The Practicality of the Use of Liquid Biopsy in Early Diagnosis and Treatment Monitoring of Oral Cancer in Resource-Limited Settings
Abstract
:Simple Summary
Abstract
1. Introduction
2. Liquid Biopsy and Cancer Management
2.1. Circulating Cell-Free DNA (cfDNA) and Circulating Tumor DNA (ctDNA) in OSCC
2.2. Circulating Tumor Cells (CTCs) in OSCC
2.3. Exosomes in OSCC
2.4. Messenger Ribonucleic Acid in OSCC
2.5. Saliva as a Liquid Biopsy Substrate
2.6. Novel Molecular Techniques for Application of Liquid Biopsy
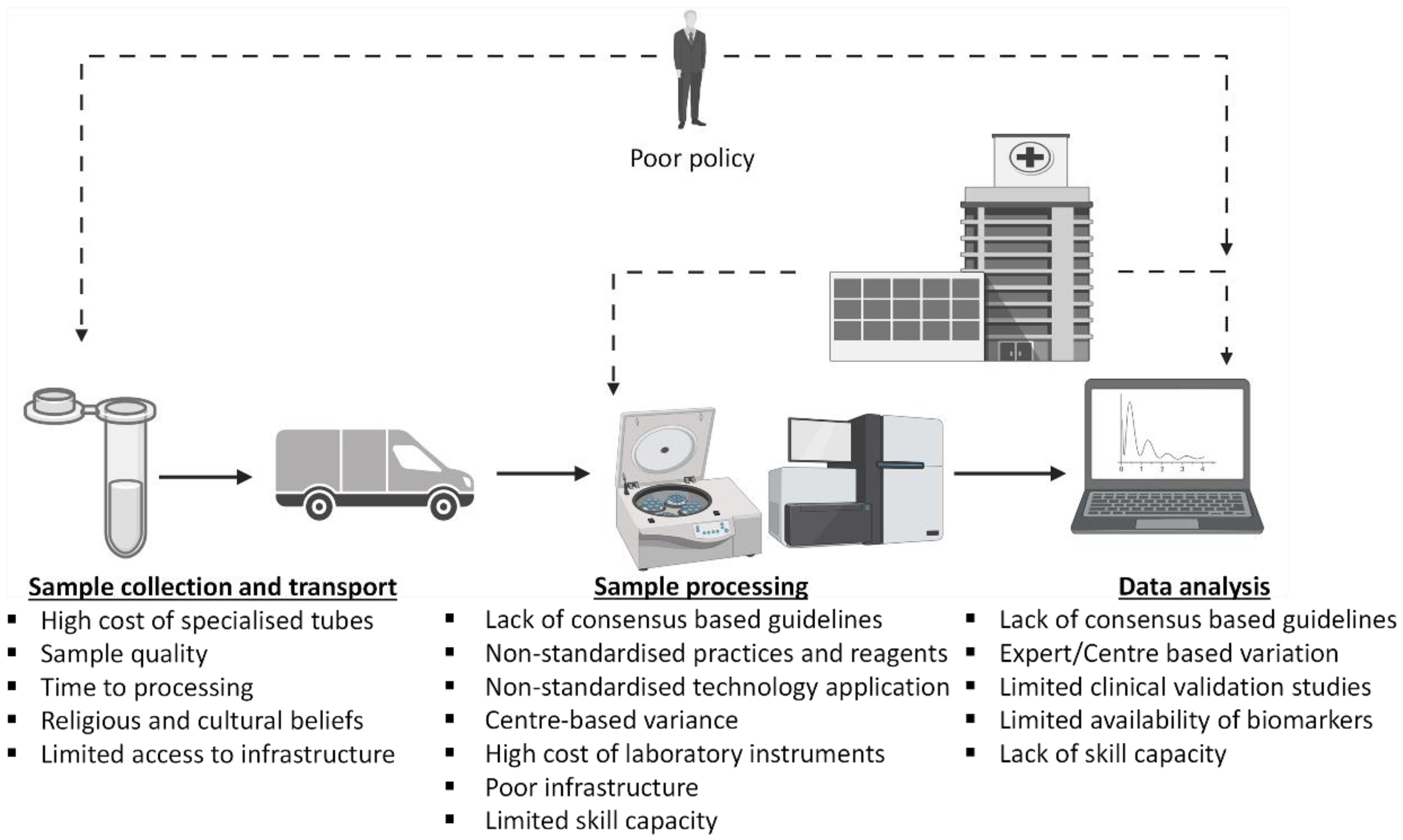
3. The Challenges of Utilizing Liquid Biopsy as an OSCC Diagnostic and Management Tool in Africa: Pre-Analytic, Analytic Processing, and Post-Analytic Processing Phase Considerations
3.1. Pre-Analytic Phase Considerations
3.2. Analytic Processing Phase Considerations
3.2.1. Next Generation Sequencing (NGS)
3.2.2. Digital Droplet PCR (ddPCR)
3.3. Post-Processing Quality Check, Data Analysis and Bioinformatics Considerations
4. Future Perspectives of Liquid Biopsy in Africa
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hulsen, T.; Jamuar, S.S.; Moody, A.R.; Karnes, J.H.; Varga, O.; Hedensted, S.; Spreafico, R.; Hafler, D.A.; McKinney, E.F. From Big Data to Precision Medicine. Front. Med. 2019, 6, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temilola, D.O.; Wium, M.; Coulidiati, T.H.; Adeola, H.A.; Carbone, G.M.; Catapano, C.V.; Zerbini, L.F. The Prospect and Challenges to the Flow of Liquid Biopsy in Africa. Cells 2019, 8, 862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. World Health Organization Regional Office for Africa Noncommunicable Diseases (NCD) Cluster; World Health Organization: Brazzaville, Congo, 2016. [Google Scholar]
- Van Zyl, A.W.; Marnewick, J. Aetiology of oral cancer: Clinical review. S. Afr. Dent. J. 2012, 67, 554–556. [Google Scholar]
- WHO. Early Cancer Diagnosis Saves Lives, Cuts Treatment Costs; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2013, 10, 472–484. [Google Scholar] [CrossRef] [PubMed]
- De Rubis, G.; Rajeev Krishnan, S.; Bebawy, M. Liquid Biopsies in Cancer Diagnosis, Monitoring, and Prognosis. Trends Pharmacol. Sci. 2019, 40, 172–186. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabieres, C. Liquid biopsy: Potential and challenges. Mol. Oncol. 2016, 10, 371–373. [Google Scholar] [CrossRef] [Green Version]
- Neumann, M.H.; Bender, S.; Krahn, T.; Schlange, T. ctDNA and CTCs in liquid biopsy–current status and where we need to progress. Comput. Struct. Biotechnol. J. 2018, 16, 190–195. [Google Scholar] [CrossRef]
- Lousada-Fernandez, F.; Rapado-Gonzalez, O.; Lopez-Cedrun, J.L.; Lopez-Lopez, R.; Muinelo-Romay, L.; Suarez-Cunqueiro, M.M. Liquid Biopsy in Oral Cancer. Int. J. Mol. Sci. 2018, 19, 1704. [Google Scholar] [CrossRef] [Green Version]
- Liskova, A.; Samec, M.; Koklesova, L.; Giordano, F.A.; Kubatka, P.; Golubnitschaja, O. Liquid Biopsy is Instrumental for 3PM Dimensional Solutions in Cancer Management. J. Clin. Med. 2020, 9, 2749. [Google Scholar] [CrossRef]
- Bronkhorst, A.J.; Ungerer, V.; Holdenrieder, S. The emerging role of cell-free DNA as a molecular marker for cancer management. Biomol. Detect. Quantif. 2019, 17, 100087. [Google Scholar] [CrossRef]
