Clinical Significance of Tumor-Infiltrating Conventional and Plasmacytoid Dendritic Cells in Pancreatic Ductal Adenocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples
2.2. Classical Immunohistochemistry
2.3. Multiplex Immunohistochemistry
2.4. Quantification of PDAC-Infiltrating cDC1s, cDC2s, and pDCs
2.5. Statistical Analysis
3. Results
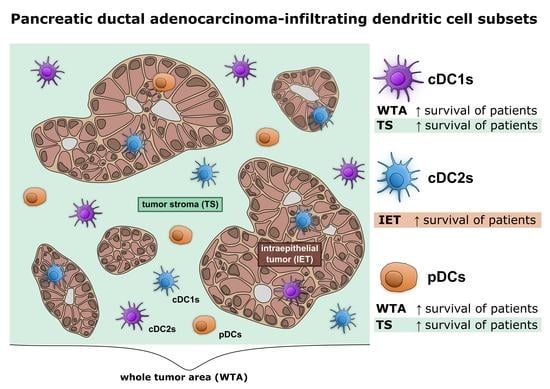
3.1. cDC1s, cDC2s, and pDCs Infiltrate PDAC
3.2. Levels of PDAC-Infiltrating cDCs Correlate with Favorable Pathological Tumor Features and with Increased Survival of Patients
3.3. Neoadjuvant Chemotherapy Does Not Influence the Frequency of DCs in PDAC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Brezgyte, G.; Shah, V.; Jach, D.; Crnogorac-Jurcevic, T. Non-invasive biomarkers for earlier detection of pancreatic cancer—A comprehensive review. Cancers 2021, 13, 2722. [Google Scholar] [CrossRef]
- Singh, R.R.; O’Reilly, E.M. New treatment strategies for metastatic pancreatic ductal adenocarcinoma. Drugs 2020, 80, 647–669. [Google Scholar] [CrossRef]
- Christenson, E.S.; Jaffee, E.; Azad, N.S. Current and emerging therapies for patients with advanced pancreatic ductal adenocarcinoma: A bright future. Lancet Oncol. 2020, 21, e135–e145. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Principe, D.R.; Underwood, P.W.; Korc, M.; Trevino, J.G.; Munshi, H.G.; Rana, A. The current treatment paradigm for pancreatic ductal adenocarcinoma and barriers to therapeutic efficacy. Front. Oncol. 2021, 11, 2773. [Google Scholar] [CrossRef]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The tumour microenvironment in pancreatic cancer—Clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef]
- Awaji, M.; Singh, R.K. Cancer-associated fibroblasts’ functional heterogeneity in pancreatic ductal adenocarcinoma. Cancers 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Zhang, C.; Jiang, K.; Werner, J.; Bazhin, A.V.; D’Haese, J.G. The role of stellate cells in pancreatic ductal adenocarcinoma: Targeting perspectives. Front. Oncol. 2021, 10, 3044. [Google Scholar] [CrossRef] [PubMed]
- Sperb, N.; Tsesmelis, M.; Wirth, T. Crosstalk between tumor and stromal cells in pancreatic ductal adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 5486. [Google Scholar] [CrossRef]
- Wu, Q.; Tian, Y.; Zhang, J.; Zhang, H.; Gu, F.; Lu, Y.; Zou, S.; Chen, Y.; Sun, P.; Xu, M.; et al. Functions of pancreatic stellate cell-derived soluble factors in the microenvironment of pancreatic ductal carcinoma. Oncotarget 2017, 8, 102721–102738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunardi, S.; Jamieson, N.B.; Lim, S.Y.; Griffiths, K.L.; Carvalho-Gaspar, M.; Al-Assar, O.; Yameen, S.; Carter, R.C.; McKay, C.J.; Spoletini, G.; et al. IP-10/CXCL10 induction in human pancreatic cancer stroma influences lymphocytes recruitment and correlates with poor survival. Oncotarget 2014, 5, 11064–11080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balachandran, V.P.; Beatty, G.L.; Dougan, S.K. Broadening the impact of immunotherapy to pancreatic cancer: Challenges and opportunities. Gastroenterology 2019, 156, 2056–2072. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Böttcher, J.P.; Reis e Sousa, C. The role of type 1 conventional dendritic cells in cancer immunity. Trends Cancer 2018, 4, 784–792. [Google Scholar] [CrossRef] [Green Version]
