The BRCA Gene in Epithelial Ovarian Cancer
Abstract
:Simple Summary
Abstract
1. BRCA Gene as Therapeutic Target
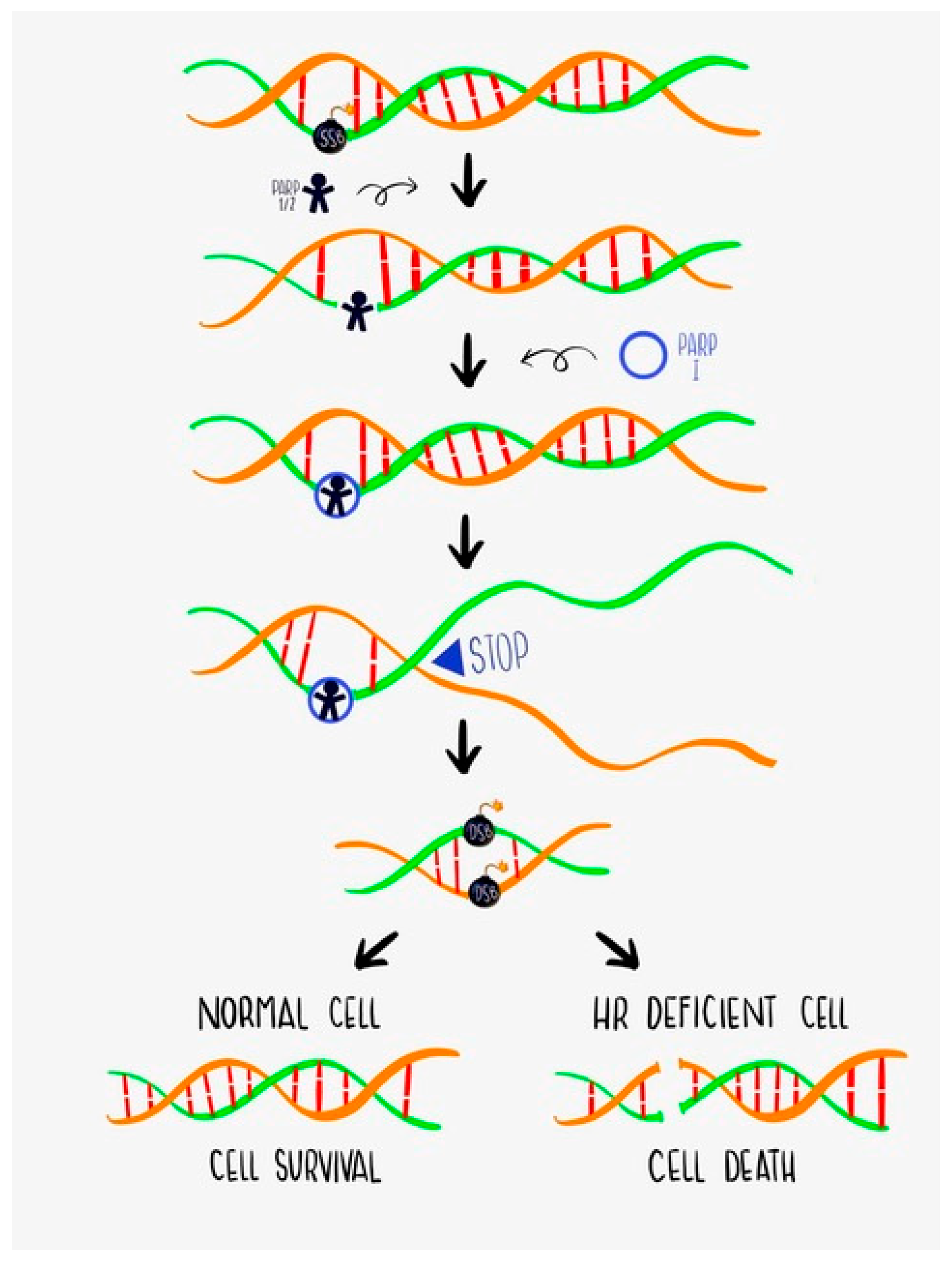
1.1. DNA Damage Repair (DDR) and BRCA Function
1.2. Challenges of BRCA Testing: Germline vs. Somatic vs. Both
2. BRCA Gene and Hereditary Ovarian Cancer Syndrome
3. BRCA Gene in the Clinic
3.1. Prognostic Implication
3.2. Predictive Factor of PARPi Sensitivity
4. Clinical Data with PARPi in BRCA-Mutated OC Patients
4.1. Maintenance Therapy
4.2. PARPi Single Agent as Treatment
4.3. Challenges and Future Approaches
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haunschild, C.E.; Tewari, K.S. The current landscape of molecular profiling in the treatment of epithelial ovarian cancer. Gynecol. Oncol. 2021, 160, 333–345. [Google Scholar] [CrossRef]
- Gudmundsdottir, K.; Ashworth, A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene 2006, 25, 5864–5874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagenesis 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turk, A.A.; Wisinski, K.B. PARP inhibitors in breast cancer: Bringing synthetic lethality to the bedside. Cancer 2018, 124, 2498–2506. [Google Scholar] [CrossRef]
- Byrum, A.; Vindigni, A.; Mosammaparast, N. Defining and Modulating ‘BRCAness’. Trends Cell Biol. 2019, 29, 740–751. [Google Scholar] [CrossRef]
- Palacios, J.; De La Hoya, M.; Bellosillo, B.; De Juan, I.; Matías-Guiu, X.; Lázaro, C.; Palanca, S.; Osorio, A.; Rojo, F.; Rosa-Rosa, J.; et al. Mutational Screening of BRCA1/2 Genes as a Predictive Factor for Therapeutic Response in Epithelial Ovarian Cancer: A Consensus Guide from the Spanish Society of Pathology (SEAP-IAP) and the Spanish Society of Human Genetics (AEGH). Virchows Arch. 2020, 476, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eccles, D.M.; Mitchell, G.; Monteiro, A.; Schmutzler, R.; Couch, F.J.; Spurdle, A.B.; Gómez-García, E.B.; Driessen, R.; Lindor, N.; Blok, M.; et al. BRCA1 and BRCA2 genetic testing—pitfalls and recommendations for managing variants of uncertain clinical significance. Ann. Oncol. 2015, 26, 2057–2065. [Google Scholar] [CrossRef]
- Hinchcliff, E.M.; Bednar, E.; Lu, K.H.; Rauh-Hain, J.A. Disparities in gynecologic cancer genetics evaluation. Gynecol. Oncol. 2019, 153, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Kanchi, K.L.; Johnson, K.J.; Lu, C.; McLellan, M.D.; Leiserson, M.D.M.; Wendl, M.C.; Zhang, Q.; Koboldt, D.C.; Xie, M.; Kandoth, C.; et al. Integrated analysis of germline and somatic variants in ovarian cancer. Nat. Commun. 2014, 5, 3156. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bae, E.; Zhang, L.; Hughes, K.; Parmigiani, G.; Braun, D.; Rebbeck, T.R. Penetrance of Breast and Ovarian Cancer in Women Who Carry a BRCA1/2 Mutation and Do Not Use Risk-Reducing Salpingo-Oophorectomy: An Updated Meta-Analysis. JNCI Cancer Spectr. 2020, 4, pkaa029. [Google Scholar] [CrossRef]