- Baby, N.T.; Abdullah, A.; Kannan, S. The scope of liquid biopsy in the clinical management of oral cancer. Int. J. Oral Maxillofac. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224ra224. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.H.; Chang, K.W.; Kao, S.Y.; Cheng, H.W.; Liu, C.J. Increased Plasma Circulating Cell-Free DNA Could Be a Potential Marker for Oral Cancer. Int. J. Mol. Sci. 2018, 19, 3303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Ginkel, J.H.; Huibers, M.M.H.; Noorlag, R.; de Bree, R.; van Es, R.J.; Willems, S.M. Liquid Biopsy: A Future Tool for Posttreatment Surveillance in Head and Neck Cancer? Pathobiol. J. Immunopathol. Mol. Cell. Biol. 2017, 84, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Galot, R.; Machiels, J.H. Current applications and challenges of circulating tumor DNA (ctDNA) in squamous cell carcinoma of the head and neck (SCCHN). Cancer Treat. Rev. 2020, 85, 101992. [Google Scholar] [CrossRef] [PubMed]
- Shohdy, K.S.; West, H.J. Circulating Tumor DNA Testing-Liquid Biopsy of a Cancer. JAMA Oncol. 2020, 6, 792. [Google Scholar] [CrossRef]
- Patel, S.; Shah, K.; Mirza, S.; Shah, K.; Rawal, R. Circulating tumor stem like cells in oral squamous cell carcinoma: An unresolved paradox. Oral. Oncol 2016, 62, 139–146. [Google Scholar] [CrossRef]
- Economopoulou, P.; Kotsantis, I.; Kyrodimos, E.; Lianidou, E.S.; Psyrri, A. Liquid biopsy: An emerging prognostic and predictive tool in Head and Neck Squamous Cell Carcinoma (HNSCC). Focus on Circulating Tumor Cells (CTCs). Oral Oncol. 2017, 74, 83–89. [Google Scholar] [CrossRef]
- Kaldjian, E.P.; Ramirez, A.B.; Sun, Y.; Campton, D.E.; Werbin, J.L.; Varshavskaya, P.; Quarre, S.; George, T.; Madan, A.; Blau, C.A.; et al. The RareCyte® platform for next-generation analysis of circulating tumor cells. Cytomet. Part A J. Int. Soc. Anal. Cytol. 2018, 93, 1220–1225. [Google Scholar] [CrossRef] [Green Version]
- Magbanua, M.J.M.; Park, J.W. Isolation and Molecular Characterization of Circulating Tumor Cells; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Partridge, M.B.R.; Phillips, E.; Ali, K.; Francis, R.; Hooper, R.; Lavery, K.; Brown, A.; Langdon, J. Detection of rare disseminated tumor cells identifies head and neck cancer patients at risk of treatment failure. Clin. Cancer Res 2003, 9, 5287–5294. [Google Scholar]
- Oliveira-Costa, J.P.; de Carvalho, A.F.; da Silveira da, G.G.; Amaya, P.; Wu, Y.; Park, K.-J.J.; Gigliola, M.P.; Lustberg, M.; Buim, M.E.C.; Ferreira, E.N.; et al. Gene expression patterns through oral squamous cell carcinoma development: PD-L1 expression in primary tumor and circulating tumor cells. Oncotarget 2015, 6, 20902–20920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inhestern, J.; Oertel, K.; Stemmann, V.; Schmalenberg, H.; Dietz, A.; Rotter, N.; Veit, J.; Görner, M.; Sudhoff, H.; Junghanß, C.; et al. Prognostic Role of Circulating Tumor Cells during Induction Chemotherapy Followed by Curative Surgery Combined with Postoperative Radiotherapy in Patients with Locally Advanced Oral and Oropharyngeal Squamous Cell Cancer. PLoS ONE 2015, 10, e0132901. [Google Scholar] [CrossRef] [PubMed]
- Kapeleris, J.; Kulasinghe, A.; Warkiani, M.E.; Vela, I.; Kenny, L.; O’Byrne, K.; Punyadeera, C. The Prognostic Role of Circulating Tumor Cells (CTCs) in Lung Cancer. Front. Oncol. 2018, 8, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanos, R.; Thierry, A.R. Clinical relevance of liquid biopsy for cancer screening. Transl. Cancer Res. 2018, 7, S105–S129. [Google Scholar] [CrossRef]
