Impact of UGT1A1 Polymorphisms on Febrile Neutropenia in Pancreatic Cancer Patients Receiving FOLFIRINOX: A Single-Center Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Chemotherapy Schedule and Response Evaluation
2.3. Assessment of Chemotherapy-Related Adverse Events
2.4. Febrile Neutropenia and Grade 4 Neutropenia
2.5. UGT1A1 Polymorphisms
2.6. Overall Survival and Progression-Free Survival
2.7. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Risk Factors for the Development of Febrile Neutropenia
3.3. Risk Factors for the Development of Grade 4 Neutropenia
3.4. Relationship between UGT1A1 Phenotypes and Hematologic Toxicities
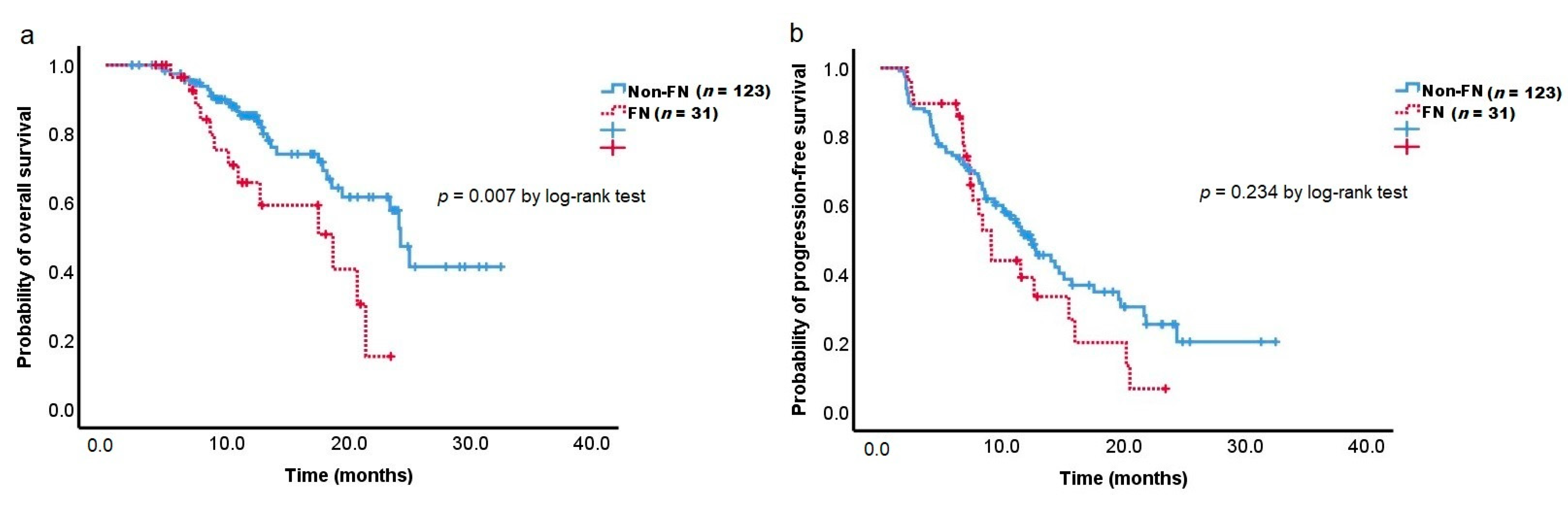
3.5. Overall Survival and Progression-Free Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. Folfirinox versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Mangu, P.B.; Khorana, A.A.; Shah, M.A.; Philip, P.A.; O’Reilly, E.M.; Uronis, H.E.; Ramanathan, R.K.; Crane, C.H.; Engebretson, A.; et al. Metastatic pancreatic cancer: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2016, 34, 2784–2796. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic adenocarcinoma, version 2.2021, nccn clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef]
- Okusaka, T.; Ikeda, M.; Fukutomi, A.; Ioka, T.; Furuse, J.; Ohkawa, S.; Isayama, H.; Boku, N. Phase ii study of FOLFIRINOX for chemotherapy-naïve Japanese patients with metastatic pancreatic cancer. Cancer Sci. 2014, 105, 1321–1326. [Google Scholar] [CrossRef]
- Hosein, P.J.; Macintyre, J.; Kawamura, C.; Maldonado, J.C.; Ernani, V.; Loaiza-Bonilla, A.; Narayanan, G.; Ribeiro, A.; Portelance, L.; Merchan, J.R.; et al. A retrospective study of neoadjuvant FOLFIRINOX in unresectable or borderline-resectable locally advanced pancreatic adenocarcinoma. BMC Cancer 2012, 12, 199. [Google Scholar] [CrossRef]
- Kang, H.; Jo, J.H.; Lee, H.S.; Chung, M.J.; Bang, S.; Park, S.W.; Song, S.Y.; Park, J.Y. Comparison of efficacy and safety between standard-dose and modified-dose FOLFIRINOX as a first-line treatment of pancreatic cancer. World J. Gastrointest. Oncol. 2018, 10, 421–430. [Google Scholar] [CrossRef]
- Cho, I.R.; Kang, H.; Jo, J.H.; Lee, H.S.; Chung, M.J.; Park, J.Y.; Park, S.W.; Song, S.Y.; An, C.; Park, M.S.; et al. Folfirinox vs gemcitabine/nab-paclitaxel for treatment of metastatic pancreatic cancer: Single-center cohort study. World J. Gastrointest. Oncol. 2020, 12, 182–194. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Hematopoietic Growth Factors, Version 4. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf (accessed on 5 October 2021).
- Sasaki, M.; Ueno, H.; Kuchiba, A.; Koga, F.; Shiba, S.; Sakamoto, Y.; Kondo, S.; Morizane, C.; Okusaka, T. P-167risk factors for febrile neutropenia in patients with unresectable pancreatic cancer receiving FOLFIRINOX as the first-line treatment. Ann. Oncol. 2015, 26, iv48. [Google Scholar] [CrossRef][Green Version]
