Survival Benefit of Resection Surgery for Pancreatic Ductal Adenocarcinoma with Liver Metastases: A Propensity Score-Matched SEER Database Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Cohort
2.3. Variables Collected
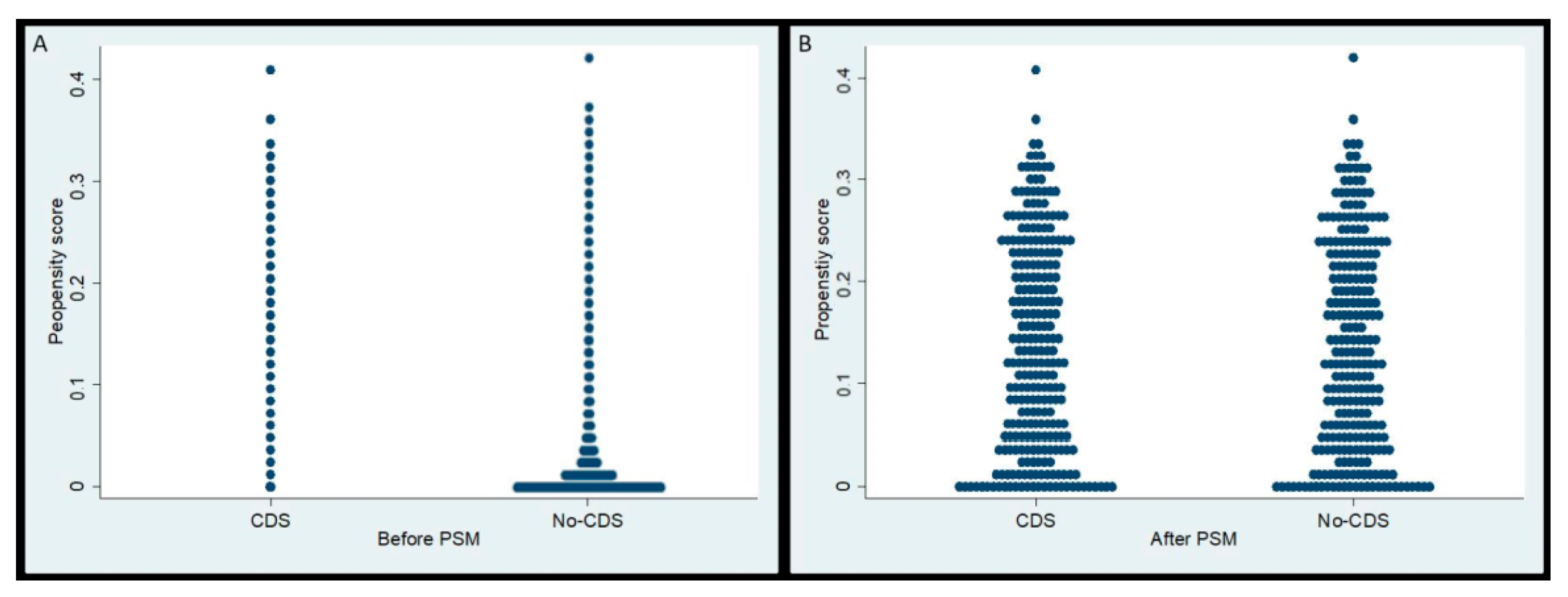
2.4. Propensity Score-Matching (PSM)
2.5. Survival Analysis
3. Results
3.1. Selection of Study Cohort and Propensity Score Matching
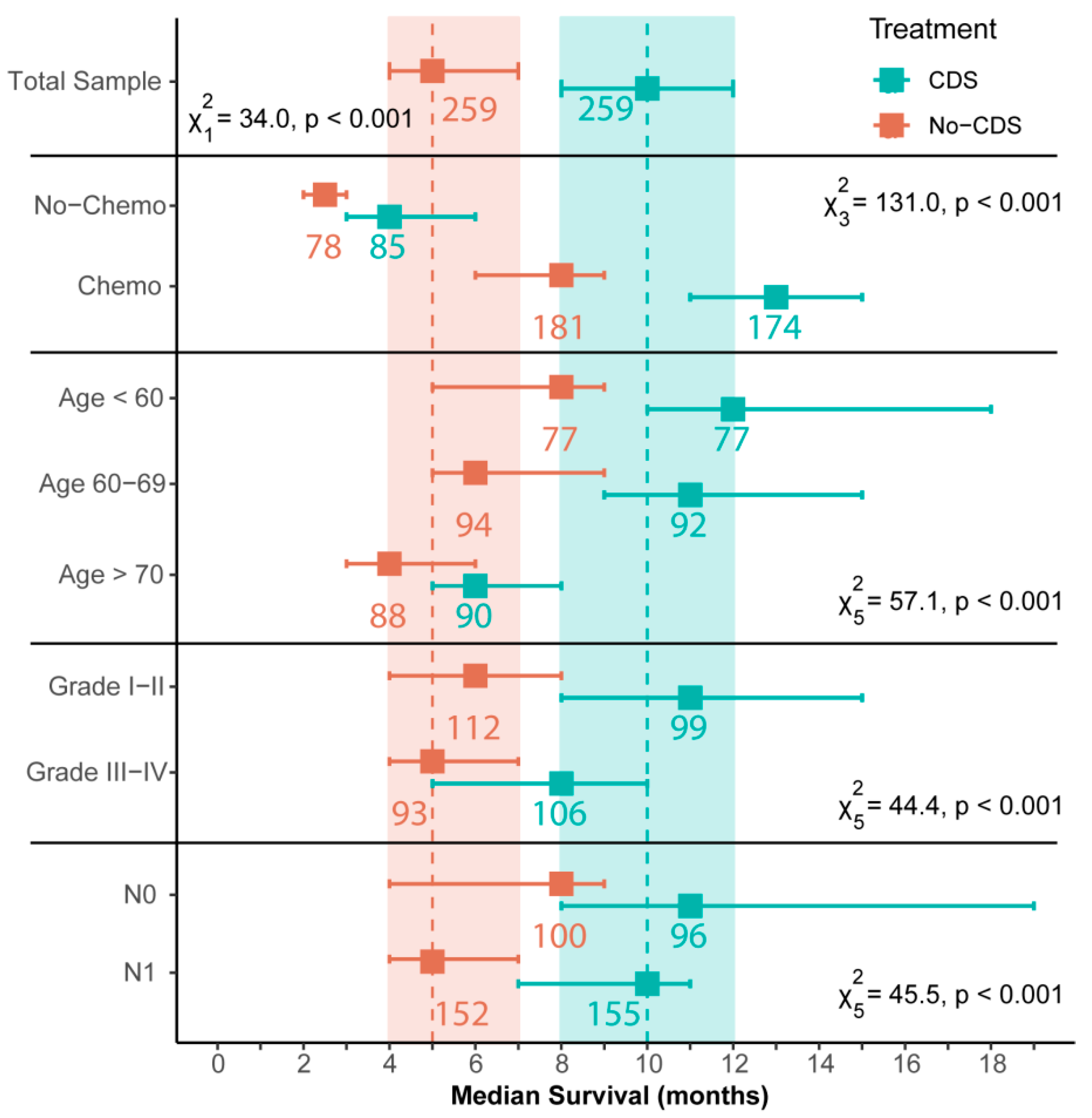
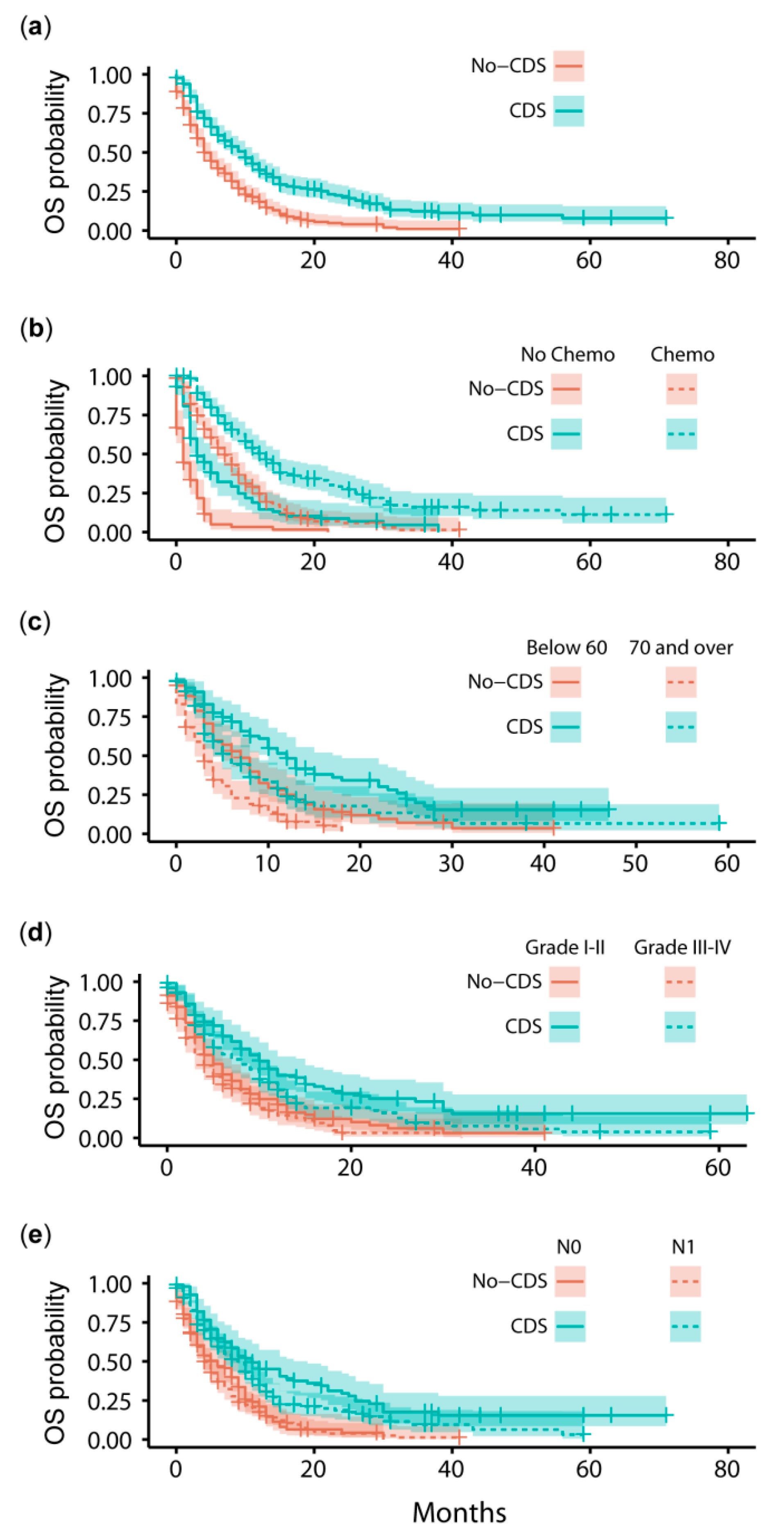
3.2. Survival Outcomes after Propensity Score-Matching
4. Discussion
4.1. Comparison to Previous SEER Analyses
4.2. Limitations of SEER Analyses
4.3. The Need for Evidence-Backed Guidance When Selecting Patients for CDS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci. 2017, 18, 1338. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.B.F. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.fr/today (accessed on 6 January 2020).
- Seufferlein, T.; Bachet, J.B.; Van Cutsem, E.; Rougier, P. Pancreatic adenocarcinoma: ESMO-ESDO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23, vii33–vii40. [Google Scholar] [CrossRef] [PubMed]
- Sohal, D.P.S.; Mangu, P.B.; Khorana, A.A.; Shah, M.A.; Philip, P.A.; O’Reilly, E.M.; Uronis, H.E.; Ramanathan, R.K.; Crane, C.H.; Engebretson, A.; et al. Metastatic pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2016, 34, 2784–2796. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, V.; Boeck, S. Perioperative management of pancreatic cancer. Ann. Oncol. 2008, 19, 273–278. [Google Scholar] [CrossRef]
