Machine Learning Model to Stratify the Risk of Lymph Node Metastasis for Early Gastric Cancer: A Single-Center Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
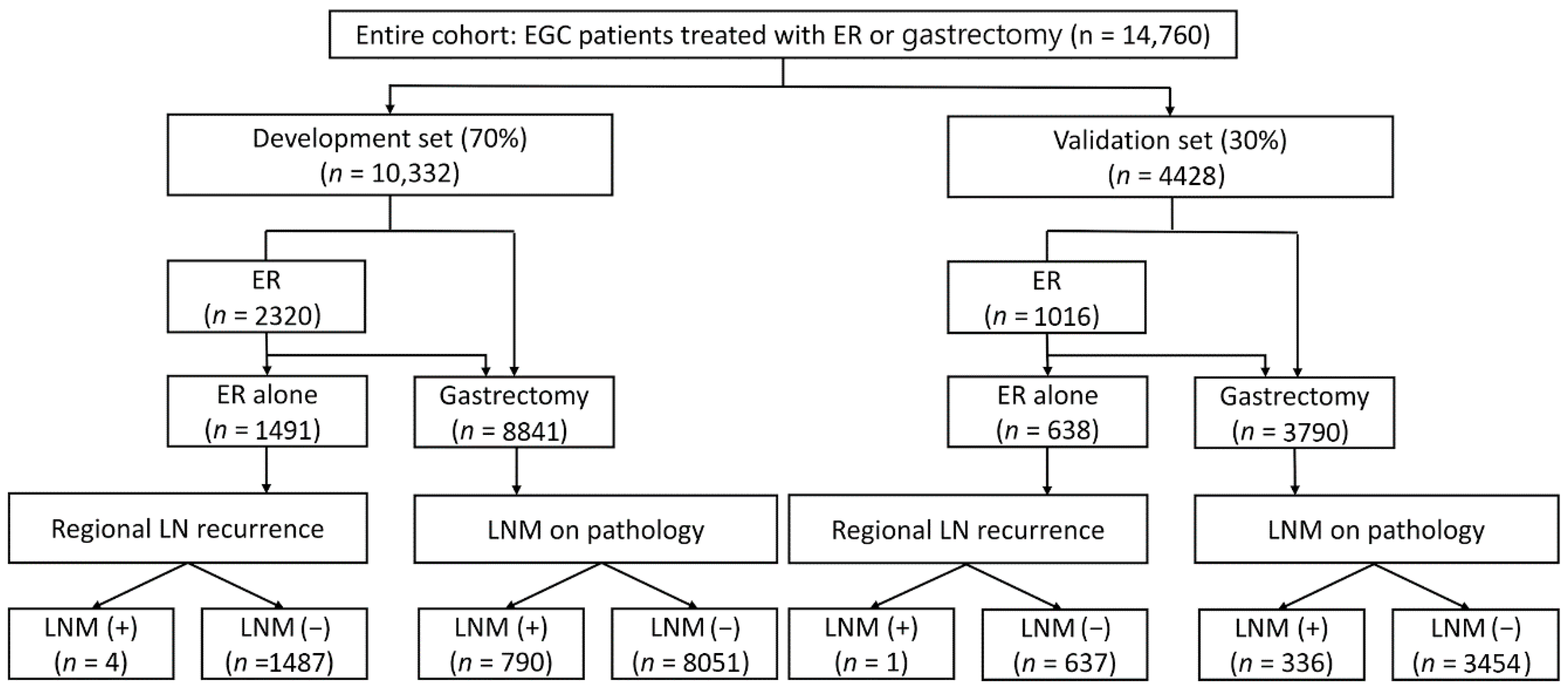
2.1. Patients
2.2. Definition, Outcome, Data Sources, and Study Variables
2.3. Establishment of the Machine Learning Model
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
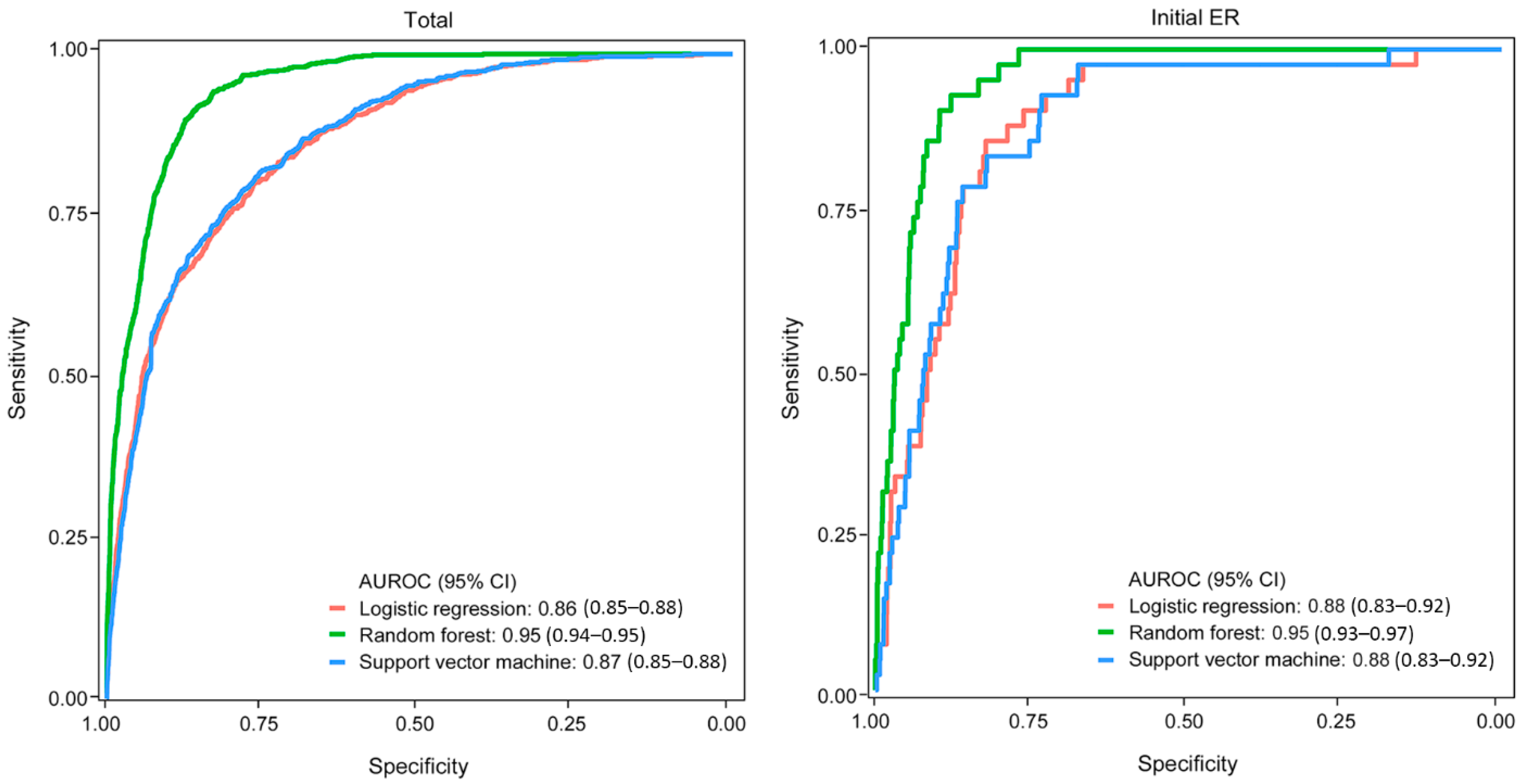
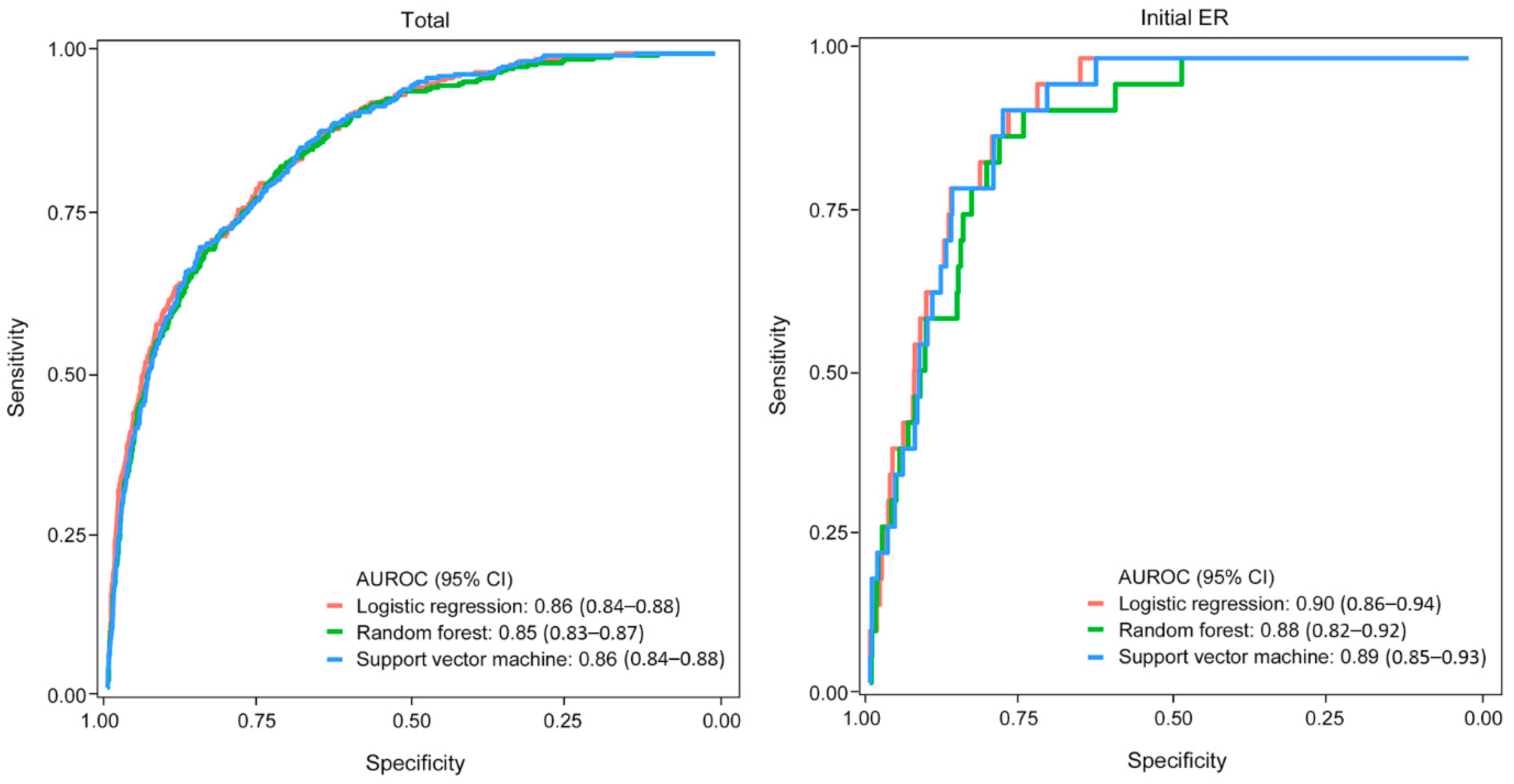
3.2. Derivation of the Machine Learning Model
3.3. Validation of the Machine Learning Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, C.H.; Yang, D.H.; Kim, J.W.; Kim, J.H.; Kim, J.H.; Min, Y.W.; Lee, S.H.; Bae, J.H.; Chung, H.; Choi, K.D.; et al. Clinical Practice Guideline for Endoscopic Resection of Early Gastrointestinal Cancer. Clin. Endosc. 2020, 53, 142–166. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draganov, P.V.; Wang, A.Y.; Othman, M.O.; Fukami, N. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin. Gastroenterol. Hepatol. 2019, 17, 16–25.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Ponchon, T.; Repici, A.; Vieth, M.; De Ceglie, A.; Amato, A.; Berr, F.; Bhandari, P.; Bialek, A.; et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015, 47, 829–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, S.; Gotoda, T.; Hatta, W.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Nakagawa, M.; Hirano, M.; et al. Survival Benefit of Additional Surgery after Non-Curative Endoscopic Submucosal Dissection for Early Gastric Cancer: A Propensity Score Matching Analysis. Ann. Surg. Oncol. 2017, 24, 3353–3360. [Google Scholar] [CrossRef]
- Li, D.; Luan, H.; Wang, S.; Zhou, Y. Survival benefits of additional surgery after non-curative endoscopic resection in patients with early gastric cancer: A meta-analysis. Surg. Endosc. 2019, 33, 711–716. [Google Scholar] [CrossRef]