- Alix-Panabieres, C.; Pantel, K. Real-time liquid biopsy: Circulating tumor cells versus circulating tumor DNA. Ann. Transl. Med. 2013, 1, 18. [Google Scholar]
- Gao, Y.; Zhu, Y.; Yuan, Z. Circulating Tumor Cells and Circulating Tumor DNA Provide New Insights into Pancreatic Cancer. Int. J. Med. Sci. 2016, 13, 902–913. [Google Scholar] [CrossRef] [Green Version]
- Heitzer, E.; Perakis, S.; Geigl, J.B.; Speicher, M.R. The potential of liquid biopsies for the early detection of cancer. NPJ Precis. Oncol. 2017, 1, 36. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, C.; Wang, S.; Wang, Z.; Jiang, J.; Wang, W.; Li, X.; Chen, J.; Liu, K.; Li, C.; et al. Exosomes Derived from Hypoxic Oral Squamous Cell Carcinoma Cells Deliver miR-21 to Normoxic Cells to Elicit a Prometastatic Phenotype. Cancer Res. 2016, 76, 1770–1780. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.; Wang, S.S.; Zhang, M.; Jiang, J.; Fan, H.; Wu, J.; Wang, H.; Liang, X.; Tang, Y. OSCC cell-secreted exosomal CMTM6 induced M2-like macrophages polarization via ERK1/2 signaling pathway. Cancer Immunol. Immunother. 2021, 70, 1015–1029. [Google Scholar] [CrossRef]
- Mori, K.; Hiroi, M.; Shimada, J.; Ohmori, Y. Infiltration of m2 tumor-associated macrophages in oral squamous cell carcinoma correlates with tumor malignancy. Cancers 2011, 3, 3726–3739. [Google Scholar] [CrossRef]
- Lu, Y.; Zheng, Z.; Yuan, Y.; Pathak, J.L.; Yang, X.; Wang, L.; Ye, Z.; Cho, W.C.; Zeng, M.; Wu, L. The Emerging Role of Exosomes in Oral Squamous Cell Carcinoma. Front. Cell Dev. Biol. 2021, 9, 628103. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Kang, S.M.; Kang, S.H.; Lee, H.J.; Kwon, T.; Kim, J.; Lee, S.; Choi, S.; Hong, S. Potential Salivary mRNA Biomarkers for Early Detection of Oral Cancer. J. Clin. Med. 2020, 9, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.C.; Chang, J.T.; Huang, Y.C.; Huang, C.C.; Chen, W.-H.; Lee, L.-Y.; Huang, B.-S.; Chen, Y.-J.; Li, H.-F.; Cheng, A.-J. Combined determination of circulating miR-196a and miR-196b levels produces high sensitivity and specificity for early detection of oral cancer. Clin. Biochem. 2015, 48, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Kao, S.Y.; Tu, H.F.; Tsai, M.M.; Chang, K.W.; Lin, S.C. Increase of microRNA miR-31 level in plasma could be a potential marker of oral cancer. Oral Dis. 2010, 16, 360–364. [Google Scholar] [CrossRef]
- Cristaldi, M.; Mauceri, R.; Di Fede, O.; Giuliana, G.; Campisi, G.; Panzarella, V. Salivary Biomarkers for Oral Squamous Cell Carcinoma Diagnosis and Follow-Up: Current Status and Perspectives. Front. Physiol. 2019, 10, 1476. [Google Scholar] [CrossRef]
- Cheng, J.; Nonaka, T.; Ye, Q.; Wei, F.; Wong, D.T. Salivaomics, saliva-exosomics, and saliva liquid biopsy. Salivary Biosci. 2020, 157–175. [Google Scholar]