- LevyLahad, E.; Friedman, E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br. J. Cancer 2007, 96, 11–15. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Mitra, N.; Wan, F.; Sinilnikova, O.M.; Healey, S.; McGuffog, L.; Mazoyer, S.; Chenevix-Trench, G.; Easton, D.F.; Antoniou, A.C.; et al. Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA 2015, 313, 1347–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLeonardis, K.; Hogan, L.; Cannistra, S.A.; Rangachari, D.; Tung, N. When Should Tumor Genomic Profiling Prompt Consideration of Germline Testing? J. Oncol. Pr. 2019, 15, 465–473. [Google Scholar] [CrossRef] [PubMed]
- González-Santiago, S.; the SEOM Hereditary Cancer Working Group; Cajal, T.R.Y.; Aguirre, E.; Alés-Martínez, J.E.; Andrés, R.; Balmaña, J.; Graña, B.; Herrero, A.; Llort, G.; et al. SEOM clinical guidelines in hereditary breast and ovarian cancer (2019). Clin. Transl. Oncol. 2020, 22, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Mavaddat, N.; Barrowdale, D.; Andrulis, I.L.; Domchek, S.M.; Eccles, D.; Nevanlinna, H.; Ramus, S.J.; Spurdle, A.; Robson, M.; Sherman, M.; et al. Consortium of Investigators of Modifiers of BRCA1/2. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: Results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol. Prev. Biomark. 2012, 21, 134–147. [Google Scholar] [CrossRef] [Green Version]
- Shaw, P.A.; McLaughlin, J.R.; Zweemer, R.P.; Narod, S.A.; Risch, H.; Verheijen, R.H.; Ryan, A.; Menko, F.H.; Kenemans, P.; Jacobs, I.J. Histopathologic features of genetically determined ovarian cancer. Int. J. Gynecol. Pathol. 2002, 21, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Giusti, I.; Bianchi, S.; Nottola, S.A.; Macchiarelli, G.; Dolo, V. Clinical Electron Microscopy in the Study of Human Ovarian Tissues. EuroMediterranean Biomed. J. 2019, 14, 145–151. [Google Scholar]
- Schrader, K.; Hurlburt, J.; Kalloger, S.E.; Hansford, S.; Young, S.; Huntsman, D.G.; Gilks, C.B.; McAlpine, J.N. Germline BRCA1 and BRCA2 Mutations in Ovarian Cancer. Obstet. Gynecol. 2012, 120, 235–240. [Google Scholar] [CrossRef]
- King, M.-C.; Marks, J.H.; Mandell, J.B. Breast and Ovarian Cancer Risks Due to Inherited Mutations in BRCA1 and BRCA2. Science 2003, 302, 643–646. [Google Scholar] [CrossRef]
- Finch, A.P.; Lubinski, J.; Møller, P.; Singer, C.F.; Karlan, B.; Senter, L.; Rosen, B.; Maehle, L.; Ghadirian, P.; Cybulski, C.; et al. Impact of Oophorectomy on Cancer Incidence and Mortality in Women With a BRCA1 or BRCA2 Mutation. J. Clin. Oncol. 2014, 32, 1547–1553. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.-L.; Wang, K.; Liu, Q.; Li, J.; Zhang, X.; Li, H.-Y. Risk Reduction and Survival Benefit of Risk-Reducing Salpingo-oophorectomy in Hereditary Breast Cancer: Meta-analysis and Systematic Review. Clin. Breast Cancer 2019, 19, e48–e65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eleje, G.U.; Eke, A.C.; Ezebialu, I.U.; Ikechebelu, J.I.; Ugwu, E.O.; Okonkwo, O.O. Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations. Cochrane Database Syst. Rev. 2018, 8, CD012464. [Google Scholar] [CrossRef] [PubMed]