- Keum, J.; Lee, H.S.; Kang, H.; Jo, J.H.; Chung, M.J.; Park, J.Y.; Park, S.W.; Song, S.Y.; Bang, S. Single-center risk factor analysis for FOLFIRINOX associated febrile neutropenia in patients with pancreatic cancer. Cancer Chemother. Pharm. 2020, 85, 651–659. [Google Scholar] [CrossRef]
- Senter, P.D.; Beam, K.S.; Mixan, B.; Wahl, A.F. Identification and activities of human carboxylesterases for the activation of cpt-11, a clinically approved anticancer drug. Bioconjug. Chem. 2001, 12, 1074–1080. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, W.; Ma, M.K.; McLeod, H.L. Human carboxylesterase 2 is commonly expressed in tumor tissue and is correlated with activation of irinotecan. Clin. Cancer Res. 2002, 8, 2605–2611. [Google Scholar]
- Dranitsaris, G.; Shah, A.; Spirovski, B.; Vincent, M. Severe diarrhea in patients with advanced-stage colorectal cancer receiving folfox or folfiri chemotherapy: The development of a risk prediction tool. Clin. Colorectal Cancer 2007, 6, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, T.; Tadokoro, J.; Fujiki, T.; Fujino, K.; Kakihata, K.; Masatani, S.; Morita, S.; Gemma, A.; Boku, N. Risk factors for severe adverse effects and treatment-related deaths in Japanese patients treated with irinotecan-based chemotherapy: A postmarketing survey. JPN J. Clin. Oncol. 2013, 43, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, F.; Undevia, S.D.; Iyer, L.; Chen, P.X.; Das, S.; Kocherginsky, M.; Karrison, T.; Janisch, L.; Ramírez, J.; Rudin, C.M.; et al. Genetic variants in the udp-glucuronosyltransferase 1a1 gene predict the risk of severe neutropenia of irinotecan. J. Clin. Oncol. 2004, 22, 1382–1388. [Google Scholar] [CrossRef]
- Iyer, L.; King, C.D.; Whitington, P.F.; Green, M.D.; Roy, S.K.; Tephly, T.R.; Coffman, B.L.; Ratain, M.J. Genetic predisposition to the metabolism of irinotecan (cpt-11). Role of uridine diphosphate glucuronosyltransferase isoform 1a1 in the glucuronidation of its active metabolite (sn-38) in human liver microsomes. J. Clin. Investig. 1998, 101, 847–854. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, M.; Hu, M.; Cui, Y.; Zhong, Q.; Liang, L.; Huang, F. UGT1A1*6 and UGT1A1*28 polymorphisms are correlated with irinotecan-induced toxicity: A meta-analysis. Asia Pac. J. Clin. Oncol. 2018, 14, e479–e489. [Google Scholar] [CrossRef]
- Hikino, K.; Ozeki, T.; Koido, M.; Terao, C.; Kamatani, Y.; Murakami, Y.; Kubo, M.; Mushiroda, T. Comparison of effects of UGT1A1*6 and UGT1A1*28 on irinotecan-induced adverse reactions in the Japanese population: Analysis of the biobank japan project. J. Hum. Genet. 2019, 64, 1195–1202. [Google Scholar] [CrossRef]
- Ando, Y.; Saka, H.; Ando, M.; Sawa, T.; Muro, K.; Ueoka, H.; Yokoyama, A.; Saitoh, S.; Shimokata, K.; Hasegawa, Y. Polymorphisms of udp-glucuronosyltransferase gene and irinotecan toxicity: A pharmacogenetic analysis. Cancer Res. 2000, 60, 6921–6926. [Google Scholar] [PubMed]