- Del Prete, A.; Sozio, F.; Barbazza, I.; Salvi, V.; Tiberio, L.; Laffranchi, M.; Gismondi, A.; Bosisio, D.; Schioppa, T.; Sozzani, S. Functional role of dendritic cell subsets in cancer progression and clinical implications. Int. J. Mol. Sci. 2020, 21, 3930. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.E.; Barry, K.C. The natural killer–dendritic cell immune axis in anti-cancer immunity and immunotherapy. Front. Immunol. 2021, 11, 3778. [Google Scholar] [CrossRef]
- Michea, P.; Noël, F.; Zakine, E.; Czerwinska, U.; Sirven, P.; Abouzid, O.; Goudot, C.; Scholer-Dahirel, A.; Vincent-Salomon, A.; Reyal, F.; et al. Adjustment of dendritic cells to the breast-cancer microenvironment is subset specific. Nat. Immunol. 2018, 19, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 2018, 172, 1022–1037.e14. [Google Scholar] [CrossRef] [Green Version]
- Hubert, M.; Gobbini, E.; Couillault, C.; Manh, T.V.; Doffin, A.-C.; Berthet, J.; Rodriguez, C.; Ollion, V.; Kielbassa, J.; Sajous, C.; et al. IFN-III is selectively produced by cDC1 and predicts good clinical outcome in breast cancer. Sci. Immunol. 2020, 5, 3942. [Google Scholar] [CrossRef] [Green Version]
- Mattiuz, R.; Brousse, C.; Ambrosini, M.; Cancel, J.-C.; Bessou, G.; Mussard, J.; Sanlaville, A.; Caux, C.; Bendriss-Vermare, N.; Valladeau-Guilemond, J.; et al. Type 1 conventional dendritic cells and interferons are required for spontaneous CD4+ and CD8+ T-cell protective responses to breast cancer. Clin. Transl. Immunol. 2021, 10, e1305. [Google Scholar] [CrossRef] [PubMed]
- Mastelic-Gavillet, B.; Sarivalasis, A.; Lozano, L.E.; Wyss, T.; Inoges, S.; de Vries, I.J.M.; Dartiguenave, F.; Jichlinski, P.; Derrè, L.; Coukos, G.; et al. Quantitative and qualitative impairments in dendritic cell subsets of patients with ovarian or prostate cancer. Eur. J. Cancer 2020, 135, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Sosa Cuevas, E.; Ouaguia, L.; Mouret, S.; Charles, J.; De Fraipont, F.; Manches, O.; Valladeau-Guilemond, J.; Bendriss-Vermare, N.; Chaperot, L.; Aspord, C. BDCA1+ cDC2s, BDCA2+ pDCs and BDCA3+ cDC1s reveal distinct pathophysiologic features and impact on clinical outcomes in melanoma patients. Clin. Transl. Immunol. 2020, 9, e1190. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef]
- Rhodes, J.W.; Tong, O.; Harman, A.N.; Turville, S.G. Human dendritic cell subsets, ontogeny, and impact on HIV infection. Front. Immunol. 2019, 10, 1088. [Google Scholar] [CrossRef] [Green Version]
