Update on Epidemiology, Diagnosis, and Biomarkers in Gastroenteropancreatic Neuroendocrine Neoplasms
Abstract
:Simple Summary
Abstract
1. Introduction
2. Epidemiology
3. Diagnosis
3.1. Pathology
3.2. Endoscopy
3.3. CT
3.4. MRI
3.5. Functional Imaging
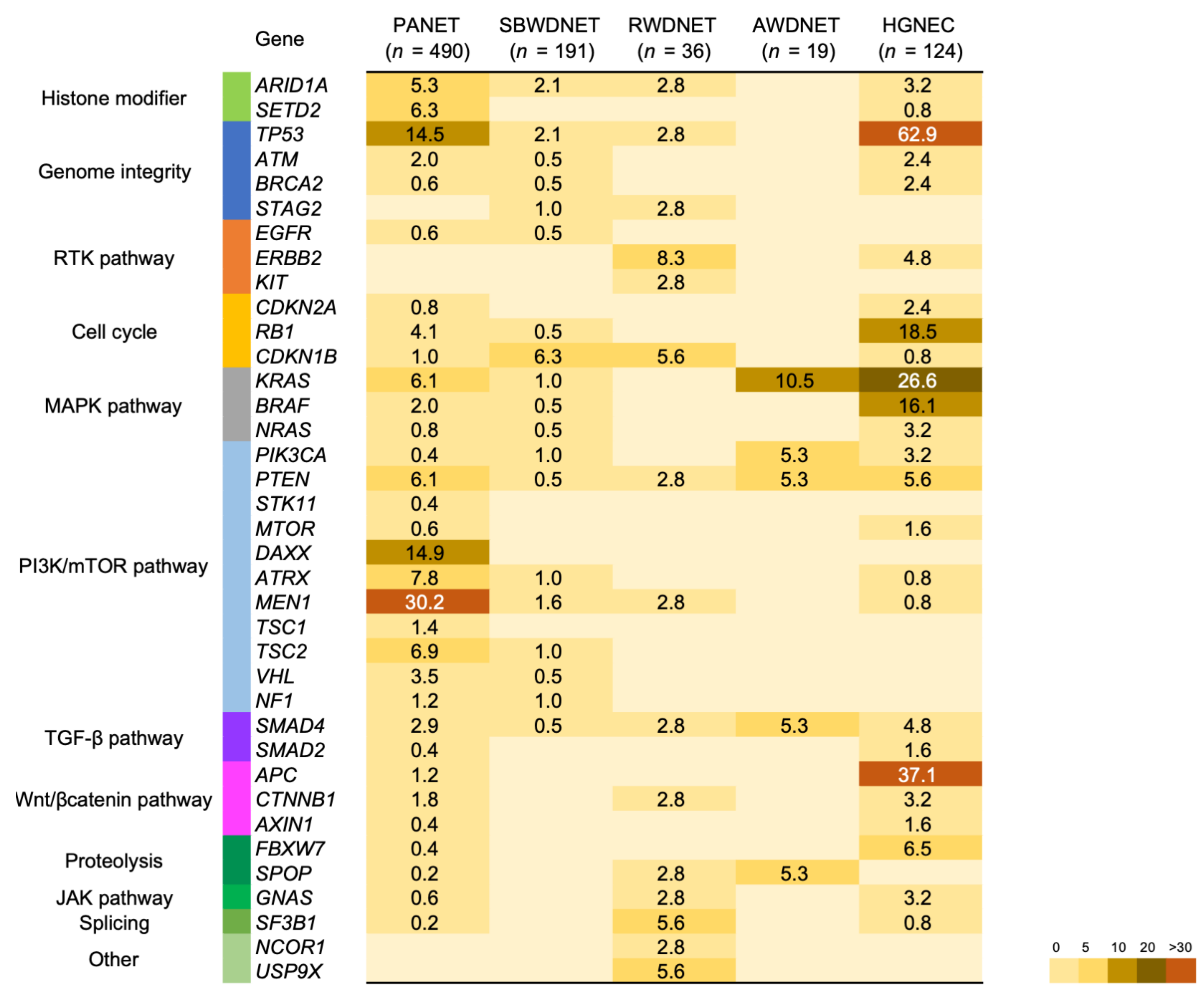
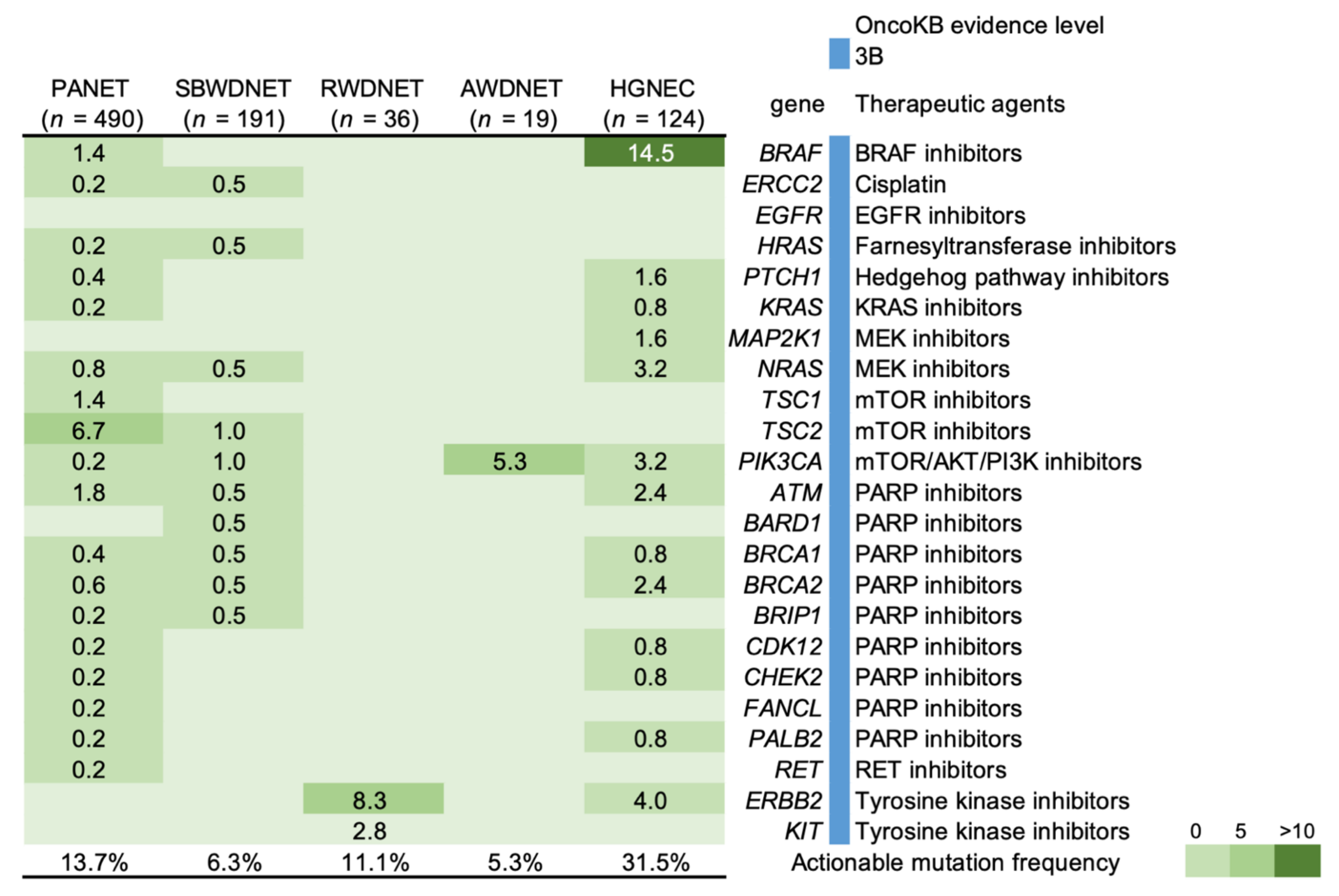
4. Genetic Features and Targeted Therapy
5. Biomarkers
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumors of the Digestive System Tumors, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Andreasi, V.; Partelli, S.; Muffatti, F.; Manzoni, M.F.; Capurso, G.; Falconi, M. Update on Gastroenteropancreatic Neuroendocrine Tumors. Dig. Liver Dis. 2021, 53, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J. Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Mafficini, A.; Scarpa, A. Genetics and Epigenetics of Gastroenteropancreatic Neuroendocrine Neoplasms. Endocr. Rev. 2019, 40, 506–536. [Google Scholar] [CrossRef]
- Sorbye, H.; Strosberg, J.; Baudin, E.; Klimstra, D.S.; Yao, J.C. Gastroenteropancreatic High-Grade Neuroendocrine Carcinoma. Cancer 2014, 120, 2814–2823. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 839–868. [Google Scholar] [CrossRef]
- Kohno, T. Implementation of “Clinical Sequencing” in Cancer Genome Medicine in Japan. Cancer Sci. 2018, 109, 507–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunami, K.; Ichikawa, H.; Kubo, T.; Kato, M.; Fujiwara, Y.; Shimomura, A.; Koyama, T.; Kakishima, H.; Kitami, M.; Matsushita, H.; et al. Feasibility and Utility of a Panel Testing for 114 Cancer-Associated Genes in a Clinical Setting: A Hospital-Based Study. Cancer Sci. 2019, 110, 1480–1490. [Google Scholar] [CrossRef] [Green Version]
- Zehir, A.; Benayed, R.; Shah, R.H.; Syed, A.; Middha, S.; Kim, H.R.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational Landscape of Metastatic Cancer Revealed from Prospective Clinical Sequencing of 10,000 Patients. Nat. Med. 2017, 23, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Kilickap, S.; Hayran, K.M. Epidemiology of Neuroendocrine Tumors. In Neuroendocrine Tumours: Diagnosis and Management; Yalcin, S., Öberg, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 23–33. [Google Scholar]