- Adeola, H.A.; Holmes, H.; Temilola, D.O. Diagnostic Potential of Salivary Exosomes in Oral Cancer. In Oral Cancer—Current Concepts and Future Perspectives; Sridharan, G., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Babiuch, K.; Kuśnierz-Cabala, B.; Kęsek, B.; Okoń, K.; Darczuk, D.; Chomyszyn-Gajewska, M. Evaluation of proinflammatory, NF-kappaB dependent cytokines: IL-1α, IL-6, IL-8, and TNF-α in tissue specimens and saliva of patients with oral squamous cell carcinoma and oral potentially malignant disorders. J. Clin. Med. 2020, 9, 867. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, T.; Taha, E.A. Extracellular Vesicle-associated Moonlighting Proteins: Heat Shock Proteins and Metalloproteinases. In Heat Shock Proteins; Asea, A.A.A., Kaur, P., Eds.; Springer: Dordrecht, The Netherlands, 2020; pp. 1–18. [Google Scholar]
- Tsai, C.K.; Lin, C.Y.; Kang, C.J.; Liao, C.T.; Wang, W.-L.; Chiang, M.-H.; Yen, T.-C.; Lin, G. Nuclear Magnetic Resonance Metabolomics Biomarkers for Identifying High Risk Patients with Extranodal Extension in Oral Squamous Cell Carcinoma. J. Clin. Med. 2020, 9, 951. [Google Scholar] [CrossRef] [Green Version]
- Iwai, K.; Minamisawa, T.; Suga, K.; Yajima, Y.; Shiba, K. Isolation of human salivary extracellular vesicles by iodixanol density gradient ultracentrifugation and their characterizations. J. Extracell Vesicles 2016, 5, 30829. [Google Scholar] [CrossRef] [Green Version]
- Ono, K.; Eguchi, T.; Sogawa, C.; Calderwood, S.K.; Futagawa, J.; Kasai, T.; Seno, M.; Okamoto, K.; Sasaki, A.; Kozaki, K. HSP-enriched properties of extracellular vesicles involve survival of metastatic oral cancer cells. J. Cell Biochem. 2018, 119, 7350–7362. [Google Scholar] [CrossRef] [Green Version]
- Ono, K.; Sogawa, C.; Kawai, H.; Tran, M.T.; Taha, E.A.; Lu, Y.; Oo, M.W.; Okusha, Y.; Okamura, H.; Ibaragi, S. Triple knockdown of CDC37, HSP90-alpha and HSP90-beta diminishes extracellular vesicles-driven malignancy events and macrophage M2 polarization in oral cancer. J. Extracell Vesicles 2020, 9, 1769373. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Sogawa, C.; Ono, K.; Matsumoto, M.; Tran, M.T.; Okusha, Y.; Lang, B.J.; Okamoto, K.; Calderwood, S.K. Cell Stress Induced Stressome Release Including Damaged Membrane Vesicles and Extracellular HSP90 by Prostate Cancer Cells. Cells 2020, 9, 755. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, T.; Eguchi, T.; Sogawa, C.; Ono, K.; Murakami, J.; Ibaragi, S.; Asaumi, J.-I.; Calderwood, S.K.; Okamoto, K.; Kozaki, K.-I. Carcinogenic epithelial-mesenchymal transition initiated by oral cancer exosomes is inhibited by anti-EGFR antibody cetuximab. Oral Oncol. 2018, 86, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Machida, T.; Tomofuji, T.; Ekuni, D.; Maruyama, T.; Yoneda, T.; Kawabata, Y.; Mizuno, H.; Miyai, H.; Kunitomo, M.; Morita, M. MicroRNAs in Salivary Exosome as Potential Biomarkers of Aging. Int. J. Mol. Sci. 2015, 16, 21294–21309. [Google Scholar] [CrossRef] [Green Version]
- Fujimori, K.; Yoneda, T.; Tomofuji, T.; Ekuni, D.; Azuma, T.; Maruyama, T.; Mizuno, H.; Sugiura, Y.; Morita, M. Detection of Salivary miRNAs Reflecting Chronic Periodontitis: A Pilot Study. Molecules 2019, 24, 1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Tomofuji, T.; Machida, T.; Yoneda, T.; Ekuni, D.; Azuma, T.; Maruyama, T.; Hirose, A.; Morita, M. Expression of Salivary miR-203a-3p Was Related with Oral Health-Related Quality of Life in Healthy Volunteers. Int. J. Mol. Sci. 2017, 18, 1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spafford, M.F.; Koch, W.M.; Reed, A.L.; Califano, J.A.; Xu, L.H.; Eisenberger, C.F.; Yip, L.; Leong, P.L.; Wu, L.; Liu, S.X.; et al. Detection of head and neck squamous cell carcinoma among exfoliated oral mucosal cells by microsatellite analysis. Clin. Cancer Res. 2001, 7, 607–612. [Google Scholar]