- Tabarkiewicz, J.; Rybojad, P.; Jablonka, A.; Rolinski, J. CD1c+ and CD303+ Dendritic cells in peripheral blood, lymph nodes and tumor tissue of patients with non-small cell lung cancer. Oncol. Rep. 2008, 19, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Jahrsdörfer, B.; Vollmer, A.; Blackwell, S.E.; Maier, J.; Sontheimer, K.; Beyer, T.; Mandel, B.; Lunov, O.; Tron, K.; Nienhaus, G.U.; et al. Granzyme B produced by human plasmacytoid dendritic cells suppresses T-cell expansion. Blood 2010, 115, 1156–1165. [Google Scholar] [CrossRef] [Green Version]
- Kalb, M.L.; Glaser, A.; Stary, G.; Koszik, F.; Stingl, G. TRAIL(+) human plasmacytoid dendritic cells kill tumor cells in vitro: Mechanisms of imiquimod- and IFN-α-mediated antitumor reactivity. J. Immunol. Baltim. Md 1950 2012, 188, 1583–1591. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Lawrence, T.; Liang, Y. The role of plasmacytoid dendritic cells in cancers. Front. Immunol. 2021, 12, 4414. [Google Scholar] [CrossRef]
- Kießler, M.; Plesca, I.; Sommer, U.; Wehner, R.; Wilczkowski, F.; Müller, L.; Tunger, A.; Lai, X.; Rentsch, A.; Peuker, K.; et al. Tumor-infiltrating plasmacytoid dendritic cells are associated with survival in human colon cancer. J. Immunother. Cancer 2021, 9, e001813. [Google Scholar] [CrossRef]
- Treilleux, I.; Blay, J.-Y.; Bendriss-Vermare, N.; Ray-Coquard, I.; Bachelot, T.; Guastalla, J.-P.; Bremond, A.; Goddard, S.; Pin, J.-J.; Barthelemy-Dubois, C.; et al. Dendritic cell infiltration and prognosis of early-stage breast cancer. Clin. Cancer Res. 2004, 10, 7466–7474. [Google Scholar] [CrossRef] [Green Version]
- Pang, L.; Ng, K.T.; Liu, J.; Yeung, W.O.; Zhu, J.; Chiu, T.S.; Liu, H.; Chen, Z.; Lo, C.-M.; Man, K. Plasmacytoid dendritic cells recruited by HIF-1α/EADO/ADORA1 signaling induce immunosuppression in hepatocellular carcinoma. Cancer Lett. 2021, 522, 80–92. [Google Scholar] [CrossRef]
- Aspord, C.; Leccia, M.-T.; Charles, J.; Plumas, J. Plasmacytoid dendritic cells support melanoma progression by promoting Th2 and regulatory immunity through OX40L and ICOSL. Cancer Immunol. Res. 2013, 1, 402–415. [Google Scholar] [CrossRef] [Green Version]
- Labidi-Galy, S.I.; Treilleux, I.; Goddard-Leon, S.; Combes, J.-D.; Blay, J.-Y.; Ray-Coquard, I.; Caux, C.; Bendriss-Vermare, N. Plasmacytoid dendritic cells infiltrating ovarian cancer are associated with poor prognosis. OncoImmunology 2012, 1, 380–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirooka, S.; Yanagimoto, H.; Satoi, S.; Yamamoto, T.; Toyokawa, H.; Yamaki, S.; Yui, R.; Inoue, K.; Michiura, T.; Kwon, A.-H. The role of circulating dendritic cells in patients with unresectable pancreatic cancer. Anticancer. Res. 2011, 31, 3827–3834. [Google Scholar]
