Neoadjuvant Pertuzumab Plus Trastuzumab in Combination with Docetaxel and Carboplatin in Patients with HER2-Positive Breast Cancer: Real-World Data from the National Institute of Oncology in Poland
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Aim
1.2. Ethics Statement
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AGO (German Gynecological Oncology Group). AGO Breast Cancer Guidelines v1. 2021. Available online: www.ago-online.de (accessed on 23 February 2022).
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Breast Cancer. Version 1. 2021. Available online: https://www.nccn.org/guidelines/category_1 (accessed on 23 February 2022).
- Gianni, L.; Pienkowski, T.; Im, Y.H.; Roman, L.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Staroslawska, E.; de la Haba-Rodriguez, J.; Im, S.A.; et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere)–A randomized multicenter, open-label, phase 2 trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.H.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Starosławska, E.; de la Haba-Rodriguez, J.; Im, S.A.; Pedrini, J.L.; et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef]
- Yamashita, Y.; Tanaka, Y.; Kono, S.; Nishimura, M.; Mukohara, T.; Morinaga, Y.; Hara, S.; Takao, S. Effectiveness of Pertuzumab, Trastuzumab, and Docetaxel Combination Neoadjuvant Chemotherapy for HER2-Positive Inflammatory Breast Cancer: A Case Report. Breast Care 2017, 12, 45–47. [Google Scholar] [CrossRef] [Green Version]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer–The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Hegg, R.; Tausch, C.; Seo, J.H.; Tsai, Y.F.; Ratnayake, J.; et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer–A randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 2013, 24, 2278–2284. [Google Scholar] [CrossRef]
- González-Santiago, S.; Saura, C.; Ciruelos, E.; Alonso, J.L.; de la Morena, P.; Santisteban Eslava, M.; Gallegos Sancho, M.I.; de Luna, A.; Dalmau, E.; Servitja, S.; et al. Real-world effectiveness of dual HER2 blockade with pertuzumab and trastuzumab for neoadjuvant treatment of HER2-positive early breast cancer (The NEOPETRA Study). Breast Cancer Res. Treat. 2020, 184, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Berg, T.; Jensen, M.-B.; Jakobsen, E.H.; Al-Rawi, S.; Kenholm, J.; Andersson, M. Neoadjuvant chemotherapy and HER2 dual blockade including biosimilar trastuzumab (SB3) for HER2-positive early breast cancer–Population-based real-world data from the Danish Breast Cancer Group (DBCG). Breast 2020, 54, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.R.; Calhoun, B.; Abraham, J.; Budd, G.T.; Moore, H.C.; Fanning, A.; Valente, S.; Stewart, R.; Andresen, S.W.; LeGrand, S.B.; et al. Efficacy and safety of neoadjuvant docetaxel, carboplatin, trastuzumab/pertuzumab [TCH-P] in non-metastatic HER2+ breast cancer–The Cleveland Clinic experience. J. Clin. Oncol. 2015, 33 (Suppl. 15), 531. [Google Scholar] [CrossRef]
- Katayama, A.; Miligy, I.M.; Shiino, S.; Toss, M.S.; Eldib, K.; Kurozumi, S.; Quinn, C.M.; Badr, N.; Murray, C.; Provenzano, E.; et al. Predictors of pathological complete response to neoadjuvant treatment and changes to post-neoadjuvant HER2 status in HER2-positive invasive breast cancer. Mod. Pathol. 2021, 34, 1271–1281. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Jackisch, C.; Cortazar, P.; Geyer, C.E., Jr.; Gianni, L.; Gligorov, J.; Machackova, Z.; Perez, E.A.; Schneeweiss, A.; Tolaney, S.M.; Untch, M.; et al. Risk-based decision-making in the treatment of HER2-positive early breast cancer: Recommendations based on the current state of knowledge. Cancer Treat. Rev. 2021, 99, 102229. [Google Scholar] [CrossRef] [PubMed]
- Yau, C.; Osdoit, M.; van der Noordaa, M.; Shad, S.; Wei, J.; de Croze, D.; Hamy, A.S.; Laé, M.; Reyal, F.; Sonke, G.S.; et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: A multicentre pooled analysis of 5161 patients. Lancet Oncol. 2022, 23, 149–160. [Google Scholar] [CrossRef]
- Smith, B.D.; Jiang, J.; McLaughlin, S.S.; Hurria, A.; Smith, G.L.; Giordano, S.H.; Buchholz, T.A. Improvement in breast cancer outcomes over time: Are older women missing out? ” J. Clin. Oncol. 2011, 29, 4647–4653. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska Urszula, Didkowska Joanna. Zachorowania i Zgony na Nowotwory Złośliwe w Polsce. Krajowy Rejestr Nowotworów, Narodowy Instytut Onkologii im. Marii Skłodowskiej-Curie–Państwowy Instytut Badawczy. Available online: http://onkologia.org.pl/raporty/dost%C4%99pzdnia26/12/2021 (accessed on 15 January 2022).