- Paulson, A.S.; Tran Cao, H.S.; Tempero, M.A.; Lowy, A.M. Therapeutic advances in pancreatic cancer. Gastroenterology 2013, 144, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Stocken, D.D.; Friess, H.; Bassi, C.; Dunn, J.A.; Hickey, H.; Beger, H.; Fernandez-Cruz, L.; Dervenis, C.; Lacaine, F.; et al. A Randomized Trial of Chemoradiotherapy and Chemotherapy after Resection of Pancreatic Cancer. N. Engl. J. Med. 2004, 350, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Bittoni, A.; Sebastiani, E.; Partelli, S.; Zanon, S.; Lanese, A.; Andrikou, K.; Muffatti, F.; Balzano, G.; Reni, M.; et al. Is there a role for surgical resection in patients with pancreatic cancer with liver metastases responding to chemotherapy? Eur. J. Surg. Oncol. 2016, 42, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, I.; Regi, P.; Giardino, A.; Scopelliti, F.; Girelli, R.; Bassi, C.; Gobbo, S.; Martini, P.T.; Capelli, P.; D’Onofrio, M.; et al. Downstaging in Stage IV Pancreatic Cancer: A New Population Eligible for Surgery? Ann. Surg. Oncol. 2017, 24, 2397–2403. [Google Scholar] [CrossRef] [PubMed]
- Shrikhande, S.V.; Kleeff, J.; Reiser, C.; Weitz, J.; Hinz, U.; Esposito, I.; Schmidt, J.; Friess, H.; Büchler, M.W. Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 2007, 14, 118–127. [Google Scholar] [CrossRef]