- Hoteya, S.; Iizuka, T.; Kikuchi, D.; Ogawa, O.; Mitani, T.; Matsui, A.; Furuhata, T.; Yamashita, S.; Yamada, A.; Kaise, M. Clinicopathological Outcomes of Patients with Early Gastric Cancer after Non-Curative Endoscopic Submucosal Dissection. Digestion 2016, 93, 53–58. [Google Scholar] [CrossRef]
- Hatta, W.; Gotoda, T.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Nakamura, K.; Hirano, M.; Esaki, M.; et al. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? A multi-center retrospective study in Japan. J. Gastroenterol. 2017, 52, 175–184. [Google Scholar] [CrossRef]
- Suzuki, H.; Oda, I.; Abe, S.; Sekiguchi, M.; Nonaka, S.; Yoshinaga, S.; Saito, Y.; Fukagawa, T.; Katai, H. Clinical outcomes of early gastric cancer patients after noncurative endoscopic submucosal dissection in a large consecutive patient series. Gastric Cancer 2017, 20, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.J.; Kim, S.G.; Lim, J.H.; Choi, J.; Im, J.P.; Kim, J.S.; Kim, W.H.; Jung, H.C. Predictors of lymph node metastasis in patients with non-curative endoscopic resection of early gastric cancer. Surg. Endosc. 2015, 29, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Hatta, W.; Gotoda, T.; Koike, T.; Masamune, A. History and future perspectives in Japanese guidelines for endoscopic resection of early gastric cancer. Dig. Endosc. 2020, 32, 180–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatta, W.; Gotoda, T.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Nakagawa, M.; Hirano, M.; Esaki, M.; et al. A Scoring System to Stratify Curability after Endoscopic Submucosal Dissection for Early Gastric Cancer: “eCura system”. Am. J. Gastroenterol. 2017, 112, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Niwa, H.; Ozawa, R.; Kurahashi, Y.; Kumamoto, T.; Nakanishi, Y.; Okumura, K.; Matsuda, I.; Ishida, Y.; Hirota, S.; Shinohara, H. The eCura system as a novel indicator for the necessity of salvage surgery after non-curative ESD for gastric cancer: A case-control study. PLoS ONE 2018, 13, e0204039. [Google Scholar] [CrossRef]