- Owusu, C.; Lash, T.L.; Silliman, R.A. Effect of undertreatment on the disparity in age-related breast cancer-specific survival among older women. Breast Cancer Res. Treat. 2007, 102, 227–236. [Google Scholar] [CrossRef]
- Williams, G.R.; Jones, E.; Muss, H.B. Challenges in the Treatment of Older Breast Cancer Patients. Hematol. Oncol. Clin. N. Am. 2013, 27, 785–804. [Google Scholar] [CrossRef]
- Cote, G.M.; Sawyer, D.B.; Chabner, B.A. ERBB2 inhibition and heart failure. N. Engl. J. Med. 2012, 367, 2150–2153. [Google Scholar] [CrossRef]
- Dang, C.; Guo, H.; Najita, J.; Yardley, D.; Marcom, K.; Albain, K.; Rugo, H.; Miller, K.; Ellis, M.; Shapira, I.; et al. Cardiac outcomes of patients receiving adjuvant weekly paclitaxel and trastuzumab for node negative, ERBB2-positive breast cancer. JAMA Oncol. 2016, 2, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Swain, S.M.; Ewer, M.S.; Cortés, J.; Amadori, D.; Miles, D.; Knott, A.; Clark, E.; Benyunes, M.C.; Ross, G.; Baselga, J. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: A randomized, double-blind, placebo-controlled phase III study. Oncologist 2013, 18, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50 (accessed on 23 February 2022).
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant Trastuzumab in HER2-positive Breast Cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef] [Green Version]
- Swain, S.M.; Kim, S.B.; Cortés, J.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Knott, A.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef]
- Shao, Z.; Pang, D.; Yang, H.; Li, W.; Wang, S.; Cui, S.; Liao, N.; Wang, Y.; Wang, C.; Chang, Y.C.; et al. Efficacy, Safety, and Tolerability of Pertuzumab, Trastuzumab, and Docetaxel for Patients With Early or Locally Advanced ERBB2- Positive Breast Cancer in Asia: The PEONY Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e193692. [Google Scholar] [CrossRef] [PubMed]
- van Ramshorst, M.S.; van der Voort, A.; van Werkhoven, E.D.; Mandjes, I.A.; Kemper, I.; Dezentjé, V.O.; Oving, I.M.; Honkoop, A.H.; Tick, L.W.; van de Wouw, A.J.; et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1630–1640. [Google Scholar] [CrossRef]