- Fujita, K.; Sparreboom, A. Pharmacogenetics of irinotecan disposition and toxicity: A review. Curr. Clin. Pharm. 2010, 5, 209–217. [Google Scholar] [CrossRef]
- Nelson, R.S.; Seligson, N.D.; Bottiglieri, S.; Carballido, E.; Cueto, A.D.; Imanirad, I.; Levine, R.; Parker, A.S.; Swain, S.M.; Tillman, E.M. UGT1A1 guided cancer therapy: Review of the evidence and considerations for clinical implementation. Cancers 2021, 13, 1566. [Google Scholar] [CrossRef] [PubMed]
- Gammal, R.S.; Court, M.H.; Haidar, C.E.; Iwuchukwu, O.F.; Gaur, A.H.; Alvarellos, M.; Guillemette, C.; Lennox, J.L.; Whirl-Carrillo, M.; Brummel, S.S.; et al. Clinical pharmacogenetics implementation consortium (cpic) guideline for UGT1A1 and atazanavir prescribing. Clin. Pharm. Ther. 2016, 99, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.R.; Kang, H.; Jo, J.H.; Lee, H.S.; Chung, M.J.; Park, J.Y.; Park, S.W.; Song, S.Y.; Chung, J.B.; An, C.; et al. Efficacy and treatment-related adverse events of gemcitabine plus nab-paclitaxel for treatment of metastatic pancreatic cancer “in a Korean” population: A single-center cohort study. Semin. Oncol. 2017, 44, 420–427. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services; National Institutes of Health; National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) V5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 31 August 2021).
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.-A.H.; Wingard, J.R. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin. Infect. Dis. 2011, 52, e56–e93. [Google Scholar] [CrossRef]
- Toffoli, G.; Cecchin, E.; Corona, G.; Russo, A.; Buonadonna, A.; D’Andrea, M.; Pasetto, L.M.; Pessa, S.; Errante, D.; De Pangher, V.; et al. The role of UGT1A1*28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer. J. Clin. Oncol. 2006, 24, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Toffoli, G.; Cecchin, E.; Gasparini, G.; D’Andrea, M.; Azzarello, G.; Basso, U.; Mini, E.; Pessa, S.; De Mattia, E.; Lo Re, G.; et al. Genotype-driven phase i study of irinotecan administered in combination with fluorouracil/leucovorin in patients with metastatic colorectal cancer. J. Clin. Oncol. 2010, 28, 866–871. [Google Scholar] [CrossRef]
- Dias, M.M.; Pignon, J.P.; Karapetis, C.S.; Boige, V.; Glimelius, B.; Kweekel, D.M.; Lara, P.N.; Laurent-Puig, P.; Martinez-Balibrea, E.; Páez, D.; et al. The effect of the UGT1A1*28 allele on survival after irinotecan-based chemotherapy: A collaborative meta-analysis. Pharm. J. 2014, 14, 424–431. [Google Scholar] [CrossRef]
- Innocenti, F.; Schilsky, R.L.; Ramírez, J.; Janisch, L.; Undevia, S.; House, L.K.; Das, S.; Wu, K.; Turcich, M.; Marsh, R.; et al. Dose-finding and pharmacokinetic study to optimize the dosing of irinotecan according to the UGT1A1 genotype of patients with cancer. J. Clin. Oncol. 2014, 32, 2328–2334. [Google Scholar] [CrossRef]