- Kong, L.; Birkeland, A.C. Liquid Biopsies in Head and Neck Cancer: Current State and Future Challenges. Cancers 2021, 13, 1874. [Google Scholar] [CrossRef] [PubMed]
- Babayan, A.; Pantel, K. Advances in liquid biopsy approaches for early detection and monitoring of cancer. Genome Med. 2018, 10, 21. [Google Scholar] [CrossRef]
- Aro, K.; Wei, F.; Wong, D.T.; Tu, M. Saliva Liquid Biopsy for Point-of-Care Applications. Front. Public Health 2017, 5, 77. [Google Scholar] [CrossRef] [Green Version]
- Pantel, K.; Brakenhoff, R.H.; Brandt, B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat. Rev. Cancer 2008, 8, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Manicone, M.; Poggiana, C.; Facchinetti, A.; Zamarchi, R. Critical issues in the clinical application of liquid biopsy in non-small cell lung cancer. J. Thorac. Dis. 2017, 9, S1346–S1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geeurickx, E.; Hendrix, A. Targets, pitfalls and reference materials for liquid biopsy tests in cancer diagnostics. Mol. Asp. Med. 2020, 72, 100828. [Google Scholar] [CrossRef]
- Sisson, B.A.; Uvalic, J.; Kelly, K.; Selvam, P.; Ananda, G.; Chandok, H.; Bergeron, D.; Holinka, L.; Reddi, H.V. Technical and Regulatory Considerations for Taking Liquid Biopsy to the Clinic: Validation of the JAX PlasmaMonitor(TM) Assay. Biomark. Insights 2019, 14, 1177271919826545. [Google Scholar] [CrossRef] [Green Version]
- Shankar, G.M.; Balaj, L.; Stott, S.L.; Nahed, B.; Carter, B.S. Liquid biopsy for brain tumors. Expert Rev. Mol. Diagn. 2017, 17, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Ardington, C.; Lam, D.; Leibbrandt, M.; Welch, M. The sensitivity to key data imputations of recent estimates of income poverty and inequality in South Africa. Econ. Model. 2006, 23, 822–835. [Google Scholar] [CrossRef] [Green Version]
- Adeola, H.A.; Blackburn, J.M.; Rebbeck, T.R.; Zerbini, L.F. Emerging proteomics biomarkers and prostate cancer burden in Africa. Oncotarget 2017, 8, 37991–38007. [Google Scholar] [CrossRef] [Green Version]
- Green, S.F. The cost of poor blood specimen quality and errors in preanalytical processes. Clin. Biochem. 2013, 46, 1175–1179. [Google Scholar] [CrossRef]
- Salvianti, F.; Gelmini, S.; Costanza, F.; Mancini, I.; Sonnati, G.; Simi, L.; Pazzagli, M.; Pinzani, P. The pre-analytical phase of the liquid biopsy. New Biotechnol. 2020, 55, 19–29. [Google Scholar] [CrossRef]
- Page, K.; Shaw, J.A.; Guttery, D.S. The liquid biopsy: Towards standardisation in preparation for prime time. Lancet Oncol. 2019, 20, 758–760. [Google Scholar] [CrossRef] [Green Version]
- Karachaliou, N.; Mayo-de las Casas, C.; Queralt, C.; de Aguirre, I.; Melloni, B.; Cardenal, F.; Garcia-Gomez, R.; Massuti, B.; Sánchez, J.M. Ruth Porta Association of EGFR L858R Mutation in Circulating Free DNA With Survival in the EURTAC Trial. JAMA Oncol. 2015, 1, 149–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arechederra, M.; Ávila, M.A.; Berasain, C. Liquid biopsy for cancer management: A revolutionary but still limited new tool for precision medicine. Adv. Lab. Med. Av. Med. Lab. 2020, 1, 20200009. [Google Scholar] [CrossRef]