- Siegel, R.; DeSantis, C.; Virgo, K.; Stein, K.; Mariotto, A.; Smith, T.; Cooper, D.; Gansler, T.; Lerro, C.; Fedewa, S.; et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J. Clin. 2012, 62, 220–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, G.T.; Oeffinger, K.C.; Chen, Y.; Kawashima, T.; Yasui, Y.; Leisenring, W.; Stovall, M.; Chow, E.J.; Sklar, C.A.; Mulrooney, D.A.; et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J. Clin. Oncol. 2013, 31, 3673–3680. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Procter, M.; De Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant pertuzumab and trastuzumab in early HER2- positive breast cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef]
- Mantarro, S.; Rossi, M.; Bonifazi, M.; D’Amico, R.; Blandizzi, C.; La Vecchia, C.; Negri, E.; Moja, L. Risk of severe cardiotoxicity following treatment with trastuzumab: A meta-analysis of randomized and cohort studies of 29,000 women with breast cancer. Intern. Emerg. Med. 2016, 11, 123–140. [Google Scholar] [CrossRef]
- Lv, M.; Guo, H.; Wang, C.; Tian, P.; Ma, Y.; Chen, X.; Luo, S. Neoadjuvant docetaxel with or without carboplatin plus dual HER2 blockade for HER2-positive breast cancer: A retrospective multi-centre Chinese study. Gland Surg. 2020, 9, 2079–2090. [Google Scholar] [CrossRef]
- Arora, S.; Gogia, D.A.; Deo, S.; Sharma, D.; Mathur, S.R. Neoadjuvant pertuzumab plus trastuzumab in combination with anthracycline- free chemotherapy regimen in patients with HER2 positive breast cancer-Real-world data from a single centre in India. Cancer Treat. Res. Commun. 2021, 29, 100483. [Google Scholar] [CrossRef]
| Variable | Parameter | N = 34 | % |
|---|---|---|---|
| Age at diagnosis of breast cancer (years) | Median (IQR) | 46 (39.25–54.25) | |
| Range | 30–68 | ||
| Age at diagnosis of breast cancer (years)—breakdown | <60 years old | 27 | 79.4% |
| ≥60 years old | 7 | 20.6% | |
| cT | 0 | 1 | 2.9% |
| 1 | 3 | 8.8% | |
| 2 | 21 | 61.8% | |
| 3 | 6 | 17.6% | |
| 4d | 3 | 8.8% | |
| cN | 0 | 13 | 38.2% |
| 1 | 14 | 41.2% | |
| 2 | 5 | 14.7% | |
| 3 | 2 | 5.9% | |