- Matanes, E.; Volodarsky-Perel, A.; Eisenberg, N.; Rottenstreich, M.; Yasmeen, A.; Mitric, C.; Lau, S.; Salvador, S.; Gotlieb, W.H.; Kogan, L. Endometrial Cancer in Germline BRCA Mutation Carriers: A Systematic Review and Meta-analysis. J. Minim. Invasive Gynecol. 2020, 28, 947–956. [Google Scholar] [CrossRef]
- Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet. Gynecol. 2017, 130, e110–e126. [CrossRef]
- Colombo, N.; Sessa, C.; Du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO–ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int. J. Gynecol. Cancer 2019, 29, 728–760. [Google Scholar] [CrossRef] [Green Version]
- Paluch-Shimon, S.; Cardoso, F.; Sessa, C.; Balmaña, J.; Cardoso, M.J.; Gilbert, F.; Senkus, E. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann. Oncol. 2017, 27, v103–v110. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.S.; Rothermundt, C.; Thomas, K.; Bancroft, E.; Eeles, R.; Shanley, S.; Ardern-Jones, A.; Norman, A.; Kaye, S.B.; Gore, M.E. “BRCAness” Syndrome in Ovarian Cancer: A Case-Control Study Describing the Clinical Features and Outcome of Patients With Epithelial Ovarian Cancer Associated WithBRCA1andBRCA2Mutations. J. Clin. Oncol. 2008, 26, 5530–5536. [Google Scholar] [CrossRef] [PubMed]
- Alsop, K.; Fereday, S.; Meldrum, C.; DeFazio, A.; Emmanuel, C.; George, J.; Dobrovic, A.; Birrer, M.J.; Webb, P.M.; Stewart, C.; et al. BRCA Mutation Frequency and Patterns of Treatment Response in BRCA Mutation–Positive Women With Ovarian Cancer: A Report From the Australian Ovarian Cancer Study Group. J. Clin. Oncol. 2012, 30, 2654–2663. [Google Scholar] [CrossRef] [Green Version]
- Ledermann, J.A.; Gabra, H.; Jayson, G.; Spanswick, V.J.; Rustin, G.J.; Jitlal, M.; James, L.E.; Hartley, J.A. Inhibition of Carboplatin-Induced DNA Interstrand Cross-link Repair by Gemcitabine in Patients Receiving these Drugs for Platinum-Resistant Ovarian Cancer. Clin. Cancer Res. 2010, 16, 4899–4905. [Google Scholar] [CrossRef] [Green Version]
- Artioli, G.; Giannone, G.; Valabrega, G.; Maggiorotto, F.; Genta, S.; Pignata, S.; Lorusso, D.; Cormio, G.; Scalone, S.; Nicoletto, M.O.; et al. Characteristics and outcome of BRCA mutated epithelial ovarian cancer patients in Italy: A retrospective multicenter study (MITO 21). Gynecol. Oncol. 2021, 161, 755–761. [Google Scholar] [CrossRef]