- Buc, E.; Orry, D.; Antomarchi, O.; Gagnière, J.; Da Ines, D.; Pezet, D. Resection of pancreatic ductal adenocarcinoma with synchronous distant metastasis: Is it worthwhile? World J. Surg. Oncol. 2014, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, E.; Margonis, G.A.; Sasaki, K.; Andreatos, N.; Polychronidis, G.; Pawlik, T.M.; Pikoulis, E. Is resection of pancreatic adenocarcinoma with synchronous hepatic metastasis justified? A review of current literature. ANZ J. Surg. 2016, 86, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Tachezy, M.; Gebauer, F.; Janot, M.; Uhl, W.; Zerbi, A.; Montorsi, M.; Perinel, J.; Adham, M.; Dervenis, C.; Agalianos, C.; et al. Synchronous resections of hepatic oligometastatic pancreatic cancer: Disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery 2016, 160, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Hackert, T.; Niesen, W.; Hinz, U.; Tjaden, C.; Strobel, O.; Ulrich, A.; Michalski, C.W.; Büchler, M.W. Radical surgery of oligometastatic pancreatic cancer. Eur. J. Surg. Oncol. 2017, 43, 358–363. [Google Scholar] [CrossRef]
- Tao, L.; Yuan, C.; Ma, Z.; Jiang, B.; Xiu, D. Surgical resection of a primary tumor improves survival of metastatic pancreatic cancer: A population-based study. Cancer Manag. Res. 2017, 9, 471–479. [Google Scholar] [CrossRef]