- Fujii, H.; Yamada, Y.; Watanabe, D.; Matsuhashi, N.; Takahashi, T.; Yoshida, K.; Suzuki, A. Dose adjustment of irinotecan based on UGT1A1 polymorphisms in patients with colorectal cancer. Cancer Chemother. Pharm. 2019, 83, 123–129. [Google Scholar] [CrossRef]
- Etienne-Grimaldi, M.C.; Boyer, J.C.; Thomas, F.; Quaranta, S.; Picard, N.; Loriot, M.A.; Narjoz, C.; Poncet, D.; Gagnieu, M.C.; Ged, C.; et al. UGT1A1 genotype and irinotecan therapy: General review and implementation in routine practice. Fundam. Clin. Pharm. 2015, 29, 219–237. [Google Scholar] [CrossRef]
- Swen, J.J.; Nijenhuis, M.; de Boer, A.; Grandia, L.; Maitland-van der Zee, A.H.; Mulder, H.; Rongen, G.A.; van Schaik, R.H.; Schalekamp, T.; Touw, D.J.; et al. Pharmacogenetics: From bench to byte—An update of guidelines. Clin. Pharm. Ther. 2011, 89, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.O.; Armstrong, K.; Botkin, J.; Calonge, N.; Haddow, J.; Hayes, M.; Kaye, C.; Phillips, K.A.; Piper, M.; Richards, C.S.; et al. Recommendations from the egapp working group: Can UGT1A1 genotyping reduce morbidity and mortality in patients with metastatic colorectal cancer treated with irinotecan? Genet. Med. 2009, 11, 15–20. [Google Scholar]
- Shirasu, H.; Todaka, A.; Omae, K.; Fujii, H.; Mizuno, N.; Ozaka, M.; Ueno, H.; Kobayashi, S.; Uesugi, K.; Kobayashi, N.; et al. Impact of UGT1A1 genetic polymorphism on toxicity in unresectable pancreatic cancer patients undergoing FOLFIRINOX. Cancer Sci. 2019, 110, 707–716. [Google Scholar] [CrossRef]
- Sharma, M.R.; Joshi, S.S.; Karrison, T.G.; Allen, K.; Suh, G.; Marsh, R.; Kozloff, M.F.; Polite, B.N.; Catenacci, D.V.T.; Kindler, H.L. A UGT1A1 genotype-guided dosing study of modified FOLFIRINOX in previously untreated patients with advanced gastrointestinal malignancies. Cancer 2019, 125, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Catenacci, D.V.T.; Karrison, T.G.; Peterson, J.D.; Zalupski, M.M.; Sehdev, A.; Wade, J.; Sadiq, A.; Picozzi, V.J.; Amico, A.; et al. Clinical assessment of 5-fluorouracil/leucovorin, nab-paclitaxel, and irinotecan (FOLFIRABRAX) in untreated patients with gastrointestinal cancer using UGT1A1 genotype-guided dosing. Clin. Cancer Res. 2020, 26, 18–24. [Google Scholar] [CrossRef]
- de Man, F.M.; Goey, A.K.L.; van Schaik, R.H.N.; Mathijssen, R.H.J.; Bins, S. Individualization of irinotecan treatment: A review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin. Pharm. 2018, 57, 1229–1254. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, M.; Hu, J.; Ren, W.; Xie, L.; Sun, Z.P.; Liu, B.R.; Xu, G.X.; Dong, X.L.; Qian, X.P. UGT1A1*6 polymorphisms are correlated with irinotecan-induced toxicity: A system review and meta-analysis in Asians. Cancer Chemother. Pharm. 2014, 73, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Oh, J.; Kim, Y.; Lee, K.A. Genetic spectrum of UGT1A1 in Korean patients with unconjugated hyperbilirubinemia. Ann. Lab. Med. 2020, 40, 281–283. [Google Scholar] [CrossRef]