- Korse, C.M.; Taal, B.G.; van Velthuysen, M.L.; Visser, O. Incidence and Survival of Neuroendocrine Tumours in the Netherlands According to Histological Grade: Experience of Two Decades of Cancer Registry. Eur. J. Cancer 2013, 49, 1975–1983. [Google Scholar] [CrossRef]
- Fraenkel, M.; Faggiano, A.; Valk, G.D. Epidemiology of Neuroendocrine Tumors. Front. Horm. Res. 2015, 44, 1–23. [Google Scholar] [CrossRef]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]
- Leoncini, E.; Boffetta, P.; Shafir, M.; Aleksovska, K.; Boccia, S.; Rindi, G. Increased incidence Trend of Low-Grade and High-Grade Neuroendocrine Neoplasms. Endocrine 2017, 58, 368–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genus, T.S.E.; Bouvier, C.; Wong, K.F.; Srirajaskanthan, R.; Rous, B.A.; Talbot, D.C.; Valle, J.W.; Khan, M.; Pearce, N.; Elshafie, M.; et al. Impact of Neuroendocrine Morphology on Cancer Outcomes and Stage at Diagnosis: A UK Nationwide Cohort Study 2013-2015. Br. J. Cancer 2019, 121, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsdottir, H.; Möller, P.H.; Jonasson, J.G.; Björnsson, E.S. Gastroenteropancreatic Neuroendocrine Tumors in Iceland: A Population-Based Study. Scand. J. Gastroenterol. 2019, 54, 69–75. [Google Scholar] [CrossRef]
- Sandvik, O.M.; Søreide, K.; Gudlaugsson, E.; Kvaløy, J.T.; Søreide, J.A. Epidemiology and Classification of Gastroenteropancreatic Neuroendocrine Neoplasms Using Current Coding Criteria. Br. J. Surg. 2016, 103, 226–232. [Google Scholar] [CrossRef] [Green Version]
- Scherübl, H.; Streller, B.; Stabenow, R.; Herbst, H.; Höpfner, M.; Schwertner, C.; Steinberg, J.; Eick, J.; Ring, W.; Tiwari, K.; et al. Clinically Detected Gastroenteropancreatic Neuroendocrine Tumors are on the Rise: Epidemiological Changes in Germany. World J. Gastroenterol. 2013, 19, 9012–9019. [Google Scholar] [CrossRef]