- Tjomsland, V.; Sandström, P.; Spångeus, A.; Messmer, D.; Emilsson, J.; Falkmer, U.; Falkmer, S.; Magnusson, K.-E.; Borch, K.; Larsson, M. Pancreatic adenocarcinoma exerts systemic effects on the peripheral blood myeloid and plasmacytoid dendritic cells: An indicator of disease severity? BMC Cancer 2010, 10, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, T.; Yanagimoto, H.; Satoi, S.; Toyokawa, H.; Yamao, J.; Kim, S.; Terakawa, N.; Takahashi, K.; Kwon, A.-H. Circulating myeloid dendritic cells as prognostic factors in patients with pancreatic cancer who have undergone surgical resection. J. Surg. Res. 2012, 173, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; WILEY Blackwell: Oxford, UK, 2017; pp. 126–129. [Google Scholar]
- Opzoomer, J.W.; Sosnowska, D.; Anstee, J.E.; Spicer, J.F.; Arnold, J.N. Cytotoxic chemotherapy as an immune stimulus: A molecular perspective on turning up the immunological heat on cancer. Front. Immunol. 2019, 10, 1654. [Google Scholar] [CrossRef] [Green Version]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.-L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N.; et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007, 13, 54–61. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 2015, 28, 690–714. [Google Scholar] [CrossRef] [Green Version]
- Kwong, T.T.; Wong, C.H.; Zhou, J.Y.; Cheng, A.S.L.; Sung, J.J.Y.; Chan, A.W.H.; Chan, S.L. Chemotherapy-induced recruitment of myeloid-derived suppressor cells abrogates efficacy of immune checkpoint blockade. JHEP Rep. 2020, 3, 100224. [Google Scholar] [CrossRef] [PubMed]
- Kanterman, J.; Sade-Feldman, M.; Biton, M.; Ish-Shalom, E.; Lasry, A.; Goldshtein, A.; Hubert, A.; Baniyash, M. Adverse immunoregulatory effects of 5FU and CPT11 chemotherapy on myeloid-derived suppressor cells and colorectal cancer outcomes. Cancer Res. 2014, 74, 6022–6035. [Google Scholar] [CrossRef] [Green Version]
- Michelakos, T.; Cai, L.; Villani, V.; Sabbatino, F.; Kontos, F.; Fernández-Del Castillo, C.; Yamada, T.; Neyaz, A.; Taylor, M.S.; Deshpande, V.; et al. Tumor microenvironment immune response in pancreatic ductal adenocarcinoma patients treated with neoadjuvant therapy. J. Natl. Cancer Inst. 2021, 113, 182–191. [Google Scholar] [CrossRef]
- Peng, H.; James, C.A.; Cullinan, D.R.; Hogg, G.D.; Mudd, J.L.; Zuo, C.; Takchi, R.; Caldwell, K.E.; Liu, J.; DeNardo, D.G.; et al. Neoadjuvant FOLFIRINOX therapy is associated with increased effector T cells and reduced suppressor cells in patients with pancreatic cancer. Clin. Cancer Res. 2021, 27, 6761–6771. [Google Scholar] [CrossRef] [PubMed]
- Mota Reyes, C.; Teller, S.; Muckenhuber, A.; Konukiewitz, B.; Safak, O.; Weichert, W.; Friess, H.; Ceyhan, G.O.; Demir, I.E. Neoadjuvant therapy remodels the pancreatic cancer microenvironment via depletion of protumorigenic immune cells. Clin. Cancer Res. 2020, 26, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, K.C.; Goel, V.K.; Xiong, W.; Sham, J.G.; Pollack, S.M.; Leahy, A.M.; Whiting, S.H.; Yeh, M.M.; Yee, C.; Riddell, S.R.; et al. Pancreatic ductal adenocarcinoma contains an effector and regulatory immune cell infiltrate that is altered by multimodal neoadjuvant treatment. PLoS ONE 2014, 9, e96565. [Google Scholar] [CrossRef]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Zitvogel, L.; Sautès–Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Tumor immunology and tumor evolution: Intertwined histories. Immunity 2020, 52, 55–81. [Google Scholar] [CrossRef] [PubMed]