- Minami, H.; Sai, K.; Saeki, M.; Saito, Y.; Ozawa, S.; Suzuki, K.; Kaniwa, N.; Sawada, J.; Hamaguchi, T.; Yamamoto, N.; et al. Irinotecan pharmacokinetics/pharmacodynamics and ugt1a genetic polymorphisms in Japanese: Roles of UGT1A1*6 and *28. Pharm. Genom. 2007, 17, 497–504. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Yu, Q.; Pei, Q.; Guo, C. Dose-dependent association between UGT1A1*28 genotype and irinotecan-induced neutropenia: Low doses also increase risk. Clin. Cancer Res. 2010, 16, 3832–3842. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, J.M.; Goldberg, R.M.; Qu, P.; Ibrahim, J.G.; McLeod, H.L. UGT1A1*28 genotype and irinotecan-induced neutropenia: Dose matters. J. Natl. Cancer Inst. 2007, 99, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, K.; Takahashi, H.; Morizane, C.; Yamada, I.; Shimizu, S.; Shioji, K.; Yoshida, Y.; Motoya, M.; Mizuno, N.; Kojima, Y.; et al. Folfirinox in advanced pancreatic cancer patients with the double-variant type of UGT1A1*28 and *6 polymorphism: A multicenter, retrospective study. Cancer Chemother. Pharm. 2021, 87, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, F.; Kroetz, D.L.; Schuetz, E.; Dolan, M.E.; Ramírez, J.; Relling, M.; Chen, P.; Das, S.; Rosner, G.L.; Ratain, M.J. Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J. Clin. Oncol. 2009, 27, 2604–2614. [Google Scholar] [CrossRef]
- Crawford, J.; Glaspy, J.A.; Stoller, R.G.; Tomita, D.K.; Vincent, M.E.; McGuire, B.W.; Ozer, H. Final results of a placebo-controlled study of filgrastim in small-cell lung cancer: Exploration of risk factors for febrile neutropenia. Support Cancer Ther. 2005, 3, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Abella, E.; Pettengell, R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: A systematic review. Crit. Rev Oncol. Hematol. 2014, 90, 190–199. [Google Scholar] [CrossRef]
- Jung, J.H.; Shin, D.W.; Kim, J.; Lee, J.C.; Hwang, J.H. Primary granulocyte colony-stimulating factor prophylaxis in metastatic pancreatic cancer patients treated with FOLFIRINOX as the first-line treatment. Cancers 2020, 12, 3137. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Chen, P.M.; Chiou, T.J.; Liu, J.H.; Lin, J.K.; Lin, T.C.; Chen, W.S.; Jiang, J.K.; Wang, H.S.; Wang, W.S. UGT1A1*28 polymorphism predicts irinotecan-induced severe toxicities without affecting treatment outcome and survival in patients with metastatic colorectal carcinoma. Cancer 2008, 112, 1932–1940. [Google Scholar] [CrossRef]
- Loh, M.; Chua, D.; Yao, Y.; Soo, R.A.; Garrett, K.; Zeps, N.; Platell, C.; Minamoto, T.; Kawakami, K.; Iacopetta, B.; et al. Can population differences in chemotherapy outcomes be inferred from differences in pharmacogenetic frequencies? Pharm. J. 2013, 13, 423–429. [Google Scholar] [CrossRef]
