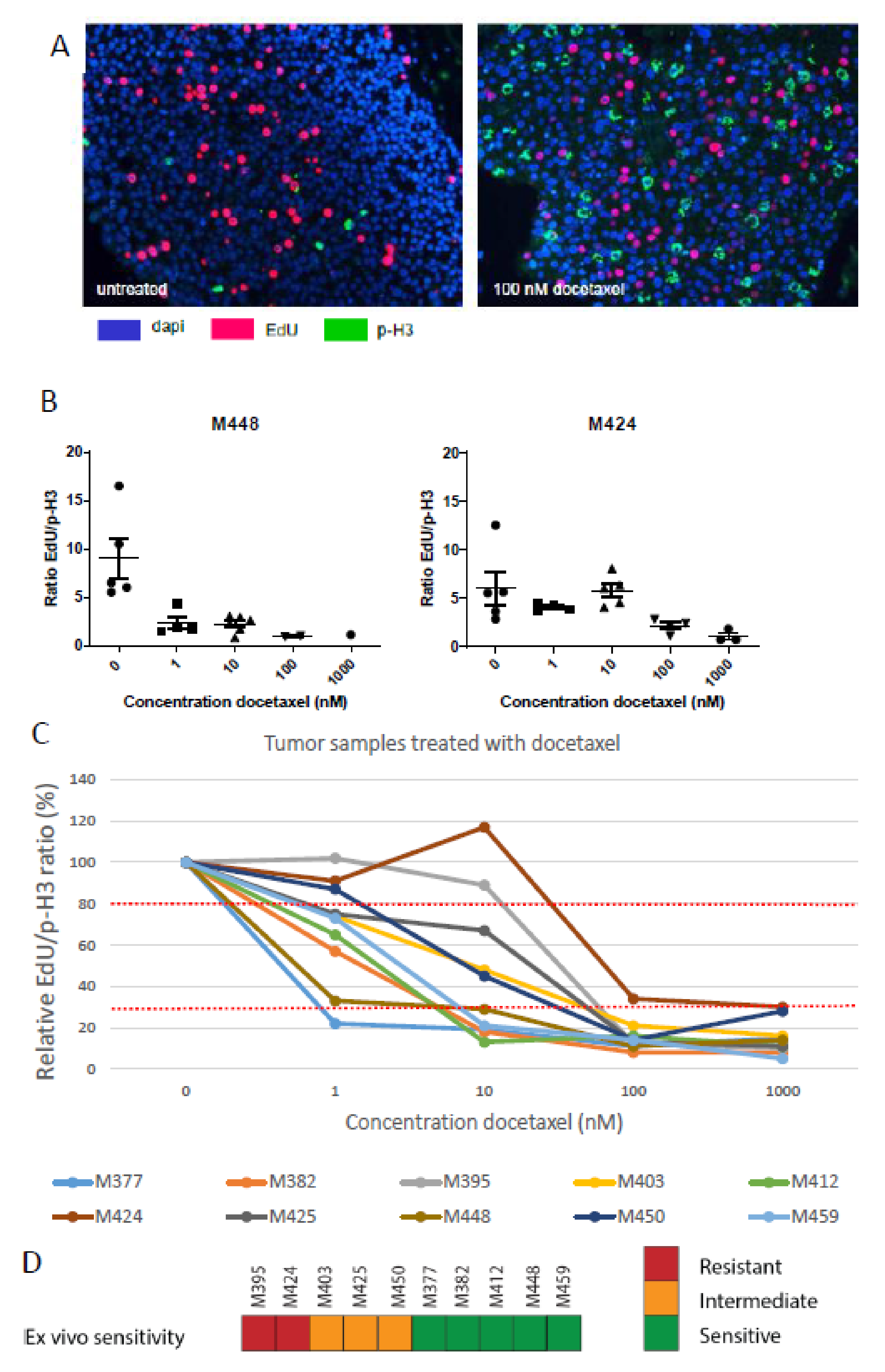
3.3. Docetaxel Sensitivity Assay
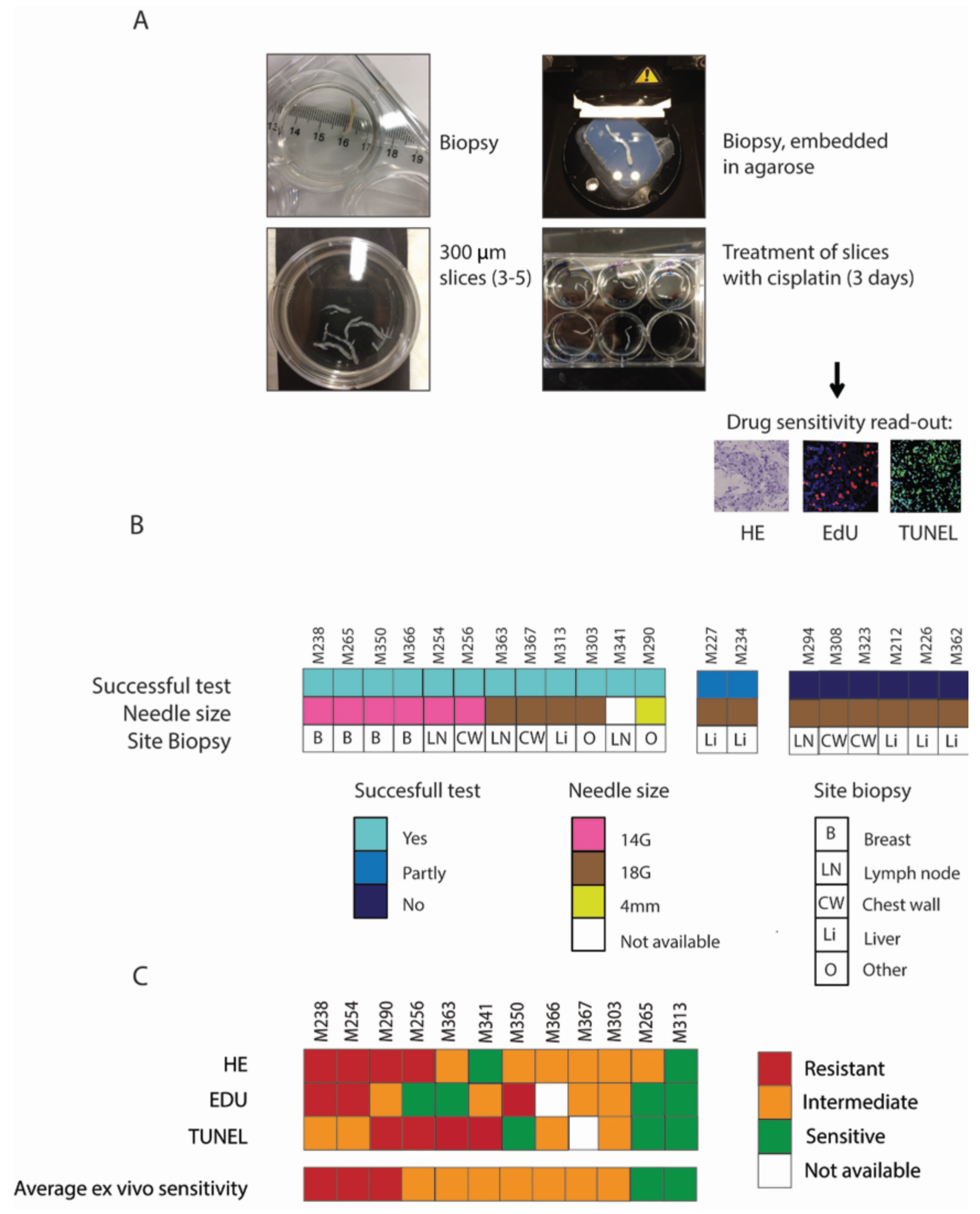
3.4. Sensitivity Assays on Biopsies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicolini, A.; Ferrari, P.; Duffy, M.J. Prognostic and predictive biomarkers in breast cancer: Past, present and future. Semin. Cancer Biol. 2018, 52, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Naipal, K.A.; Verkaik, N.S.; Sanchez, H.; van Deurzen, C.H.; den Bakker, M.A.; Hoeijmakers, J.H.; Kanaar, R.; Vreeswijk, M.P.; Jager, A.; van Gent, D.C. Tumor slice culture system to assess drug response of primary breast cancer. BMC Cancer 2016, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Holliday, D.L.; Moss, M.A.; Pollock, S.; Lane, S.; Shaaban, A.M.; Millican-Slater, R.; Nash, C.; Hanby, A.M.; Speirs, V. The practicalities of using tissue slices as preclinical organotypic breast cancer models. J. Clin. Pathol. 2013, 66, 253–255. [Google Scholar] [CrossRef] [PubMed]
- van der Kuip, H.; Murdter, T.E.; Sonnenberg, M.; McClellan, M.; Gutzeit, S.; Gerteis, A.; Simon, W.; Fritz, P.; Aulitzky, W.E. Short term culture of breast cancer tissues to study the activity of the anticancer drug taxol in an intact tumor environment. BMC Cancer 2006, 6, 86. [Google Scholar] [CrossRef]
- Carranza-Torres, I.E.; Guzman-Delgado, N.E.; Coronado-Martinez, C.; Banuelos-Garcia, J.I.; Viveros-Valdez, E.; Moran-Martinez, J.; Carranza-Rosales, P. Organotypic culture of breast tumor explants as a multicellular system for the screening of natural compounds with antineoplastic potential. Biomed. Res. Int. 2015, 2015, 618021. [Google Scholar] [CrossRef]
- Isakoff, S.J.; Mayer, E.L.; He, L.; Traina, T.A.; Carey, L.A.; Krag, K.J.; Rugo, H.S.; Liu, M.C.; Stearns, V.; Come, S.E.; et al. Tbcrc009: A multicenter phase ii clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J. Clin. Oncol. 2015, 33, 1902–1909. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, Y.; Sun, X.J.; Wang, B.Y.; Wang, Z.H.; Luo, J.F.; Wang, L.P.; Zhang, S.; Cao, J.; Tao, Z.H.; et al. Biomarker assessment of the cbcsg006 trial: A randomized phase III trial of cisplatin plus gemcitabine compared with paclitaxel plus gemcitabine as first-line therapy for patients with metastatic triple-negative breast cancer. Ann. Oncol. 2018, 29, 1741–1747. [Google Scholar] [CrossRef]
- Meijer, T.G.; Verkaik, N.S.; Sieuwerts, A.M.; van Riet, J.; Naipal, K.A.T.; van Deurzen, C.H.M.; den Bakker, M.A.; Sleddens, H.; Dubbink, H.J.; den Toom, T.D.; et al. Functional ex vivo assay reveals homologous recombination deficiency in breast cancer beyond brca gene defects. Clin. Cancer Res. 2018, 24, 6277–6287. [Google Scholar] [CrossRef]
- Ladan, M.M.; van Gent, D.C.; Jager, A. Homologous recombination deficiency testing for brca-like tumors: The road to clinical validation. Cancers 2021, 13, 1004. [Google Scholar] [CrossRef]
- Schiff, P.B.; Horwitz, S.B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc. Natl. Acad. Sci. USA 1980, 77, 1561–1565. [Google Scholar] [CrossRef]
- Fekete, J.T.; Osz, A.; Pete, I.; Nagy, G.R.; Vereczkey, I.; Gyorffy, B. Predictive biomarkers of platinum and taxane resistance using the transcriptomic data of 1816 ovarian cancer patients. Gynecol. Oncol. 2020, 156, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Ter Brugge, P.; Kristel, P.; van der Burg, E.; Boon, U.; de Maaker, M.; Lips, E.; Mulder, L.; de Ruiter, J.; Moutinho, C.; Gevensleben, H.; et al. Mechanisms of therapy resistance in patient-derived xenograft models of brca1-deficient breast cancer. J. Natl. Cancer Inst. 2016, 108, djw148. [Google Scholar] [CrossRef] [PubMed]
- Meijer, T.G.; Verkaik, N.S.; van Deurzen, C.H.M.; Dubbink, H.J.; den Toom, T.D.; Sleddens, H.F.B.M.; Oomen-de Hoop, E.; Dinjens, W.N.M.; Kanaar, R.; van Gent, D.C.; et al. Direct ex vivo observation of homologous recombination defect reversal after DNA-damaging chemotherapy in patients with metastatic breast cancer. JCO Precis. Oncol. 