- Hatta, W.; Gotoda, T.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Nakagawa, M.; Hirano, M.; Esaki, M.; et al. Is the eCura system useful for selecting patients who require radical surgery after noncurative endoscopic submucosal dissection for early gastric cancer? A comparative study. Gastric Cancer 2018, 21, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Song, K.Y.; Lee, H.J.; Han, S.U.; Hyung, W.J.; Cho, G.S. The impact of comorbidity on surgical outcomes in laparoscopy-assisted distal gastrectomy: A retrospective analysis of multicenter results. Ann. Surg. 2008, 248, 793–799. [Google Scholar] [CrossRef]
- Kunisaki, C.; Makino, H.; Takagawa, R.; Sato, K.; Kawamata, M.; Kanazawa, A.; Yamamoto, N.; Nagano, Y.; Fujii, S.; Ono, H.A.; et al. Predictive factors for surgical complications of laparoscopy-assisted distal gastrectomy for gastric cancer. Surg. Endosc. 2009, 23, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.N.; Das, D.; Turrentine, F.E.; Bauer, T.W.; Adams, R.B.; Zaydfudim, V.M. Morbidity and Mortality after Gastrectomy: Identification of Modifiable Risk Factors. J. Gastrointest. Surg. 2016, 20, 1554–1564. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.W.; Kim, Y.W.; Lee, J.H.; Nam, B.H.; Kook, M.C.; Choi, I.J.; Bae, J.M. Surgical complications and the risk factors of laparoscopy-assisted distal gastrectomy in early gastric cancer. Ann. Surg. Oncol. 2008, 15, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Kurita, N.; Miyata, H.; Gotoh, M.; Shimada, M.; Imura, S.; Kimura, W.; Tomita, N.; Baba, H.; Kitagawa, Y.; Sugihara, K.; et al. Risk Model for Distal Gastrectomy When Treating Gastric Cancer on the Basis of Data from 33,917 Japanese Patients Collected Using a Nationwide Web-Based Data Entry System. Ann. Surg. 2015, 262, 295–303. [Google Scholar] [CrossRef]