- Oweira, H.; Petrausch, U.; Helbling, D.; Schmidt, J.; Mannhart, M.; Mehrabi, A.; Schöb, O.; Giryes, A.; Decker, M.; Abdel-Rahman, O. Prognostic value of site-specific metastases in pancreatic adenocarcinoma: A Surveillance Epidemiology and End Results database analysis. World J. Gastroenterol. 2017, 23, 1872–1880. [Google Scholar] [CrossRef]
- Gebauer, F.; Damanakis, A.; Quaas, A.; Kütting, F.; Göser, T.; Popp, F.; Waldschmidt, D.; Bruns, C. Non-randomised, open phase II trial, investigating liposomal irinotecan and 5-fluorouraci followed by surgical resection in patients with hepatic oligometastatic pancreatic cancer (HOLIPANC). Eur. J. Surg. Oncol. 2020, 46, e6. [Google Scholar] [CrossRef]
- Ghadmimi, M.; Pelzer, U.; Siveke, J.T. METAPANC (AIO-PAK-0219). Available online: https://www.aio-portal.de/index.php/informationen-194.html (accessed on 29 September 2021).
- Andreou, A.; Knitter, S.; Klein, F.; Malinka, T.; Schmelzle, M.; Struecker, B.; Schmuck, R.B.; Noltsch, A.R.; Lee, D.; Pelzer, U.; et al. The role of hepatectomy for synchronous liver metastases from pancreatic adenocarcinoma. Surg. Oncol. 2018, 27, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K.A.; Ries, L.A.G.; Edwards, B.K. Preface. Cancer 2014, 120, 3755–3757. [Google Scholar] [CrossRef] [PubMed]
- SEER*Stat Database: Incidence-SEER Research Data; 9 Registries, Nov 2017 Sub (1973–2015); National Cancer Institute: Bethesda, MA, USA, 2018; SEER 9.