- Merker, J.D.; Oxnard, G.R.; Compton, C.; Diehn, M.; Hurley, P.; Lazar, A.; Lindeman, N.; Lockwood, C.; Rai, A.J.; Schilsky, R.L.; et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists joint review. Arch. Pathol. Lab. Med. 2018, 142, 1242–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, T.-D.; Kim, M.H.; Park, S.; Chung, H.-S.; Lee, J.W.; Chang, J.H.; Huh, J. Effects of pre-analytical variables on cell-free DNA extraction for liquid biopsy. Lab. Med. Online 2019, 9, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Franczak, C.; Filhine-Tresarrieu, P.; Gilson, P.; Merlin, J.L.; Au, L.; Harlé, A. Technical considerations for circulating tumor DNA detection in oncology. Expert Rev. Mol. Diagn. 2019, 19, 121–135. [Google Scholar] [CrossRef]
- Medina Diaz, I.; Nocon, A.; Mehnert, D.H.; Fredebohm, J.; Diehl, F.; Holtrup, F. Performance of Streck cfDNA Blood Collection Tubes for Liquid Biopsy Testing. PLoS ONE 2016, 11, e0166354. [Google Scholar]
- Sorber, L.; Zwaenepoel, K.; Jacobs, J.; De Winne, K.; Van Casteren, K.; Augustus, E.; Lardon, F.; Prenen, H.; Peeters, M.; Van Meerbeeck, J.; et al. Specialized Blood Collection Tubes for Liquid Biopsy: Improving the Pre-analytical Conditions. Mol. Diagn. Ther. 2020, 24, 113–124. [Google Scholar] [CrossRef]
- Thierry, A.R.; Mouliere, F.; El Messaoudi, S.; Mollevi, C.; Lopez-Crapez, E.; Rolet, F.; Gillet, B.; Gongora, C.; Dechelotte, P.; Robert, B.; et al. Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat. Med. 2014, 20, 430–435. [Google Scholar] [CrossRef]
- Gobbini, E.; Swalduz, A.; Levra, M.G.; Ortiz-Cuaran, S.; Toffart, A.-C.; Pérol, M.; Moro-Sibilot, D.; Saintigny, P. Implementing ctDNA Analysis in the Clinic: Challenges and Opportunities in Non-Small Cell Lung Cancer. Cancers 2020, 12, 3112. [Google Scholar] [CrossRef]
- Torga, G.; Pienta, K.J. Patient-Paired Sample Congruence Between 2 Commercial Liquid Biopsy Tests. JAMA Oncol. 2018, 4, 868–870. [Google Scholar] [CrossRef]
- Esagian, S.M.; Grigoriadou, G.; Nikas, I.P.; Boikou, V.; Sadow, P.M.; Won, J.-K.; Economopoulos, K.P. Comparison of liquid-based to tissue-based biopsy analysis by targeted next generation sequencing in advanced non-small cell lung cancer: A comprehensive systematic review. J. Cancer Res. Clin. Oncol. 2020, 146, 2051–2066. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.J.; Arcila, M.E.; Corless, C.; Kamel-Reid, S.; Lubin, I.M.; Pfeifer, J.; Temple-Smolkin, R.L.; Voelkerding, K.V.; Nikiforova, M.N. Guidelines for validation of next-generation sequencing–based oncology panels: A joint consensus recommendation of the Association for Molecular Pathology and College of American Pathologists. J. Mol. Diagn. 2017, 19, 341–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrackova, A.; Vasinek, M.; Sedlarikova, L.; Dyskova, T.; Schneiderova, P.; Novosad, T.; Papajik, T.; Kriegova, E. Standardization of Sequencing Coverage Depth in NGS: Recommendation for Detection of Clonal and Subclonal Mutations in Cancer Diagnostics. Front. Oncol. 2019, 9, 851. [Google Scholar] [CrossRef]