- Watanabe, M.; Miyata, H.; Gotoh, M.; Baba, H.; Kimura, W.; Tomita, N.; Nakagoe, T.; Shimada, M.; Kitagawa, Y.; Sugihara, K.; et al. Total gastrectomy risk model: Data from 20,011 Japanese patients in a nationwide internet-based database. Ann. Surg. 2014, 260, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, H.J.; Oh, S.Y.; Park, S.H.; Berlth, F.; Son, Y.G.; Kim, T.H.; Huh, Y.J.; Yang, J.Y.; Lee, K.G.; et al. Prediction of Postoperative Mortality in Patients with Organ Failure after Gastric Cancer Surgery. World J. Surg. 2020, 44, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Yoo, S.H.; Sunwoo, S.; Yoo, M.W. Management of long-term gastric cancer survivors in Korea. J. Korean Med. Assoc. 2016, 59, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.W.; Suh, B.; Lim, H.; Suh, Y.S.; Choi, Y.J.; Jeong, S.M.; Yun, J.M.; Song, S.O.; Park, Y. Increased Risk of Osteoporotic Fracture in Postgastrectomy Gastric Cancer Survivors Compared with Matched Controls: A Nationwide Cohort Study in Korea. Am. J. Gastroenterol. 2019, 114, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Kudo, S.E.; Ichimasa, K.; Villard, B.; Mori, Y.; Misawa, M.; Saito, S.; Hotta, K.; Saito, Y.; Matsuda, T.; Yamada, K.; et al. Artificial Intelligence System to Determine Risk of T1 Colorectal Cancer Metastasis to Lymph Node. Gastroenterology 2021, 160, 1075–1084.e2. [Google Scholar] [CrossRef] [PubMed]
- Labianca, R.; Nordlinger, B.; Beretta, G.D.; Mosconi, S.; Mandala, M.; Cervantes, A.; Arnold, D. ESMO Guidelines Working Group. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. 6), vi64–vi72. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaukat, A.; Kaltenbach, T.; Dominitz, J.A.; Robertson, D.J.; Anderson, J.C.; Cruise, M.; Burke, C.A.; Gupta, S.; Lieberman, D.; Syngal, S.; et al. Endoscopic Recognition and Management Strategies for Malignant Colorectal Polyps: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020, 159, 1916–1934.e2. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Min, B.H.; Ahn, J.H.; Jung, S.H.; An, J.Y.; Choi, M.G.; Sohn, T.S.; Bae, J.M.; Kim, S.; Lee, H.; et al. Nomogram to predict lymph node metastasis in patients with early gastric cancer: A useful clinical tool to reduce gastrectomy after endoscopic resection. Endoscopy 2020, 52, 435–444. [Google Scholar] [CrossRef] [PubMed]