- Fritz, A.; Percy, C.; Jack, A.; Shanmugaratnam, K.; Sobin, L.; Parkin, D.; Whelan, S. (Eds.) International Classification of Diseases for Oncology, 3rd ed.; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Shi, S.; Hua, J.; Liang, C.; Meng, Q.; Liang, D.; Xu, J.; Ni, Q.; Yu, X. Proposed Modification of the 8th Edition of the AJCC Staging System for Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2019, 269, 944–950. [Google Scholar] [CrossRef]
- Ansari, D.; Bauden, M.; Bergström, S.; Rylance, R.; Marko-Varga, G.; Andersson, R. Relationship between tumour size and outcome in pancreatic ductal adenocarcinoma. Br. J. Surg. 2017, 104, 600–607. [Google Scholar] [CrossRef]
- Voss, N.; Izbicki, J.R.; Nentwich, M.F. Oligometastases in pancreatic cancer (Synchronous resections of hepatic oligometastatic pancreatic cancer: Disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis). Ann. Gastroenterol. Surg. 2019, 3, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Damanakis, A.I.; Ostertag, L.; Waldschmidt, D.; Kütting, F.; Quaas, A.; Plum, P.; Bruns, C.J.; Gebauer, F.; Popp, F. Proposal for a definition of “Oligometastatic disease in pancreatic cancer”. BMC Cancer 2019, 19, 1261. [Google Scholar] [CrossRef]
- Adamo, A.M.; Dickie, L.; Ruhl, J. SEER Program Coding and Staging Manual 2015; National Cancer Institute: Bethesda, MD, USA, 2015; pp. 20850–29765.
- Edge, S.; Byrd, D.; Compton, C.; Fritz, A.; Greene, F.; Trotti, A. (Eds.) AJCC Cancer Staging Handbook, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Austin, P. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am. J. Epidemiol. 2010, 172, 1092–1097. [Google Scholar] [CrossRef]
- Ekman, A. Variable Selection for the Cox Proportional Hazards Model: A Simulation Study Comparing the Stepwise, Lasso and Bootstrap Approach; Umeå University: Umeå, Sweden, 2017. [Google Scholar]
- Burnham, K.P.; Anderson, R.P. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Symonds, M.R.E.; Moussalli, A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav. Ecol. Sociobiol. 2010, 65, 13–21. [Google Scholar] [CrossRef]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.J.M.; van Dam, R.M.; et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020, 38, 1763–1773. [Google Scholar] [CrossRef]
- Klaiber, U.; Schnaidt, E.S.; Hinz, U.; Gaida, M.M.; Heger, U.; Hank, T.; Strobel, O.; Neoptolemos, J.P.; Mihaljevic, A.L.; Büchler, M.W.; et al. Prognostic Factors of Survival After Neoadjuvant Treatment and Resection for Initially Unresectable Pancreatic Cancer. Ann. Surg. 2021, 273, 154–162. [Google Scholar] [CrossRef]
- Strobel, O.; Neoptolemos, J.; Jäger, D.; Büchler, M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Shi, S.; Hua, J.; Xu, J.; Yu, X. Simultaneous resection of the primary tumour and liver metastases after conversion chemotherapy versus standard therapy in pancreatic cancer with liver oligometastasis: Protocol of a multicentre, prospective, randomised phase III control trial (CSPAC-1). BMJ Open 2019, 9, e033452. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Uesaka, K.; Kanemoto, H.; Mizuno, T.; Sasaki, K.; Furukawa, H.; Matsunaga, K.; Maeda, A. Serum CA19-9 is a significant predictor among preoperative parameters for early recurrence after resection of pancreatic adenocarcinoma. J. Gastrointest. Surg. 2012, 16, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Halloran, C.M.; Ghaneh, P.; Connor, S.; Sutton, R.; Neoptolemos, J.P.; Raraty, M.G.T. Carbohydrate antigen 19.9 accurately selects patients for laparoscopic assessment to determine resectability of pancreatic malignancy. Br. J. Surg. 2008, 95, 453–459. [Google Scholar] [CrossRef]