- Zhong, Q.; Peng, H.-L.; Zhao, X.; Zhang, L.; Hwang, W.-T. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: A meta-analysis. Clin. Cancer Res. 2015, 21, 211–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vencken, P.M.L.H.; Kriege, M.; Hoogwerf, D.; Beugelink, S.; van der Burg, M.E.L.; Hooning, M.J.; Berns, E.M.; Jager, A.; Collée, M.; Burger, C.W.; et al. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann. Oncol. 2011, 22, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, D.; Sun, Y.; Shmulevich, I.; Xue, F.; Sood, A.K.; Zhang, W. Differing clinical impact of BRCA1 and BRCA2 mutations in serous ovarian cancer. Pharmacogenomics 2012, 13, 1523–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyman, D.M.; Zhou, Q.; Iasonos, A.; Grisham, R.N.; Arnold, A.G.; Phillips, M.F.; Bhatia, J.; Levine, D.A.; Aghajanian, C.; Offit, K.; et al. Improved survival forBRCA2-associated serous ovarian cancer compared with bothBRCA-negative andBRCA1-associated serous ovarian cancer. Cancer 2012, 118, 3703–3709. [Google Scholar] [CrossRef] [Green Version]
- Bolton, K.L.; Chenevix-Trench, G.; Goh, C.; Sadetzki, S.; Ramus, S.J.; Karlan, B.Y.; Lambrechts, D.; Despierre, E.; Barrowdale, D.; McGuffog, L.; et al. Association Between BRCA1 and BRCA2 Mutations and Survival in Women With Invasive Epithelial Ovarian Cancer. JAMA 2012, 307, 382–390. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.K.; Matulonis, U.A. PARP Inhibitor Resistance Mechanisms and Implications for Post-Progression Combination Therapies. Cancers 2020, 12, 2054. [Google Scholar] [CrossRef]
- Li, S.; Tao, L.; Dai, H.; Gong, X.; Zhuo, Y.; Xiang, H.; Zhao, Y.; Gao, Q.; Deng, L. BRCA1 Versus BRCA2 and PARP Inhibitors Efficacy in Solid Tumors:A Meta-Analysis of Randomized Controlled Trials. Front. Oncol. 2021, 11, 718871. [Google Scholar] [CrossRef]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib Maintenance Therapy in Platinum-Sensitive Relapsed Ovarian Cancer. N. Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef] [Green Version]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.L.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2015, 15, 852–861. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef] [Green Version]
- Poveda, A.; Floquet, A.; Ledermann, J.A.; Asher, R.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Pignata, S.; Friedlander, M.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 620–631. [Google Scholar] [CrossRef]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhu, J.; Yin, R.; Yang, J.; Liu, J.; Wang, J.; Wu, L.; Liu, Z.; Gao, Y.; Wang, D.; et al. Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA): A randomized, double-blind, placebo-controlled phase III trial. Ann. Oncol. 2021, 32, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 390, 1949–1961, Erratum in Lancet 2017, 390, 1948. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef] [Green Version]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Coleman, R.L.; Fleming, G.F.; Brady, M.F.; Swisher, E.M.; Steffensen, K.D.; Friedlander, M.; Okamoto, A.; Moore, K.N.; Efrat Ben-Baruch, N.; Werner, T.L.; et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2403–2415. [Google Scholar] [CrossRef]