| Variable | Development (n = 10,332) | Validation (n = 4428) | p Value a |
|---|---|---|---|
| Age † | 58 ± 11 | 58 ± 11 | 0.413 |
| Gender | 0.789 | ||
| Male | 6697 (65) | 2881 (65) | |
| Female | 3635 (35) | 1547 (35) | |
| tumors | 512 (5) | 201 (5) | |
| Location | 0.013 | ||
| Upper | 1083 (11) | 483 (11) | |
| Middle | 4773 (46) | 1929 (44) | |
| Lower | 4476 (43) | 2016 (45) | |
| Size (mm) † | 27 ± 18 | 27 ± 18 | 0.645 |
| Gross type | 0.823 | ||
| Non-depressed | 2568 (25) | 1109 (25) | |
| Depressed | 7764 (75) | 3319 (75) | |
| Differentiation | 0.999 | ||
| Well | 1214 (12) | 523 (12) | |
| Moderate | 4053 (39) | 1741 (39) | |
| Signet | 2315 (22) | 989 (22) | |
| Poorly | 2750 (27) | 1175 (27) | |
| Histologic type by Lauren | 0.122 | ||
| Intestinal | 5198 (50) | 2271 (51) | |
| Diffuse | 3867 (38) | 1666 (38) | |
| Mixed | 1267 (12) | 491 (11) | |
| Depth of invasion | 0.983 | ||
| Lamina propria | 2568 (25) | 1114 (25) | |
| Muscularis mucosa | 3767 (37) | 1612 (37) | |
| SM1 | 1069 (10) | 455 (10) | |
| SM2/3 | 2928 (28) | 1247 (28) | |
| Lymphatic invasion, present | 1571 (15) | 682 (15) | 0.780 |
| Venous invasion, present | 154 (2) | 72 (2) | 0.588 |
| Perineural invasion, present | 232 (2) | 96 (2) | 0.817 |
| (A) Total Patients (n = 10,332) and LNM (n = 794) | ||||
| Logistic regression | ||||
| n of patients | n of LNM | Rate (%) | Risk probability | Risk category |
| 1863 | 3 | 0.2 | <1% | Very low |
| 3105 | 42 | 1.4 | ≥1% to <3% | Low |
| 1656 | 67 | 4.1 | ≥3% to <7% | Intermediate |
| 3708 | 682 | 18.4 | ≥7% | High |
| Random forest | ||||
| n of patients | n of LNM | Rate (%) | Risk probability | Risk category |
| 5589 | 2 | <0.1 | <1% | Very low |
| 1859 | 24 | 1.3 | ≥1% to <3% | Low |
| 412 | 18 | 4.4 | ≥3% to <7% | Intermediate |
| 2472 | 750 | 30.3 | ≥7% | High |
| Support vector machine | ||||