- Bruni, D.; Angell, H.K.; Galon, J. The immune contexture and immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef]
- Carstens, J.L.; Correa de Sampaio, P.; Yang, D.; Barua, S.; Wang, H.; Rao, A.; Allison, J.P.; LeBleu, V.S.; Kalluri, R. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat. Commun. 2017, 8, 15095. [Google Scholar] [CrossRef]
- Kiryu, S.; Ito, Z.; Suka, M.; Bito, T.; Kan, S.; Uchiyama, K.; Saruta, M.; Hata, T.; Takano, Y.; Fujioka, S.; et al. Prognostic value of immune factors in the tumor microenvironment of patients with pancreatic ductal adenocarcinoma. BMC Cancer 2021, 21, 1197. [Google Scholar] [CrossRef] [PubMed]
- Seifert, L.; Plesca, I.; Müller, L.; Sommer, U.; Heiduk, M.; von Renesse, J.; Digomann, D.; Glück, J.; Klimova, A.; Weitz, J.; et al. LAG-3-expressing tumor-infiltrating T cells are associated with reduced disease-free survival in pancreatic cancer. Cancers 2021, 13, 1297. [Google Scholar] [CrossRef]
- Tang, R.; Liu, X.; Liang, C.; Hua, J.; Xu, J.; Wang, W.; Meng, Q.; Liu, J.; Zhang, B.; Yu, X.; et al. Deciphering the prognostic implications of the components and signatures in the immune microenvironment of pancreatic ductal adenocarcinoma. Front. Immunol. 2021, 12, 575. [Google Scholar] [CrossRef]
- Mahajan, U.M.; Langhoff, E.; Goni, E.; Costello, E.; Greenhalf, W.; Halloran, C.; Ormanns, S.; Kruger, S.; Boeck, S.; Ribback, S.; et al. Immune cell and stromal signature associated with progression-free survival of patients with resected pancreatic ductal adenocarcinoma. Gastroenterology 2018, 155, 1625–1639.e2. [Google Scholar] [CrossRef] [Green Version]
- Tahkola, K.; Mecklin, J.-P.; Wirta, E.-V.; Ahtiainen, M.; Helminen, O.; Böhm, J.; Kellokumpu, I. High immune cell score predicts improved survival in pancreatic cancer. Virchows Arch. 2018, 472, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Wartenberg, M.; Zlobec, I.; Perren, A.; Koelzer, V.H.; Gloor, B.; Lugli, A.; Karamitopoulou, E. Accumulation of FOXP3+T-cells in the tumor microenvironment is associated with an epithelial-mesenchymal-transition-type tumor budding phenotype and is an independent prognostic factor in surgically resected pancreatic ductal adenocarcinoma. Oncotarget 2015, 6, 4190–4201. [Google Scholar] [CrossRef] [Green Version]
- Seifert, A.M.; Eymer, A.; Heiduk, M.; Wehner, R.; Tunger, A.; von Renesse, J.; Decker, R.; Aust, D.E.; Welsch, T.; Reissfelder, C.; et al. PD-1 expression by lymph node and intratumoral regulatory T cells is associated with lymph node metastasis in pancreatic cancer. Cancers 2020, 12, 2756. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Q.; Liao, Q. Tumor-associated macrophages in pancreatic ductal adenocarcinoma: Origin, polarization, function, and reprogramming. Front. Cell Dev. Biol. 2021, 8, 607209. [Google Scholar] [CrossRef]