| ER | Positive | 20 | 58.8% |
| Negative | 14 | 41.2% | |
| PR | Positive | 15 | 44.1% |
| Negative | 19 | 55.9% | |
| Type of treatment—breast | Mastectomy | 12 | 35.3% |
| Mastectomy + immediate breast reconstruction | 8 | 23.5% | |
| Breast-conserving surgery (BCS) | 13 | 38.3% | |
| No treatment (T0 patient) | 1 | 2.9% | |
| Type of treatment—axilla | Axillary dissection | 18 | 53% |
| Sentinel node (SN) | 13 | 38.2% | |
| Targeted axillary dissection (TAD) | 3 | 8.9% |
| Toxicity | Incidence (Any Grade) | Grade 3 or 4 | ||||
|---|---|---|---|---|---|---|
| Any | Patients < 60 | Patients ≥ 60 | Any | Patients < 60 | Patients ≥ 60 | |
| Diarrhoea | 9 (26%) | 4 (15%) | 5 (71%) | 2 (6%) | 0 | 2 (28%) |
| Thrombocytopenia | 13 (38%) | 9 (33%) | 4 (57%) | 0 | 0 | 0 |
| Neutropenia | 14 (41%) | 10 (37%) | 4 (57%) | 4 (12%) | 2 (7%) | 2 (28%) |
| Febrile neutropenia | 4 (12%) | 2 (7%) | 2 (28%) | 4 (12%) | 2 (7%) | 2 (28%) |
| Anaemia | 27 (79%) | 23 (85%) | 4 (57%) | 2 (6%) | 1 (3.5%) | 1 (14%) |
| Fatigue | 24 (70%) | 19 (70%) | 5 (71%) | 2 (6%) | 1 (3.5%) | 1 (14%) |
| Neuropathy | 22 (65%) | 17 (63%) | 5 (71%) | 0 | 0 | 0 |
| Mucositis | 3 (9%) | 2 (7%) | 1 (14%) | 0 | 0 | 0 |
| Cardiac dysfunction | 0 | 0 | 0 | 0 | 0 | 0 |
| Characteristic | Parameter | % (N = 34) |
|---|---|---|
| Achieved response to neoadjuvant treatment | pCR | 52.9% (N = 18) |
| non pCR | 47.1% (N = 16) | |
| Residual cancer burden (RCB)—only patients with non-pCR | I | 20.6% (N = 7) |
| II | 11.8% (N = 4) | |
| III | 14.7% (N = 5) |
| Variable | Parameter | pCR (N = 18) | Non-pCR (N = 16) | p-Value |
|---|---|---|---|---|
| Age at diagnosis of breast cancer (years) | N | 18 | 16 | 0.07512 |
| Median | 43 | 50 | ||
| Range | 30–67 | 33–68 | ||
| Age at diagnosis of breast cancer (years)—breakdown | <60 years old | 88.9% (N = 16) | 68.8% (N = 11) | 0.2143 |
| ≥60 years old | 11.1% (N = 2) | 31.2% (N = 5) | ||
| cT | 0 | 5.6% (N = 1) | 0% (N = 0) | 0.8128 |
| 1 | 11.1% (N = 2) | 6.2% (N = 1) | ||
| 2 | 61.1% (N = 11) | 62.5% (N = 10) | ||
| 3 | 11.1% (N = 2) | 25% (N = 4) | ||
| 4d | 11.1% (N = 2) | 6.2% (N = 1) | ||