- Rosmarin, D.; Palles, C.; Church, D.; Domingo, E.; Jones, A.; Johnstone, E.; Wang, H.; Love, S.; Julier, P.; Scudder, C.; et al. Genetic markers of toxicity from capecitabine and other fluorouracil-based regimens: Investigation in the quasar2 study, systematic review, and meta-analysis. J. Clin. Oncol. 2014, 32, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.S.; Kwok, L.L.; Syn, N.; Chay, W.Y.; Chia, J.W.K.; Tham, C.K.; Wong, N.S.; Lo, S.K.; Dent, R.A.; Tan, S.; et al. Predictors of hand-foot syndrome and pyridoxine for prevention of capecitabine-induced hand-foot syndrome: A randomized clinical trial. JAMA Oncol. 2017, 3, 1538–1545. [Google Scholar] [CrossRef] [PubMed]

| Variable | All Patients (n = 154) | FN Group (n = 31) | Non-FN Group (n = 123) | p-Value | |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Age, years | 62 (55–67) | 66 (62–70) | 60 (55–66) | <0.001 | |
| Sex, no. (%) | |||||
| Male | 95 (61.7) | 13 (41.9) | 82 (66.7) | 0.011 | |
| Female | 59 (38.3) | 18 (58.1) | 41 (33.3) | ||
| ECOG PS | |||||
| 0 | 130 (84.4) | 21 (67.7) | 109 (88.6) | 0.010 | |
| 1 | 24 (15.6) | 10 (32.3) | 14 (11.4) | ||
| BMI, median (kg/m2) | 22.7 (21.2–25.0) | 23.6 (21.2–26.1) | 22.3 (21.2–24.9) | 0.499 | |
| DM | 50 (32.5) | 13 (41.9) | 37 (30.1) | 0.208 | |
| UGT1A1 | |||||
| Extensive metabolizer | 71 (46.1) | 5 (16.1) | 66 (53.7) | <0.001 | |
| Intermediate metabolizer | 66 (42.9) | 21 (67.7) | 45 (36.6) | ||
| Poor metabolizer | 17 (11.0) | 5 (16.1) | 12 (9.8) | ||
| Tumor characteristics | |||||
| Location | |||||
| Head | 83 (53.9) | 23 (74.2) | 60 (48.8) | 0.011 | |
| Body/Tail | 71 (46.1) | 8 (25.8) | 63 (51.2) | ||
| Stage | |||||
| Resectable | 12 (7.8) | 3 (9.7) | 9 (7.3) | 0.449 | |
| Borderline Resectable | 24 (15.6) | 2 (6.5) | 22 (17.9) | ||
| Locally advanced | 55 (35.7) | 12 (38.7) | 43 (35.0) | ||
| Metastatic | 63 (40.9) | 14 (45.2) | 49 (39.8) | ||
| Laboratory characteristics | |||||
| WBC per μL | 6860.0 (5565.0–8192.5) | 7590.0 (5590.0–8660.0) | 6810.0 (5550.0–7920.0) | 0.212 | |
| Neutrophils per μL | 4200.0 (3172.5–5422.5) | 4600.0 (3390.0–6490.0) | 4030.0 (3150.0–5200.0) | 0.103 | |
| Lymphocytes per μL | 1630.0 (1310.0–2040.0) | 1600.0 (1140.0–2030.0) | 1630.0 (1310.0–2060.0) | 0.442 | |
| NLR | 2.4 (1.8–3.6) | 2.6 (2.0–4.3) | 2.3 (1.7–3.3) | 0.159 | |
| Hemoglobin, g/dL | 12.6 (11.6–13.6) | 12.6 (11.2–13.2) | 12.5 (11.6–13.7) | 0.429 | |
| Platelets, 103/μL | 242.5 (198.8–316.6) | 273.0 (220.0–346.0) | 228.0 (196.0–308.0) | 0.033 | |
| Total bilirubin, mg/dL | 0.6 (0.5–1.0) | 0.8 (0.6–2.0) | 0.6 (0.4–0.9) | 0.002 | |
| AST, IU/L | 20.0 (16.0–34.0) | 25.0 (15.0–46.0) | 20.0 (16.0–30.0) | 0.168 | |
| ALT, IU/L | 21.0 (14.8–40.5) | 27.0 (17.0–48.0) | 19.0 (14.0–38.0) | 0.091 | |
| CA 19-9, U/mL | 245.0 (28.5–1370.8) | 611.0 (135.0–2364.0) | 209.0 (20.8–1247.0) | 0.160 | |
| Albumin, g/dL | 4.1 (3.7–4.4) | 4.0 (3.5–4.2) | 4.1 (3.7–4.5) | 0.151 | |
| Variables | All Patients (n = 154) | FN Group (n = 31) | Non-FN Group * (n = 123) | p-Value |