- Yu, M.; Guan, R.; Hong, W.; Zhou, Y.; Lin, Y.; Jin, H.; Hou, B.; Jian, Z. Prognostic value of tumor-associated macrophages in pancreatic cancer: A meta-analysis. Cancer Manag. Res. 2019, 11, 4041–4058. [Google Scholar] [CrossRef] [Green Version]
- Brunner, M.; Maier, K.; Rümmele, P.; Jacobsen, A.; Merkel, S.; Benard, A.; Krautz, C.; Kersting, S.; Grützmann, R.; Weber, G.F. Upregulation of CD20 positive B-cells and B-cell aggregates in the tumor infiltration zone is associated with better survival of patients with pancreatic ductal adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castino, G.F.; Cortese, N.; Capretti, G.; Serio, S.; Di Caro, G.; Mineri, R.; Magrini, E.; Grizzi, F.; Cappello, P.; Novelli, F.; et al. Spatial distribution of B cells predicts prognosis in human pancreatic adenocarcinoma. OncoImmunology 2015, 5, e1085147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegde, S.; Krisnawan, V.E.; Herzog, B.H.; Zuo, C.; Breden, M.A.; Knolhoff, B.L.; Hogg, G.D.; Tang, J.P.; Baer, J.M.; Mpoy, C.; et al. Dendritic cell paucity leads to dysfunctional immune surveillance in pancreatic cancer. Cancer Cell 2020, 37, 289–307.e9. [Google Scholar] [CrossRef]
- Lin, J.H.; Huffman, A.P.; Wattenberg, M.M.; Walter, D.M.; Carpenter, E.L.; Feldser, D.M.; Beatty, G.L.; Furth, E.E.; Vonderheide, R.H. Type 1 conventional dendritic cells are systemically dysregulated early in pancreatic carcinogenesis. J. Exp. Med. 2020, 217, e20190673. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-E.; Hajdu, C.H.; Liot, C.; Miller, G.; Dustin, M.L.; Bar-Sagi, D. Crosstalk between regulatory T cells and tumor-associated dendritic cells negates anti-tumor immunity in pancreatic cancer. Cell Rep. 2017, 20, 558–571. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.A.; Baer, J.M.; Knolhoff, B.L.; Nywening, T.M.; Panni, R.Z.; Su, X.; Weilbaecher, K.N.; Hawkins, W.G.; Ma, C.; Fields, R.C.; et al. Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance. Nat. Commun. 2018, 9, 1250. [Google Scholar] [CrossRef]
- Kenkel, J.A.; Tseng, W.W.; Davidson, M.G.; Tolentino, L.L.; Choi, O.; Bhattacharya, N.; Seeley, E.S.; Winer, D.A.; Reticker-Flynn, N.E.; Engleman, E.G. An immunosuppressive dendritic cell subset accumulates at secondary sites and promotes metastasis in pancreatic cancer. Cancer Res. 2017, 77, 4158–4170. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Sui, S.; Shang, Y.; Yu, Z.; Han, J.; Zhang, G.; Ntim, M.; Hu, M.; Gong, P.; Chen, H.; et al. The landscape of immune cell infiltration and its clinical implications of pancreatic ductal adenocarcinoma. J. Adv. Res. 2020, 24, 139–148. [Google Scholar] [CrossRef]