| cN | 0 | 38.9% (N = 7) | 37.5% (N = 6) | 1 |
| 1 | 38.9% (N = 7) | 43.8% (N = 7) | ||
| 2 | 16.7% (N = 3) | 12.5% (N = 2) | ||
| 3 | 5.6% (N = 1) | 6.2% (N = 1) | ||
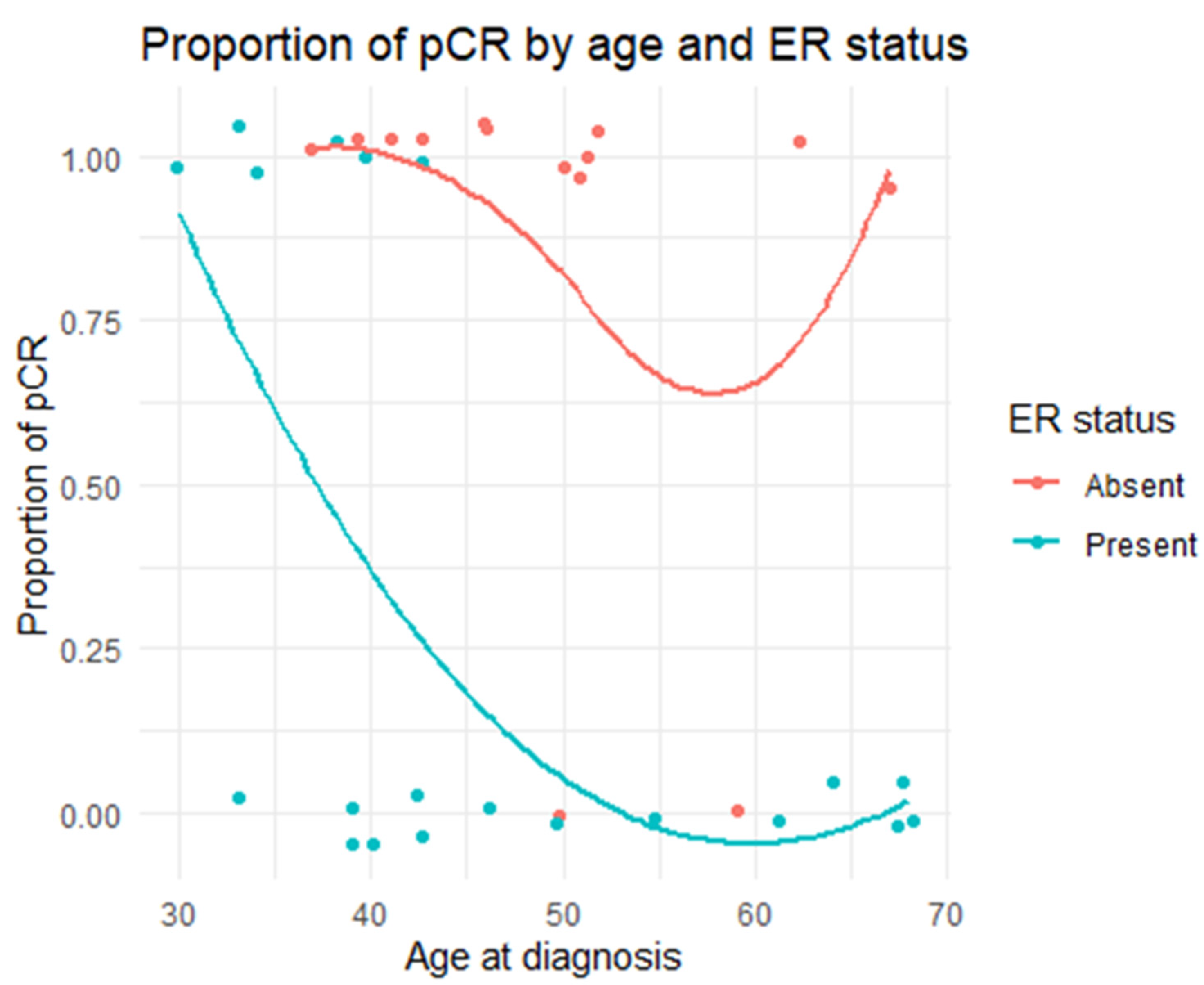
| ER | Positive | 33.3% (N = 6) | 87.5% (N = 14) | 0.0019 |
| Negative | 66.7% (N = 12) | 12.5% (N = 2) | ||
| PR | Positive | 27.8% (N = 5) | 62.5% (N = 10) | 0.0824 |
| Negative | 72.2% (N = 13) | 37.5% (N = 6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagiełło-Gruszfeld, A.I.; Rosinska, M.; Meluch, M.; Pogoda, K.; Niwinska, A.; Sienkiewicz, R.; Grous, A.; Winter, P.; Nowecki, Z.I. Neoadjuvant Pertuzumab Plus Trastuzumab in Combination with Docetaxel and Carboplatin in Patients with HER2-Positive Breast Cancer: Real-World Data from the National Institute of Oncology in Poland. Cancers 2022, 14, 1218. https://doi.org/10.3390/cancers14051218
Jagiełło-Gruszfeld AI, Rosinska M, Meluch M, Pogoda K, Niwinska A, Sienkiewicz R, Grous A, Winter P, Nowecki ZI. Neoadjuvant Pertuzumab Plus Trastuzumab in Combination with Docetaxel and Carboplatin in Patients with HER2-Positive Breast Cancer: Real-World Data from the National Institute of Oncology in Poland. Cancers. 2022; 14(5):1218. https://doi.org/10.3390/cancers14051218
Chicago/Turabian StyleJagiełło-Gruszfeld, Agnieszka Irena, Magdalena Rosinska, Małgorzata Meluch, Katarzyna Pogoda, Anna Niwinska, Renata Sienkiewicz, Aleksander Grous, Paweł Winter, and Zbigniew I. Nowecki. 2022. "Neoadjuvant Pertuzumab Plus Trastuzumab in Combination with Docetaxel and Carboplatin in Patients with HER2-Positive Breast Cancer: Real-World Data from the National Institute of Oncology in Poland" Cancers 14, no. 5: 1218. https://doi.org/10.3390/cancers14051218
APA StyleJagiełło-Gruszfeld, A. I., Rosinska, M., Meluch, M., Pogoda, K., Niwinska, A., Sienkiewicz, R., Grous, A., Winter, P., & Nowecki, Z. I. (2022). Neoadjuvant Pertuzumab Plus Trastuzumab in Combination with Docetaxel and Carboplatin in Patients with HER2-Positive Breast Cancer: Real-World Data from the National Institute of Oncology in Poland. Cancers, 14(5), 1218. https://doi.org/10.3390/cancers14051218