- Hartwig, W.; Strobel, O.; Hinz, U.; Fritz, S.; Hackert, T.; Roth, C.; Büchler, M.W.; Werner, J. CA 19-9 in potentially resectable pancreatic cancer: Perspective to adjust surgical and perioperative therapy. Ann. Surg. Oncol. 2013, 20, 2188–2196. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Luo, G.; Liu, C.; Guo, M.; Cheng, H.; Lu, Y.; Jin, K.; Liu, L.; Long, J.; Xu, J.; Lu, R.; et al. Potential biomarkers in lewis negative patients with pancreatic cancer. Ann. Surg. 2017, 265, 800–805. [Google Scholar] [CrossRef]
- Groot, V.P.; Rezaee, N.; Wu, W.; Cameron, J.L.; Fishman, E.K.; Hruban, R.H.; Weiss, M.J.; Zheng, L.; Wolfgang, C.L.; He, J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2018, 267, 936–945. [Google Scholar] [CrossRef]
- Gaitanidis, A.; Alevizakos, M.; Tsalikidis, C.; Tsaroucha, A.; Simopoulos, C.; Pitiakoudis, M. Refusal of Cancer-Directed Surgery by Breast Cancer Patients: Risk Factors and Survival Outcomes. Clin. Breast Cancer 2018, 18, e469–e476. [Google Scholar] [CrossRef] [PubMed]
- Gray, B.N. Colorectal Cancer: The Natural History of Disseminated Disease—A Review. Aust. N. Z. J. Surg. 1980, 50, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Hackl, C.; Neumann, P.; Gerken, M.; Loss, M.; Klinkhammer-Schalke, M.; Schlitt, H.J. Treatment of colorectal liver metastases in Germany: A ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer 2014, 14, 810. [Google Scholar] [CrossRef] [PubMed]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef]
- Bagante, F.; Spolverato, G.; Merath, K.; Postlewait, L.M.L.; Poultsides, G.A.; Mullen, M.G.; Bauer, T.W.; Fields, R.C.; Lamelas, J.; Marques, H.P.; et al. Neuroendocrine liver metastasis: The chance to be cured after liver surgery. J. Surg. Oncol. 2017, 115, 687–695. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Choti, M.A. Surgical therapy for colorectal metastases to the liver. J. Gastrointest. Surg. 2007, 11, 1057–1077. [Google Scholar] [CrossRef] [PubMed]
- Akgül, Ö.; Çetinkaya, E.; Ersöz, Ş.; Tez, M. Role of surgery in colorectal cancer liver metastases. World J. Gastroenterol. 2014, 20, 6113–6122. [Google Scholar] [CrossRef]
- Meyers, M.O.; Sasson, A.R.; Sigurdson, E.R. Locoregional strategies for colorectal hepatic metastases. Clin. Colorectal Cancer 2003, 3, 34–44. [Google Scholar] [CrossRef]
- Farley, H.A.; Pommier, R.F. Treatment of Neuroendocrine Liver Metastases. Surg. Oncol. Clin. N. Am. 2016, 25, 217–225. [Google Scholar] [CrossRef]
- Modlin, I.M.; Lye, K.D.; Kidd, M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003, 97, 934–959. [Google Scholar] [CrossRef]