- Miller, R.; Leary, A.; Scott, C.; Serra, V.; Lord, C.; Bowtell, D.; Chang, D.; Garsed, D.; Jonkers, J.; Ledermann, J.; et al. ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann. Oncol. 2020, 31, 1606–1622. [Google Scholar] [CrossRef]
- Banerjee, S.; Moore, K.N.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1721–1731. [Google Scholar] [CrossRef]
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmaña, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; Hubert, A.; et al. Olaparib Monotherapy in Patients With Advanced Cancer and a Germline BRCA1/2 Mutation. J. Clin. Oncol. 2015, 33, 244–250. [Google Scholar] [CrossRef]
- Penson, R.T.; Valencia, R.V.; Cibula, D.; Colombo, N.; Leath, C.A., III; Bidziński, M.; Kim, J.-W.; Nam, J.H.; Madry, R.; Hernández, C.; et al. Olaparib Versus Nonplatinum Chemotherapy in Patients With Platinum-Sensitive Relapsed Ovarian Cancer and a Germline BRCA1/2 Mutation (SOLO3): A Randomized Phase III Trial. J. Clin. Oncol. 2020, 38, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Oza, A.M.; Tinker, A.V.; Oaknin, A.; Shapira-Frommer, R.; McNeish, I.; Swisher, E.M.; Ray-Coquard, I.; Bell-McGuinn, K.; Coleman, R.L.; O’Malley, D.M.; et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: Integrated analysis of data from Study 10 and ARIEL2. Gynecol. Oncol. 2017, 147, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristeleit, R.; Lisyanskaya, A.; Fedenko, A.; Dvorkin, M.; de Melo, A.C.; Shparyk, Y.; Rakhmatullina, I.; Bondarenko, I.; Colombo, N.; Svintsitskiy, V.; et al. Rucaparib versus chemotherapy in patients with advanced, relapsed ovarian cancer and a deleterious BRCA mutation: Efficacy and safety from ARIEL4, a randomized phase 3 study. In Proceedings of the Society of Gynecologic Oncology 2021 Virtual Annual Meeting on Women’s Cancer, Virtual, 19–25 March 2021; p. 11479. [Google Scholar]
- Moore, K.N.; Secord, A.A.; Geller, M.A.; Miller, D.S.; Cloven, N.; Fleming, G.F.; Wahner Hendrickson, A.E.; Azodi, M.; DiSilvestro, P.; Oza, A.M.; et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 636–648. [Google Scholar] [CrossRef]
- Kim, D.-S.; Camacho, C.V.; Kraus, W.L. Alternate therapeutic pathways for PARP inhibitors and potential mechanisms of resistance. Exp. Mol. Med. 2021, 53, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Lauraine, E.; Selle, F.; Scambia, G.; Asselain, B.; Marmé, F.; Lindemann, K.; Colombo, N.; Madry, R.; Glasspool, R.; Dubot, C.; et al. LBA33 Maintenance olaparib rechallenge in patients (pts) with ovarian carcinoma (OC) previously treated with a PARP inhibitor (PARPi): Phase IIIb OReO/ENGOT Ov-38 trial. Ann. Oncol. 2021, 32, S1308–S1309. [Google Scholar] [CrossRef]