- Truxova, I.; Kasikova, L.; Hensler, M.; Skapa, P.; Laco, J.; Pecen, L.; Belicova, L.; Praznovec, I.; Halaska, M.J.; Brtnicky, T.; et al. Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. J. Immunother. Cancer 2018, 6, 139. [Google Scholar] [CrossRef]
- Halse, H.; Colebatch, A.J.; Petrone, P.; Henderson, M.A.; Mills, J.K.; Snow, H.; Westwood, J.A.; Sandhu, S.; Raleigh, J.M.; Behren, A.; et al. Multiplex immunohistochemistry accurately defines the immune context of metastatic melanoma. Sci. Rep. 2018, 8, 11158. [Google Scholar] [CrossRef] [Green Version]
- Al-Shibli, K.I.; Donnem, T.; Al-Saad, S.; Persson, M.; Bremnes, R.M.; Busund, L.-T. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin. Cancer Res. 2008, 14, 5220–5227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wortman, J.C.; He, T.-F.; Solomon, S.; Zhang, R.Z.; Rosario, A.; Wang, R.; Tu, T.Y.; Schmolze, D.; Yuan, Y.; Yost, S.E.; et al. Spatial distribution of B cells and lymphocyte clusters as a predictor of triple-negative breast cancer outcome. Npj Breast Cancer 2021, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]






| n = 58 n (%) | |
|---|---|
| Age | |
| Median (Range) | 67.14 (47–79) |
| Gender | |
| Male | 30 (52) |
| Female | 28 (48) |
| pT Stage | |
| 1 | 5 (9) |
| 2 | 33 (57) |
| 3 | 20 (34) |
| pN Stage | |
| 0 | 35 (60) |
| 1 | 23 (40) |
| pM Stage | |
| 0 | 58 (100) |
| UICC Stage | |
| I | 8 (14) |
| II | 50 (86) |
| Neoadjuvant Chemotherapy | |
| Yes | 21 (36) |
| No | 37 (64) |
| DFS–WTA | n | HR | 95% CI | p-Value |
|---|---|---|---|---|
| cDC1s | 40 | 0.89 | 0.82–0.97 | 0.006 ** |
| Age | 40 | 0.92 | 0.86–0.99 | 0.017 * |
| Female vs. male | 16 vs. 24 | 1.69 | 0.64–4.47 | 0.294 |
| pT stage | ||||
| pT2 vs. pT1 | 22 vs. 5 | 2.04 | 0.52–8.02 | 0.306 |
| pT3 vs. pT1 | 13 vs. 5 | 2.26 | 0.52–9.77 | 0.273 |
| pN stage | ||||
| pN1 vs. pN0 | 14 vs. 26 | 1.79 | 0.68–4.70 | 0.239 |
| OS–WTA | ||||
| cDC1s | 40 | 0.92 | 0.85–0.98 | 0.016 * |
| Age | 40 | 0.96 | 0.91–1.01 | 0.094 |
| Female vs. male | 16 vs. 24 | 1.70 | 0.74–3.93 | 0.214 |
| pT stage | ||||
| pT2 vs. pT1 | 22 vs. 5 | 5.41 | 0.67–43.51 | 0.113 |
| pT3 vs. pT1 | 13 vs. 5 | 3.77 | 0.46–31.09 | 0.217 |
| pN stage | ||||
| pN1 vs. pN0 | 14 vs. 26 | 2.88 | 1.28–6.50 | 0.011 * |
| DFS–TS | n | HR | 95% CI | p-Value |
|---|---|---|---|---|
| cDC1s | 40 | 0.91 | 0.86–0.97 | 0.005 ** |
| Age | 40 | 0.91 | 0.85–0.98 | 0.013 * |
| Female vs. male | 16 vs. 24 | 1.84 | 0.67–5.04 | 0.237 |
| pT stage | ||||
| pT2 vs. pT1 | 22 vs. 5 | 1.88 | 0.49–7.26 | 0.361 |
| pT3 vs. pT1 | 13 vs. 5 | 1.98 | 0.46–8.55 | 0.36 |
| pN stage | ||||
| pN1 vs. pN0 | 14 vs. 26 | 1.83 | 0.70–4.84 | 0.22 |
| OS–TS | ||||
| cDC1s | 40 | 0.93 | 0.88–0.98 | 0.012 * |
| Age | 40 | 0.95 | 0.90–1.01 | 0.086 |
| Female vs. male | 16 vs. 24 | 1.70 | 0.73–3.96 | 0.22 |
| pT stage | ||||
| pT2 vs. pT1 | 22 vs. 5 | 4.91 | 0.62–39.22 | 0.133 |
| pT3 vs. pT1 | 13 vs. 5 | 3.24 | 0.39–26.86 | 0.277 |
| pN stage | ||||
| pN1 vs. pN0 | 14 vs. 26 | 2.94 | 1.30–6.69 | 0.01 ** |
| DFS–WTA | n | HR | 95% CI | p-Value |