2019, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; van Weerden, W.M.; de Ridder, C.M.A.; Erkens-Schulze, S.; Schonfeld, E.; Meijer, T.G.; Kanaar, R.; van Gent, D.C.; Nonnekens, J. Ex vivo treatment of prostate tumor tissue recapitulates in vivo therapy response. Prostate 2018, 79, 390–402. [Google Scholar] [CrossRef]
- Moreno-Aspitia, A.; Perez, E.A. Treatment options for breast cancer resistant to anthracycline and taxane. Mayo. Clin. Proc. 2009, 84, 533–545. [Google Scholar] [CrossRef]
- Urbaniak, A.; Pina-Oviedo, S.; Yuan, Y.; Huczynski, A.; Chambers, T.C. Limitations of an ex vivo breast cancer model for studying the mechanism of action of the anticancer drug paclitaxel. Eur. J. Pharmacol. 2021, 891, 173780. [Google Scholar] [CrossRef] [PubMed]
- Junk, D.; Kramer, S.; Broschewitz, J.; Laura, H.; Massa, C.; Moulla, Y.; Hoang, N.A.; Monecke, A.; Eichfeld, U.; Bechmann, I.; et al. Human tissue cultures of lung cancer predict patient susceptibility to immune-checkpoint inhibition. Cell Death Discov. 2021, 7, 264. [Google Scholar] [CrossRef]
- Alsafadi, H.N.; Uhl, F.E.; Pineda, R.H.; Bailey, K.E.; Rojas, M.; Wagner, D.E.; Konigshoff, M. Applications and approaches for three-dimensional precision-cut lung slices. Disease modeling and drug discovery. Am. J. Respir. Cell Mol. Biol. 2020, 62, 681–691. [Google Scholar] [CrossRef]
- Berger, J.; Zech, H.B.; Hoffer, K.; von Bargen, C.M.; Nordquist, L.; Bussmann, L.; Gatzemeier, F.; Busch, C.J.; Mockelmann, N.; Munscher, A.; et al. Kinomic comparison of snap frozen and ex vivo-cultured head and neck tumors. Oral Oncol. 2021, 123, 105603. [Google Scholar] [CrossRef]
- Peria, M.; Donnadieu, J.; Racz, C.; Ikoli, J.F.; Galmiche, A.; Chauffert, B.; Page, C. Evaluation of individual sensitivity of head and neck squamous cell carcinoma to cetuximab by short-term culture of tumor slices. Head Neck 2016, 38 (Suppl. 1), E911–E915. [Google Scholar] [CrossRef]