- Jin, K.; Xu, J.; Chen, J.; Chen, M.; Chen, R.; Chen, Y.; Chen, Z.; Cheng, B.; Chi, Y.; Feng, S.T.; et al. Surgical management for non-functional pancreatic neuroendocrine neoplasms with synchronous liver metastasis: A consensus from the Chinese Study Group for Neuroendocrine Tumors (CSNET). Int. J. Oncol. 2016, 49, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, I.; Bencini, L.; Risaliti, M.; Ringressi, M.N.; Moraldi, L.; Taddei, A. Current management of pancreatic neuroendocrine tumors: From demolitive surgery to observation. Gastroenterol. Res. Pract. 2018, 9647247. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Bentrem, D.J.; Ko, C.Y.; Stewart, A.K.; Winchester, D.P.; Talamonti, M.S. National failure to operate on early stage pancreatic cancer. Ann. Surg. 2007, 246, 173–180. [Google Scholar] [CrossRef]
- McDowell, B.D.; Chapman, C.G.; Smith, B.J.; Button, A.M.; Chrischilles, E.A.; Mezhir, J.J. Pancreatectomy predicts improved survival for pancreatic adenocarcinoma: Results of an instrumental variable analysis. Ann. Surg. 2015, 261, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cheng, C.S.; Chen, L.; Chen, Z. Benefit from the inclusion of surgery in the treatment of patients with stage III pancreatic cancer: A propensity-adjusted, population-based SEER analysis. Cancer Manag. Res. 2018, 10, 1907–1918. [Google Scholar] [CrossRef]
- Hackert, T.; Klaiber, U.; Pausch, T.; Mihaljevic, A.L.; Büchler, M.W. Fifty Years of Surgery for Pancreatic Cancer. Pancreas 2020, 49, 1005–1013. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Timmer, F.E.F.; Geboers, B.; Nieuwenhuizen, S.; Schouten, E.A.C.; Dijkstra, M.; de Vries, J.J.J.; van den Tol, M.P.; Meijerink, M.R.; Scheffer, H.J. Locoregional treatment of metastatic pancreatic cancer utilizing resection, ablation and embolization: A systematic review. Cancers 2021, 13, 1608. [Google Scholar] [CrossRef]


| Factor | Pre-PSM | CDS (n = 259) | Post-PSM | ||
|---|---|---|---|---|---|
| No-CDS | Comparison | Comparison | No-CDS | ||
| (n = 11,759) | (n = 259) | ||||
| Age (mean ± SD) | 67.4 ± 11.2 | t12,016 = 3.71, p < 0.001 | 64.8 ± 10.5 | t516 = 0.23, p = 0.815 | 64.5 ± 10.9 |
| Sex (n, %) | χ21 = 0.38, p = 0.539 | χ21 = 0.63, p = 0.427 | |||
| Female | 5313 (45.2) | 122 (47.1) | 113 (43.6) | ||
| Male | 6446 (54.8) | 137 (52.9) | 146 (56.4) | ||
| Ethnicity (n, %) | χ23 = 1.73, p = 0.631 | χ23 = 0.59, p = 0.745 | |||
| White | 9336 (79.4) | 209 (80.7) | 210 (81.1) | ||
| Black | 1591(13.5) | 30 (11.6) | 33 (12.7) | ||
| Other | 802 (6.8) | 20 (7.7) | 16 (6.2) | ||
| Unknown | 30 (0.3) | 0 (0) | 0 (0) | ||
| Marital status (n, %) | χ24 = 11.88, p = 0.018 | χ24 = 1.20, p = 0.879 | |||
| Single | 1689 (14.4) | 31 (12.0) | 34 (13.1) | ||
| Married | 6570 (55.9) | 170 (65.6) | 164 (63.3) | ||
| Divorced | 1227 (10.4) | 22 (8.5) | 25 (9.7) | ||
| Widowed | 1628 (13.8) | 30 (11.6) | 27 (10.4) | ||
| Others/unknown | 645 (5.3) | 6 (2.3) | 9 (3.5) | ||
| Insurance (n, %) | χ23 = 6.16, p = 0.104 | χ23 = 0.52, p = 0.915 | |||
| Any Medicaid | 1448 (12.3) | 20 (7.7) | 16 (6.2) | ||
| Insured | 7895 (67.1) | 189 (73.0) | 191 (73.7) | ||
| Insured/no specifics | 1900 (16.2) | 41 (15.8) | 42 (16.2) | ||
| Uninsured/unknown | 516 (4.4) | 9 (3.5) | 10 (3.9) | ||
| Tumor location (n, %) | χ22 = 68.43, p < 0.001 | χ22 = 1.41, p = 0.493 | |||
| Pancreatic head | 4445 (37.8) | 159 (63.4) | 172 (66.4) | ||
| Pancreatic body/tail | 4203 (35.7) | 76 (29.3) | 66 (25.5) | ||
| Pancreas other | 3111 (26.5) | 24 (9.3) | 21 (8.1) | ||