|---|---|---|---|---|
| pDCs | 58 | 0.90 | 0.82–0.98 | 0.018 * |
| Age | 58 | 0.94 | 0.90–0.99 | 0.01 * |
| Female vs. male | 28 vs. 30 | 1.38 | 0.61–3.12 | 0.445 |
| pT stage | ||||
| pT2 vs. pT1 | 33 vs. 5 | 1.74 | 0.47–6.46 | 0.405 |
| pT3 vs. pT1 | 20 vs. 5 | 2.97 | 0.74–11.91 | 0.124 |
| pN stage | ||||
| pN1 vs. pN0 | 35 vs. 23 | 3.08 | 1.35–7.03 | 0.008 ** |
| OS–WTA | ||||
| pDCs | 58 | 0.95 | 0.89–1.0 | 0.135 |
| Age | 58 | 0.98 | 0.94–1.0 | 0.28 |
| Female vs. male | 28 vs. 30 | 1.21 | 0.60–2.4 | 0.587 |
| pT stage | ||||
| pT2 vs. pT1 | 33 vs. 5 | 4.81 | 0.63–36.9 | 0.13 |
| pT3 vs. pT1 | 20 vs. 5 | 4.87 | 0.61–38.8 | 0.135 |
| pN stage | ||||
| pN1 vs. pN0 | 35 vs. 23 | 3.23 | 1.65–6.3 | <0.001 *** |
| DFS–TS | n | HR | 95% CI | p Value |
|---|---|---|---|---|
| pDCs | 58 | 0.89 | 0.81–0.98 | 0.017 * |
| Age | 58 | 0.94 | 0.90–0.99 | 0.01 * |
| Female vs. male | 28 vs. 30 | 1.38 | 0.61–3.13 | 0.445 |
| pT stage | ||||
| pT2 vs. pT1 | 33 vs. 5 | 1.74 | 0.47–6.46 | 0.405 |
| pT3 vs. pT1 | 20 vs. 5 | 2.98 | 0.74–11.95 | 0.124 |
| pN stage | ||||
| pN1 vs. pN0 | 35 vs. 23 | 3.08 | 1.35–7.04 | 0.008 ** |
| OS–TS | ||||
| pDCs | 58 | 0.95 | 0.89–1.0 | 0.144 |
| Age | 58 | 0.98 | 0.94–1.0 | 0.276 |
| Female vs. male | 28 vs. 30 | 1.21 | 0.60–2.4 | 0.588 |
| pT stage | ||||
| pT2 vs. pT1 | 33 vs. 5 | 4.79 | 0.63–36.7 | 0.131 |
| pT3 vs. pT1 | 20 vs. 5 | 4.82 | 0.61–38.3 | 0.137 |
| pN stage | ||||
| pN1 vs. pN0 | 35 vs. 23 | 3.21 | 1.65–6.3 | <0.001 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plesca, I.; Benešová, I.; Beer, C.; Sommer, U.; Müller, L.; Wehner, R.; Heiduk, M.; Aust, D.; Baretton, G.; Bachmann, M.P.; et al. Clinical Significance of Tumor-Infiltrating Conventional and Plasmacytoid Dendritic Cells in Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 1216. https://doi.org/10.3390/cancers14051216
Plesca I, Benešová I, Beer C, Sommer U, Müller L, Wehner R, Heiduk M, Aust D, Baretton G, Bachmann MP, et al. Clinical Significance of Tumor-Infiltrating Conventional and Plasmacytoid Dendritic Cells in Pancreatic Ductal Adenocarcinoma. Cancers. 2022; 14(5):1216. https://doi.org/10.3390/cancers14051216
Chicago/Turabian StylePlesca, Ioana, Iva Benešová, Carolin Beer, Ulrich Sommer, Luise Müller, Rebekka Wehner, Max Heiduk, Daniela Aust, Gustavo Baretton, Michael P Bachmann, and et al. 2022. "Clinical Significance of Tumor-Infiltrating Conventional and Plasmacytoid Dendritic Cells in Pancreatic Ductal Adenocarcinoma" Cancers 14, no. 5: 1216. https://doi.org/10.3390/cancers14051216
APA StylePlesca, I., Benešová, I., Beer, C., Sommer, U., Müller, L., Wehner, R., Heiduk, M., Aust, D., Baretton, G., Bachmann, M. P., Feldmann, A., Weitz, J., Seifert, L., Seifert, A. M., & Schmitz, M. (2022). Clinical Significance of Tumor-Infiltrating Conventional and Plasmacytoid Dendritic Cells in Pancreatic Ductal Adenocarcinoma. Cancers, 14(5), 1216. https://doi.org/10.3390/cancers14051216