- Milosevic, D.; Mills, J.R.; Campion, M.B.; Vidal-Folch, N.; Voss, J.S.; Halling, K.C.; Highsmith, W.E.; Liu, M.C.; Kipp, B.R.; Grebe, S.K. Applying Standard Clinical Chemistry Assay Validation to Droplet Digital PCR Quantitative Liquid Biopsy Testing. Clin. Chem. 2018, 64, 1732–1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Combaret, V.; Iacono, I.; Bellini, A.; Brejon, S.; Bernard, V.; Marabelle, A.; Coze, C.; Pierron, G.; Lapouble, E.; Schleiermacher, G.; et al. Detection of tumor ALK status in neuroblastoma patients using peripheral blood. Cancer Med. 2015, 4, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Lodrini, M.; Sprussel, A.; Astrahantseff, K.; Tiburtius, D.; Konschak, R.; Lode, H.; Fischer, M.; Keilholz, U.; Eggert, A.; Deubzer, H.E. Using droplet digital PCR to analyze MYCN and ALK copy number in plasma from patients with neuroblastoma. Oncotarget 2017, 8, 85234–85251. [Google Scholar] [CrossRef] [Green Version]
- Esposito, A.; Criscitiello, C.; Locatelli, M.; Milano, M.; Curigliano, G. Liquid biopsies for solid tumors: Understanding tumor heterogeneity and real time monitoring of early resistance to targeted therapies. Pharmacol. Ther. 2016, 157, 120–124. [Google Scholar] [CrossRef]
- Ishengoma, D.S.; Saidi, Q.; Sibley, C.H.; Roper, C.; Alifrangis, M. Deployment and utilization of next-generation sequencing of Plasmodium falciparum to guide anti-malarial drug policy decisions in sub-Saharan Africa: Opportunities and challenges. Malar. J. 2019, 18, 267. [Google Scholar] [CrossRef] [Green Version]
- Bacher, U.; Shumilov, E.; Flach, J.; Porret, N.; Oncourt, R.; Wiedemann, G.; Fiedler, M.; Novak, U.; Amstutz, U.; Pabst, T. Challenges in the introduction of next-generation sequencing (NGS) for diagnostics of myeloid malignancies into clinical routine use. Blood Cancer J. 2018, 8, 113. [Google Scholar] [CrossRef] [Green Version]
- Swerdlow, S.H.; Campo, E.; Harris Lee, N.; Jaffe, E.S.; Pileri, S.A.; Stein, H. WHO Classification of Tumours. Tumours of Haematopoietic and Lymphoid Tissues; IARC: Lyon, France, 2017. [Google Scholar]
- Asante, D.-B.; Calapre, L.; Ziman, M.; Meniawy, T.M.; Gray, E.S. Liquid biopsy in ovarian cancer using circulating tumor DNA and cells: Ready for prime time? Cancer Lett. 2020, 468, 59–71. [Google Scholar] [CrossRef]
- Aparicio-Puerta, E.; Jáspez, D.; Lebrón, R.; Koppers-Lalic, D.; Marchal, J.A.; Hackenberg, M. liqDB: A small-RNAseq knowledge discovery database for liquid biopsy studies. Nucleic Acids Res. 2019, 47, D113–D120. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Wu, X.; Li, T.; Luo, J.; Dong, D. ctcRbase: The gene expression database of circulating tumor cells and microemboli. Database 2020, 2020, baaa020. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liu, X.; Zheng, B.; Ke, R.; Tzeng, C.M. Liquid Biopsy, ctDNA Diagnosis through NGS. Life 2021, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Grossman, R.L.; Dry, J.R.; Hanlon, S.E.; Johann, D.J.; Kolatkar, A.; Lee, J.S.H.; Meyer, C.; Salvatore, L.; Wells, W.; Leiman, L. BloodPAC Data Commons for Liquid Biopsy Data. JCO Clin. Cancer Inform. 2021, 5, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xie, S.; Lu, C.; Zhu, L.; Zhu, L. Application of Data Science in Circulating Tumor DNA Detection: A Promising Avenue Towards Liquid Biopsy. Front. Oncol. 2021, 11, 692322. [Google Scholar] [CrossRef]
| Study | Country | Type of Cancer | Liquid Biopsy Technology | Publication Type | Reference |
|---|---|---|---|---|---|
| Lousada-Fernandez et al., 2018 | Spain | Oral cancer | Liquid Biopsy | Review | [10] |