- Donnadieu, J.; Lachaier, E.; Peria, M.; Saidak, Z.; Dakpe, S.; Ikoli, J.F.; Chauffert, B.; Page, C.; Galmiche, A. Short-term culture of tumour slices reveals the heterogeneous sensitivity of human head and neck squamous cell carcinoma to targeted therapies. BMC Cancer 2016, 16, 273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meijer, T.G.; Naipal, K.A.; Jager, A.; van Gent, D.C. Ex vivo tumor culture systems for functional drug testing and therapy response prediction. Future Sci. OA 2017, 3, FSO190. [Google Scholar] [CrossRef] [PubMed]
- Dorrigiv, D.; Simeone, K.; Communal, L.; Kendall-Dupont, J.; St-Georges-Robillard, A.; Peant, B.; Carmona, E.; Mes-Masson, A.M.; Gervais, T. Microdissected tissue vs tissue slices-a comparative study of tumor explant models cultured on-chip and off-chip. Cancers 2021, 13, 4208. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladan, M.M.; Meijer, T.G.; Verkaik, N.S.; Komar, Z.M.; van Deurzen, C.H.M.; den Bakker, M.A.; Kanaar, R.; van Gent, D.C.; Jager, A. Functional Ex Vivo Tissue-Based Chemotherapy Sensitivity Testing for Breast Cancer. Cancers 2022, 14, 1252. https://doi.org/10.3390/cancers14051252
Ladan MM, Meijer TG, Verkaik NS, Komar ZM, van Deurzen CHM, den Bakker MA, Kanaar R, van Gent DC, Jager A. Functional Ex Vivo Tissue-Based Chemotherapy Sensitivity Testing for Breast Cancer. Cancers. 2022; 14(5):1252. https://doi.org/10.3390/cancers14051252
Chicago/Turabian StyleLadan, Marjolijn M., Titia G. Meijer, Nicole S. Verkaik, Zofia M. Komar, Carolien H. M. van Deurzen, Michael A. den Bakker, Roland Kanaar, Dik C. van Gent, and Agnes Jager. 2022. "Functional Ex Vivo Tissue-Based Chemotherapy Sensitivity Testing for Breast Cancer" Cancers 14, no. 5: 1252. https://doi.org/10.3390/cancers14051252
APA StyleLadan, M. M., Meijer, T. G., Verkaik, N. S., Komar, Z. M., van Deurzen, C. H. M., den Bakker, M. A., Kanaar, R., van Gent, D. C., & Jager, A. (2022). Functional Ex Vivo Tissue-Based Chemotherapy Sensitivity Testing for Breast Cancer. Cancers, 14(5), 1252. https://doi.org/10.3390/cancers14051252