| n of patients | n of LNM | Rate (%) | Risk probability | Risk category |
| 2277 | 5 | 0.2 | <1% | Very low |
| 2691 | 35 | 1.3 | ≥1% to <3% | Low |
| 1656 | 65 | 3.9 | ≥3% to <7% | Intermediate |
| 3708 | 689 | 18.6 | ≥7% | High |
| (B) Initial ER(n = 2320) and LNM (n = 42) | ||||
| Logistic regression | ||||
| n of patients | n of LNM | Rate (%) | Risk probability | Risk category |
| 1492 | 1 | 0.1 | <1% | Very low |
| 368 | 5 | 1.4 | ≥1% to <3% | Low |
| 92 | 3 | 3.3 | ≥3% to <7% | Intermediate |
| 368 | 33 | 9.0 | ≥7% | High |
| Random forest | ||||
| n of patients | n of LNM | Rate (%) | Risk probability | Risk category |
| 1722 | 0 | 0 | <1% | Very low |
| 322 | 4 | 1.2 | ≥1% to <3% | Low |
| 46 | 2 | 4.4 | ≥3% to <7% | Intermediate |
| 230 | 36 | 15.7 | ≥7% | High |
| Support vector machine | ||||
| n of patients | n of LNM | Rate (%) | Risk probability | Risk category |
| 1491 | 1 | 0.1 | <1% | Very low |
| 136 | 2 | 1.5 | ≥1% to <3% | Low |
| 445 | 15 | 3.3 | ≥3% to <7% | Intermediate |
| 206 | 24 | 10.4 | ≥7% | High |
| (A) Total Patients (n = 4428) and LNM (n = 337) | ||||
| Logistic regression | ||||
| Risk probability | Risk category | n of patients | n of LNM | Rate (%) |
| <1% | Very low | 801 | 1 | 0.1 |
| ≥1% to <3% | Low | 1335 | 21 | 1.6 |
| ≥3% to <7% | Intermediate | 708 | 34 | 4.8 |
| ≥7% | High | 1584 | 281 | 17.7 |
| Random forest | ||||
| Risk probability | Risk category | n of patients | n of LNM | Rate (%) |
| <1% | Very low | 2403 | 30 | 1.3 |
| ≥1% to <3% | Low | 793 | 50 | 6.3 |
| ≥3% to <7% | Intermediate | 176 | 13 | 7.4 |
| ≥7% | High | 1056 | 244 | 23.1 |
| Support vector machine | ||||
| Risk probability | Risk category | n of patients | n of LNM | Rate (%) |
| <1% | Very low | 978 | 1 | 0.1 |
| ≥1% to <3% | Low | 1138 | 19 | 1.6 |
| ≥3% to <7% | Intermediate | 678 | 30 | 4.2 |
| ≥7% | High | 1297 | 287 | 18.1 |
| (B) Patients with Initial ER (n = 1016) and LNM (n = 24) | ||||
| Logistic regression | ||||
| Risk probability | Risk category | n of patients | n of LNM | Rate (%) |