| Germline HR Testing | Somatic HR Testing | |
|---|---|---|
| Sample | Blood (EDTA anticoagulated) or derivative fractions | Fresh or FFPE tissue, cytological samples |
| Processing is not very good | Important fixation, paraffination, decalcification conditions | |
| Rapid sample processing to avoid RNA degradation | Sample selection for at least 30% tumor cells (>50% ideally) | |
| Analytical considerations | Only germline variants are detected | Both germline and somatic variants are detected |
| Expected VAF, around 50% | Expected VAF from 5% | |
| Read depth 50× to 200× | Recommended read depth 500× to 2000× | |
| No variants due to technical issues | Potential false positives due to fixation | |
| More straightforward and validated NGS and pipeline analyses | Complex and difficult to implement NGS and pipelines | |
| 10% of patients with indication will be missed (somatic only) | All patients with indication will be detected | |
| Other considerations | False negative results at homopolymeric traits with mutations | |
| Significant number if VUS identified | ||
| Need for accurate detection of large genomic rearrangements and CNVs | ||
| Study | Phase | Population | Study Arm | Control Arm | Results |
|---|---|---|---|---|---|
| Study-19 NCT00753545 | II | Recurrent HG (G2 or 3) OC/FP/PPC ≥2 platinum-based chemotherapy With an objective response to the platinum regimen | Olaparib 400 mg BID | Placebo | gBRCAm PFS 11.2 vs. 4.3 m HR 0.18 (95% CI, 0.10–0.31) |
| SOLO-2 NCT01874353 | III | Recurrent OC/FP/PPC ≥2 platinum-based chemotherapy With an objective response to the platinum regimen BRCAm | Olaparib 300 mg BID | Placebo | PFS 19.1 vs. 5.5 m HR 0.30 (95% CI, 0.22–0.41) |
| NOVA NCT01847274 | III | Recurrent HGSOC/FP/PPC ≥2 platinum-based chemotherapy Platinum sensitive (>6 months) | Niraparib 300 mg daily | Placebo | gBRCAm PFS: 21.0 vs. 5.5 m HR 0.27 (95% CI, 0.17–0.41) Non-gBRCA PFS 9.3 vs. 3.9 m HR 0.45 (95% CI, 0.34–0.61) |
| ARIEL-3 NCT01968213 | III | Recurrent HGSOC/endometrioid (or FP/PPC) ≥2 platinum-based chemotherapy Platinum sensitive (>6 months) ≤1 non-platinum chemotherapy CR/PR platinum-based chemotherapy | Rucaparib 600 mg BID | Placebo | ITT PFS 10.8 m vs. 5.4 m HR 0.37 (95% CI, 0.30–0.45) BRCAm PFS 16.6 m vs. 5.4 m HR 0.23 (95% CI, 0.16–0.34) |
| Study | Phase | Population | Study Arm | Control Arm | Results |
|---|---|---|---|---|---|
| SOLO-1 NCT01844986 | III | HGSOC/endometrioid (or FP/PPC) FIGO III–IV BRCAm CR/PR platinum-based chemotherapy | Olaparib 300 mg BID | Placebo | PFS NR vs. 13.8 m HR 0.30 (95% CI 0.23–0.41) |
| PRIMA NCT02655016 | III | HGSOC/endometrioid (or FP/PPC) FIGO III–IV Regardless of BRCA status CR/PR platinum-based chemotherapy | Niraparib 300 mg daily | Placebo | ITT PFS 13.8 vs. 8.2 m HR 0.62 (95% CI 0.50–0.76) HRD PFS 21.9 vs. 10.4 m HR 0.43 (95% CI 0.31–0.59) BRCAmut 0.40 (95% CI, 0.27–0.62) |