- Ito, T.; Igarashi, H.; Nakamura, K.; Sasano, H.; Okusaka, T.; Takano, K.; Komoto, I.; Tanaka, M.; Imamura, M.; Jensen, R.T.; et al. Epidemiological Trends of Pancreatic and Gastrointestinal Neuroendocrine Tumors in Japan: A Nationwide Survey Analysis. J. Gastroenterol. 2015, 50, 58–64. [Google Scholar] [CrossRef]
- Masui, T.; Ito, T.; Komoto, I.; Uemoto, S.; JNETS Project Study Group. Recent Epidemiology of Patients with Gastro-Entero-Pancreatic Neuroendocrine Neoplasms (GEP-NEN) in Japan: A Population-Based Study. BMC Cancer 2020, 20, 1104. [Google Scholar] [CrossRef]
- Chang, J.S.; Chen, L.T.; Shan, Y.S.; Chu, P.Y.; Tsai, C.R.; Tsai, H.J. An Updated Analysis of The Epidemiologic Trends of Neuroendocrine Tumors in Taiwan. Sci. Rep. 2021, 11, 7881. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.Y.; Kim, J.M.; Sohn, J.H.; Kim, M.J.; Kim, K.M.; Kim, W.H.; Kim, H.; Kook, M.C.; Park, D.Y.; Lee, J.H.; et al. Current Trends of the Incidence and Pathological Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) in Korea 2000-2009: Multicenter Study. Cancer Res. Treat. 2012, 44, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Wyld, D.; Wan, M.H.; Moore, J.; Dunn, N.; Youl, P. Epidemiological Trends of Neuroendocrine Tumours Over Three Decades in Queensland, Australia. Cancer Epidemiol. 2019, 63, 101598. [Google Scholar] [CrossRef] [PubMed]
- Zárate, X.; Williams, N.; Herrera, M.F. Pancreatic Incidentalomas. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic Neuroendocrine Neoplasms: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Kessel, E.; Naparst, M.; Alpert, N.; Diaz, K.; Ahn, E.; Wolin, E.; Taioli, E.; Kim, M.K. Racial Differences in Gastroenteropancreatic Neuroendocrine Tumor Treatment and Survival in the United States. Pancreas 2021, 50, 29–36. [Google Scholar] [CrossRef]
- Modlin, I.M.; Lye, K.D.; Kidd, M. A 5-Decade Analysis of 13,715 Carcinoid Tumors. Cancer 2003, 97, 934–959. [Google Scholar] [CrossRef] [PubMed]