| Lin et al., 2018 | Taiwan | Oral Cancer | Cell-Free DNA Biomarker | Original Research | [15] |
| Patel et al., 2016 | India | Oral Squamous Cell Carcinoma | Circulating Tumor cells | Original Research | [19] |
| Economopoulou et al., 2017 | Greece | Head and Neck Squamous Cell Carcinoma | Circulating Tumor Cells | Review | [20] |
| Oliveira-Costa et al., 2015 | Brazil | Oral Squamous Cell Carcinoma | Circulating Tumor Cells | Original Research | [24] |
| Inhestern et al., 2015 | Germany | Oral and Oropharyngeal Squamous Cell Cancer | Circulating Tumor Cells | Original Research | [25] |
| Li et al., 2016 | China | Oral Squamous Cell Carcinoma | Exosomes | Original Research | [31] |
| Pang et al., 2021 | China | Oral Squamous Cell Carcinoma | Exosomes | Original Research | [32] |
| Lu et al., 2021 | China | Oral Squamous Cell Carcinoma | Exosomes | Review | [34] |
| Oh et al., 2020 | South Korea | Oral Cancer | Salivary mRNA Biomarker | Original Research | [35] |
| Lu et al., 2015 | Taiwan | Oral Cancer | Circulating miRNA Biomarker | Original Research | [36] |
| Liu et al., 2010 | Taiwan | Oral Cancer | Circulating miRNA Biomarker | Original Research | [37] |
| Cristaldi et al., 2019 | Italy | Oral Squamous Cell Carcinoma | Salivary Biomarker (ctDNA, EVs and miRNAs) | Review | [38] |
| Adeola et al., 2020 | South Africa | Oral Cancer | Salivary Exosomes Biomarker | Review | [40] |
| Tsai et al., 2020 | Taiwan | Oral Squamous Cell Carcinoma | Nuclear Magnetic Resonance Metabolomics Biomarker | Original Research | [43] |
| Ono et al., 2018 | Japan | Oral Cancer | HSP-Enriched Properties of Extracellular Vesicles | Original Research | [45] |
| Fujiwara et al., 2018 | Japan | Oral Cancer | Exosomes | Original Research | [48] |
| Spafford, et al., 2001 | USA | Head and Neck Squamous Cell Carcinoma | Pretreatment Oral Rinse Microsatellite Analysis. | Original Research | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeola, H.A.; Bello, I.O.; Aruleba, R.T.; Francisco, N.M.; Adekiya, T.A.; Adefuye, A.O.; Ikwegbue, P.C.; Musaigwa, F. The Practicality of the Use of Liquid Biopsy in Early Diagnosis and Treatment Monitoring of Oral Cancer in Resource-Limited Settings. Cancers 2022, 14, 1139. https://doi.org/10.3390/cancers14051139
Adeola HA, Bello IO, Aruleba RT, Francisco NM, Adekiya TA, Adefuye AO, Ikwegbue PC, Musaigwa F. The Practicality of the Use of Liquid Biopsy in Early Diagnosis and Treatment Monitoring of Oral Cancer in Resource-Limited Settings. Cancers. 2022; 14(5):1139. https://doi.org/10.3390/cancers14051139
Chicago/Turabian StyleAdeola, Henry Ademola, Ibrahim O. Bello, Raphael Taiwo Aruleba, Ngiambudulu M. Francisco, Tayo Alex Adekiya, Anthonio Oladele Adefuye, Paul Chukwudi Ikwegbue, and Fungai Musaigwa. 2022. "The Practicality of the Use of Liquid Biopsy in Early Diagnosis and Treatment Monitoring of Oral Cancer in Resource-Limited Settings" Cancers 14, no. 5: 1139. https://doi.org/10.3390/cancers14051139
APA StyleAdeola, H. A., Bello, I. O., Aruleba, R. T., Francisco, N. M., Adekiya, T. A., Adefuye, A. O., Ikwegbue, P. C., & Musaigwa, F. (2022). The Practicality of the Use of Liquid Biopsy in Early Diagnosis and Treatment Monitoring of Oral Cancer in Resource-Limited Settings. Cancers, 14(5), 1139. https://doi.org/10.3390/cancers14051139