|---|---|---|---|---|
| Accumulation Dose, mg/m2 | ||||
| 5-FU | 10,400.0 (9645.0–11,200.0) | 8400.0 (5600.0–16,800.0) | 10,500.0 (10,000.0–11,200.0) | 0.237 |
| Oxaliplatin | 340.0 (303.9–340.0) | 255.0 (170.0–510.0) | 340.0 (340.0–340.0) | 0.090 |
| Irinotecan | 720.0 (630.0–720.0) | 540.0 (360.0–1080.0) | 720.0 (675.0–720.0) | 0.110 |
| During All Cycles | |||||
|---|---|---|---|---|---|
| Variables | Univariate | Multivariate | |||
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| Age, years | |||||
| ≥65 | 2.30 (1.03–5.11) | 0.042 | |||
| Sex | |||||
| Male | 1.0 | 0.013 | 1.0 | 0.021 | |
| Female | 2.77 (1.24–6.20) | 2.87 (1.17–7.05) | |||
| ECOG | |||||
| 0 | 1.0 | 0.006 | 1.0 | 0.015 | |
| 1 | 3.71 (1.45–9.46) | 3.86 (1.31–11.42) | |||
| BMI | |||||
| ≥25 | 1.33 (0.55–3.20) | 0.530 | |||
| Tumor location | |||||
| Body/tail | 1.0 | 0.014 | 1.0 | 0.023 | |
| Head | 3.02 (1.25–7.27) | 3.06 (1.16–8.02) | |||
| Stage | |||||
| Resectable | 1.0 | ||||
| Borderline resectable | 0.27 (0.04–1.92) | 0.192 | |||
| Locally advanced | 0.84 (0.20–3.59) | 0.811 | |||
| Metastatic | 0.86 (0.20–3.60) | 0.833 | |||
| NLR | <3 | 1.0 | 0.222 | ||
| ≥3 | 1.65 (0.74–3.67) | ||||
| Hemoglobin, g/dL | ≥12 | 1.0 | 0.471 | ||
| <12 | 1.34 (0.60–3.00) | ||||
| Total bilirubin, mg/dL | ≤1.8 | 1.0 | 0.012 | ||
| >1.8 | 3.46 (1.32–9.09) | ||||
| CA 19-9, U/mL | <1000 | 1.0 | 0.151 | ||
| ≥1000 | 1.82 (0.81–4.10) | ||||
| UGT1A1 phenotype | |||||
| Extensive metabolizer | 1.0 | 1.0 | |||
| Intermediate metabolizer | 6.16 (2.16–17.54) | 0.001 | 4.78 (1.61–14.21) | 0.005 | |
| Poor metabolizer | 5.50 (1.38–21.95) | 0.016 | 4.86 (1.12–21.17) | 0.035 | |
| Variable | Febrile Neutropenia | ||||
|---|---|---|---|---|---|
| Unadjusted HR | p-Value | Adjusted HR | 95% CI | p-Value | |
| Female sex | 2.40 | 0.016 | 2.20 | 1.07–4.51 | 0.031 |
| ECOG PS = 1 | 3.29 | 0.002 | 2.83 | 1.32–6.10 | 0.008 |
| UGT1A1 IM | 5.15 | 0.001 | 4.30 | 1.61–11.52 | 0.004 |
| UGT1A1 PM | 4.49 | 0.018 | 4.03 | 1.16–14.01 | 0.028 |
| Variable | During All Cycles | ||||
|---|---|---|---|---|---|
| Univariate | Multivariate | ||||
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| Age, years | |||||
| ≥65 | 1.95 (0.96–3.94) | 0.063 | |||
| Sex | |||||
| Male | 1.0 | 0.001 | 1.0 | 0.001 | |
| Female | 3.47 (1.66–7.23) | 3.44 (1.63–7.25) | |||
| ECOG | |||||
| 0 | 1.0 | 0.767 | |||
| 1 | 0.88 (0.63–2.12) | ||||
| BMI | |||||
| ≥25 | 0.76 (0.53–2.40) | 1.127 | |||
| Tumor location | |||||
| Body/tail | 1.0 | 0.152 | |||
| Head | 1.61 (0.84–3.10) | ||||
| Stage | |||||
| Resectable | 1.0 | ||||
| Borderline resectable | 1.66 (0.41–6.71) | 0.481 | |||
| Locally advanced | 3.13 (0.87–11.28) | 0.081 | |||
| Metastatic | 2.13 (0.61–7.46) | 0.238 | |||
| NLR | <3 | 1.0 | 0.376 | ||
| ≥3 | 0.74 (0.38–1.45) | ||||
| Hemoglobin, g/dL | ≥12 | 1.0 | 0.950 | ||
| <12 | 0.98 (0.50–1.92) | ||||