| PAOLA-1 NCT02477644 | III | HGSOC/endometrioid/other epithelial non-mucinous (or FP/PPC) FIGO IIIB, IIIC or IV gBRCAm/BRCAwt if HGS CR/PR platinum-based chemotherapy | Olaparib 300 mg BID + bevacizumab 15 mg/kg/3 wks | Placebo + Bevacizumab 15 mg/kg/3 wks | ITT PFS 22.1 vs. 16.6 m HR 0.59 (95% CI 0.49–0.72) BRCAmut HR 0.31 (95% CI 0.20–0.47) |
| VELIA NCT02470585 | III | HGSOC OC (or FP/PPC) FIGO III–IV | Paclitaxel-carboplatin-veliparib (150 mg BID-2 weeks 400 mg BID) → veliparib | Paclitaxel-carboplatin-placebo → placebo | ITT PFS: 23.5 vs. 17.3 m HR 0.68 (95% CI 0.56–0.83) BRCAmut PFS: 34.7 vs. 22 m HR 0.44 (95% CI 0.28–0.68) |
| Study | Phase | Population | Study Arm | Control Arm | Results |
|---|---|---|---|---|---|
| SOLO-3 NCT02282020 | III | Recurrent HGSOC/endometrioid (or FP/PPC) ≥2 platinum-based chemotherapy Platinum sensitive (>6 months) BRCAm | Olaparib 300 mg BID | Chemotherapy | PFS 13.4 vs. 9.2 m HR 0.62 (95% CI 0.43–0.91) |
| Study-10 NCT01482715 | I/II | Recurrent HG OC/FP/PPC ≥3 platinum-based chemotherapy BRCAm | Rucaparib 600 mg BID | No comparator arm | ORR 59.5% mDOR 7.8 m (95% CI 5.6–10.5) |
| ARIEL-2 NCT01891344 | II | Recurrent HGSOC (G2 or G3)/endometrioid (or FP/PPC) Prior platinum-based chemotherapy Platinum sensitive (>6 months) (R: 8 weeks from the last cycle) | Rucaparib 600 mg BID | No comparator arm | PFS BRCAm: 12.8 m LOH low: 5.2 m LOH high: 5.7 m HR 0.27 (95% CI 0.16–0.44) |
| ARIEL-4 NCT02855944 | III | Recurrent HG OC/FP/PPC ≥2 chemotherapy regimens g/s BRCAm | Rucaparib 600 mg BID | Chemotherapy | PFS 7.4 m vs. 5.7 m HR 0.64 (95% CI 0.49–0.84) |
| QUADRA NCT02354586 | II | Recurrent HGSOC (or FP/PPC) ≥3–4 previous chemotherapy regimens Platinum sensitive (>6 months) HRD/gBRCA testing | Niraparib 300 mg daily | No comparator arm | PFS 5.5 m (95% CI 3.5–8.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Lorenzo, L.; Salas-Benito, D.; Villamayor, J.; Patiño-García, A.; González-Martín, A. The BRCA Gene in Epithelial Ovarian Cancer. Cancers 2022, 14, 1235. https://doi.org/10.3390/cancers14051235
Sánchez-Lorenzo L, Salas-Benito D, Villamayor J, Patiño-García A, González-Martín A. The BRCA Gene in Epithelial Ovarian Cancer. Cancers. 2022; 14(5):1235. https://doi.org/10.3390/cancers14051235
Chicago/Turabian StyleSánchez-Lorenzo, Luisa, Diego Salas-Benito, Julia Villamayor, Ana Patiño-García, and Antonio González-Martín. 2022. "The BRCA Gene in Epithelial Ovarian Cancer" Cancers 14, no. 5: 1235. https://doi.org/10.3390/cancers14051235
APA StyleSánchez-Lorenzo, L., Salas-Benito, D., Villamayor, J., Patiño-García, A., & González-Martín, A. (2022). The BRCA Gene in Epithelial Ovarian Cancer. Cancers, 14(5), 1235. https://doi.org/10.3390/cancers14051235