| Grade (n, %) | χ23 = 564.93, p < 0.001 | χ23 = 1.88, p = 0.757 | |||
| I | 122 (1.0) | 11 (4.3) | 13 (5.0) | ||
| II | 861 (7.3) | 88 (34.0) | 99 (38.2) | ||
| III | 1243 (10.6) | 104 (40.2) | 92 (35.5) | ||
| IV | 43 (0.4) | 2 (0.8) | 1 (0.4) | ||
| Unknown | 9490 (80.7) | 54 (20.9) | 54 (19.3) | ||
| T stage (n, %) | Χ25 = 218.81, p < 0.001 | χ25 = 2.98, p = 0.703 | |||
| T0 | 121 (1.0) | 0 (0) | 2 (0.8) | ||
| T1 | 304 (2.6) | 9 (3.5) | 10 (3.9) | ||
| T2 | 3339 (28.4) | 38 (14.7) | 41 (15.8) | ||
| T3 | 3100 (26.4) | 173 (66.8) | 166 (64.1) | ||
| T4 | 1973 (16.8) | 24 (9.3) | 21 (8.1) | ||
| Tx/NA 1 | 2921 (24.8) | 15 (5.8) | 19 (7.3) | ||
| N stage (n, %) | χ22 = 123.10, p < 0.001 | χ22 = 0.18, p = 0.915 | |||
| N0 | 6400 (54.4) | 96 (37.1) | 100 (38.6) | ||
| N1 | 3417 (29.1) | 155 (59.8) | 152 (58.7) | ||
| Nx/NA 1 | 1942 (16.5) | 8 (3.1) | 7 (2.7) | ||
| Chemotherapy (n, %) | χ21 = 12.93, p < 0.001 | χ21 = 0.44, p = 0.508 | |||
| No/unknown | 5177 (44.0) | 85 (32.8) | 78 (30.1) | ||
| Yes | 6582 (56.0) | 174 (67.2) | 181 (69.9) | ||
| chm | cds | age | grd | n | eth | ins | loc | t | mrg | sex | K | LL | AICc | ΔAIC | AICcW | ∑Wt |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | −1954.45 | 3925.29 | 0.00 | 0.42 | 0.42 | |||||||||||
| 6 | −1957.04 | 3926.30 | 1.01 | 0.25 | 0.67 | |||||||||||
| 10 | −1952.88 | 3926.36 | 1.07 | 0.24 | 0.91 | |||||||||||
| 13 | −1951.29 | 3929.59 | 4.30 | 0.05 | 0.96 | |||||||||||
| 15 | −1949.86 | 3931.06 | 5.77 | 0.02 | 0.98 | |||||||||||
| 4 | −1962.01 | 3932.13 | 6.84 | 0.01 | 1.00 | |||||||||||
| 18 | −1948.76 | 3935.43 | 10.14 | 0.00 | 1.00 | |||||||||||
| 2 | −1967.80 | 3939.64 | 14.35 | 0.00 | 1.00 | |||||||||||
| 22 | −1947.78 | 3942.44 | 17.14 | 0.00 | 1.00 | |||||||||||
| 23 | −1947.73 | 3944.60 | 19.31 | 0.00 | 1.00 | |||||||||||
| 1 | −1990.57 | 3983.14 | 57.85 | 0.00 | 1.00 | |||||||||||
| 0 | −2015.38 | 4030.76 | 105.47 | 0.00 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pausch, T.M.; Liu, X.; Cui, J.; Wei, J.; Miao, Y.; Heger, U.; Probst, P.; Heap, S.; Hackert, T. Survival Benefit of Resection Surgery for Pancreatic Ductal Adenocarcinoma with Liver Metastases: A Propensity Score-Matched SEER Database Analysis. Cancers 2022, 14, 57. https://doi.org/10.3390/cancers14010057
Pausch TM, Liu X, Cui J, Wei J, Miao Y, Heger U, Probst P, Heap S, Hackert T. Survival Benefit of Resection Surgery for Pancreatic Ductal Adenocarcinoma with Liver Metastases: A Propensity Score-Matched SEER Database Analysis. Cancers. 2022; 14(1):57. https://doi.org/10.3390/cancers14010057
Chicago/Turabian StylePausch, Thomas M., Xinchun Liu, Jiaqu Cui, Jishu Wei, Yi Miao, Ulrike Heger, Pascal Probst, Stephen Heap, and Thilo Hackert. 2022. "Survival Benefit of Resection Surgery for Pancreatic Ductal Adenocarcinoma with Liver Metastases: A Propensity Score-Matched SEER Database Analysis" Cancers 14, no. 1: 57. https://doi.org/10.3390/cancers14010057
APA StylePausch, T. M., Liu, X., Cui, J., Wei, J., Miao, Y., Heger, U., Probst, P., Heap, S., & Hackert, T. (2022). Survival Benefit of Resection Surgery for Pancreatic Ductal Adenocarcinoma with Liver Metastases: A Propensity Score-Matched SEER Database Analysis. Cancers, 14(1), 57. https://doi.org/10.3390/cancers14010057