| Total bilirubin, mg/dL | ≤1.8 | 1.0 | 0.787 | ||
| >1.8 | 1.14 (0.45–2.90) | ||||
| CA 19-9, U/mL | <1000 | 1.0 | 0.188 | ||
| ≥1000 | 1.62 (0.79–3.34) | ||||
| UGT1A1 phenotype | |||||
| Extensive metabolizer | 1.0 | 1.0 | |||
| Intermediate metabolizer | 1.52 (0.77–3.01) | 0.231 | 1.41 (0.69–2.88) | 0.343 | |
| Poor metabolizer | 4.05 (1.07–15.34) | 0.039 | 4.06 (1.04–15.87) | 0.044 | |
| Variable | Grade 4 Neutropenia | ||||
|---|---|---|---|---|---|
| Unadjusted HR | p-Value | Adjusted HR | 95% CI | p-Value | |
| Female sex | 2.06 | 0.001 | 1.98 | 1.31–2.98 | 0.001 |
| UGT1A1 IM | 1.32 | 0.217 | 1.29 | 0.83–2.00 | 0.264 |
| UGT1A1 PM | 2.01 | 0.026 | 1.79 | 0.96–3.33 | 0.066 |
| Extensive Metabolizer (n = 71) | Intermediate Metabolizer (n = 66) | Poor Metabolizer (n = 17) | All Patients (n = 154) | p-Value | |
|---|---|---|---|---|---|
| Neutropenia | 57/71 (80.3) | 58/66 (87.9) | 15/17 (88.2) | 130/154 (84.4) | 0.411 |
| Neutropenia (Grade IV) | 38/71 (53.5) | 42/66 (63.6) | 14/17 (82.4) | 94/154 (61.0) | 0.082 |
| Febrile neutropenia | 5/71 (7.0) | 21/66 (31.8) | 5/17 (29.4) | 31/154 (20.1) | 0.001 |
| Anemia | 3/71 (4.2) | 13/66 (19.7) | 4/17 (23.5) | 20/154 (13.0) | 0.011 |
| Thrombocytopenia | 4/71 (5.6) | 10/66 (15.2) | 3/17 (17.6) | 17/154 (11.0) | 0.118 |
| Extensive Metabolizer (n = 71) | Intermediate Metabolizer (n = 66) | Poor Metabolizer (n = 17) | All Patients (n = 154) | p-Value | |
|---|---|---|---|---|---|
| Diarrhea | 22/71 (31.0) | 21/66 (31.8) | 4/17 (23.5) | 47/154 (30.5) | 0.801 |
| Diarrhea (Grade III/ IV) | 2/71 (2.8) | 5/66 (7.6) | 1/17 (5.9) | 8/154 (5.2) | 0.427 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keum, J.; Lee, H.S.; Jo, J.H.; Chung, M.J.; Park, J.Y.; Park, S.W.; Song, S.Y.; Bang, S. Impact of UGT1A1 Polymorphisms on Febrile Neutropenia in Pancreatic Cancer Patients Receiving FOLFIRINOX: A Single-Center Cohort Study. Cancers 2022, 14, 1244. https://doi.org/10.3390/cancers14051244
Keum J, Lee HS, Jo JH, Chung MJ, Park JY, Park SW, Song SY, Bang S. Impact of UGT1A1 Polymorphisms on Febrile Neutropenia in Pancreatic Cancer Patients Receiving FOLFIRINOX: A Single-Center Cohort Study. Cancers. 2022; 14(5):1244. https://doi.org/10.3390/cancers14051244
Chicago/Turabian StyleKeum, Jiyoung, Hee Seung Lee, Jung Hyun Jo, Moon Jae Chung, Jeong Youp Park, Seung Woo Park, Si Young Song, and Seungmin Bang. 2022. "Impact of UGT1A1 Polymorphisms on Febrile Neutropenia in Pancreatic Cancer Patients Receiving FOLFIRINOX: A Single-Center Cohort Study" Cancers 14, no. 5: 1244. https://doi.org/10.3390/cancers14051244
APA StyleKeum, J., Lee, H. S., Jo, J. H., Chung, M. J., Park, J. Y., Park, S. W., Song, S. Y., & Bang, S. (2022). Impact of UGT1A1 Polymorphisms on Febrile Neutropenia in Pancreatic Cancer Patients Receiving FOLFIRINOX: A Single-Center Cohort Study. Cancers, 14(5), 1244. https://doi.org/10.3390/cancers14051244