| <1% | Very low | 656 | 1 | 0.2 |
| ≥1% to <3% | Low | 160 | 4 | 2.5 |
| ≥3% to <7% | Intermediate | 40 | 0 | 0 |
| ≥7% | High | 160 | 19 | 11.9 |
| Random forest | ||||
| Risk probability | Risk category | n of patients | n of LNM | Rate (%) |
| <1% | Very low | 756 | 3 | 0.4 |
| ≥1% to <3% | Low | 140 | 7 | 5.0 |
| ≥3% to <7% | Intermediate | 20 | 2 | 10.0 |
| ≥7% | High | 100 | 12 | 12.0 |
| Support vector machine | ||||
| Risk probability | Risk category | n of patients | n of LNM | Rate (%) |
| <1% | Very low | 655 | 1 | 0.2 |
| ≥1% to <3% | Low | 59 | 1 | 1.7 |
| ≥3% to <7% | Intermediate | 191 | 9 | 4.5 |
| ≥7% | High | 87 | 13 | 13.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, J.-E.; Lee, Y.-C.; Kim, T.-J.; Lee, H.; Won, H.-H.; Min, Y.-W.; Min, B.-H.; Lee, J.-H.; Rhee, P.-L.; Kim, J.J. Machine Learning Model to Stratify the Risk of Lymph Node Metastasis for Early Gastric Cancer: A Single-Center Cohort Study. Cancers 2022, 14, 1121. https://doi.org/10.3390/cancers14051121
Na J-E, Lee Y-C, Kim T-J, Lee H, Won H-H, Min Y-W, Min B-H, Lee J-H, Rhee P-L, Kim JJ. Machine Learning Model to Stratify the Risk of Lymph Node Metastasis for Early Gastric Cancer: A Single-Center Cohort Study. Cancers. 2022; 14(5):1121. https://doi.org/10.3390/cancers14051121
Chicago/Turabian StyleNa, Ji-Eun, Yeong-Chan Lee, Tae-Jun Kim, Hyuk Lee, Hong-Hee Won, Yang-Won Min, Byung-Hoon Min, Jun-Haeng Lee, Poong-Lyul Rhee, and Jae J. Kim. 2022. "Machine Learning Model to Stratify the Risk of Lymph Node Metastasis for Early Gastric Cancer: A Single-Center Cohort Study" Cancers 14, no. 5: 1121. https://doi.org/10.3390/cancers14051121
APA StyleNa, J.-E., Lee, Y.-C., Kim, T.-J., Lee, H., Won, H.-H., Min, Y.-W., Min, B.-H., Lee, J.-H., Rhee, P.-L., & Kim, J. J. (2022). Machine Learning Model to Stratify the Risk of Lymph Node Metastasis for Early Gastric Cancer: A Single-Center Cohort Study. Cancers, 14(5), 1121. https://doi.org/10.3390/cancers14051121