- Hallet, J.; Law, C.H.L.; Cukier, M.; Saskin, R.; Liu, N.; Singh, S. Exploring the Rising Incidence of Neuroendocrine Tumors: A Population-Based Analysis of Epidemiology, Metastatic Presentation, and Outcomes. Cancer 2015, 121, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Malczewska, A.; Kos-Kudła, B.; Kidd, M.; Drozdov, I.; Bodei, L.; Matar, S.; Oberg, K.; Modlin, I.M. The Clinical Applications of a Multigene Liquid Biopsy (NETest) in Neuroendocrine Tumors. Adv. Med. Sci. 2020, 65, 18–29. [Google Scholar] [CrossRef]
- Konukiewitz, B.; Schlitter, A.M.; Jesinghaus, M.; Pfister, D.; Steiger, K.; Segler, A.; Agaimy, A.; Sipos, B.; Zamboni, G.; Weichert, W.; et al. Somatostatin Receptor Expression Related to TP53 and RB1 Alterations in Pancreatic and Extrapancreatic Neuroendocrine Neoplasms with a Ki67-Index Above 20. Mod. Pathol. 2017, 30, 587–598. [Google Scholar] [CrossRef]
- Kasajima, A.; Papotti, M.; Ito, W.; Brizzi, M.P.; La Salvia, A.; Rapa, I.; Tachibana, T.; Yazdani, S.; Sasano, H.; Volante, M. High Interlaboratory and Interobserver Agreement of Somatostatin Receptor Immunohistochemical Determination and Correlation with Response to Somatostatin Analogs. Hum. Pathol. 2018, 72, 144–152. [Google Scholar] [CrossRef]
- Rosenbaum, J.N.; Guo, Z.; Baus, R.M.; Werner, H.; Rehrauer, W.M.; Lloyd, R.V. INSM1: A Novel Immunohistochemical and Molecular Marker for Neuroendocrine and Neuroepithelial Neoplasms. Am. J. Clin. Pathol. 2015, 144, 579–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delle Fave, G.; O’Toole, D.; Sundin, A.; Taal, B.; Ferolla, P.; Ramage, J.K.; Ferone, D.; Ito, T.; Weber, W.; Zheng-Pei, Z.; et al. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology 2016, 103, 119–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Mestier, L.; Lepage, C.; Baudin, E.; Coriat, R.; Courbon, F.; Couvelard, A.; Do Cao, C.; Frampas, E.; Gaujoux, S.; Gincul, R.; et al. Digestive Neuroendocrine Neoplasms (NEN): French Intergroup Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up (SNFGE, GTE, RENATEN, TENPATH, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig. Liver Dis. 2020, 52, 473–492. [Google Scholar] [CrossRef] [PubMed]
- Ramage, J.K.; de Herder, W.W.; Delle Fave, G.; Ferolla, P.; Ferone, D.; Ito, T.; Ruszniewski, P.; Sundin, A.; Weber, W.; Zheng-Pei, Z.; et al. ENETS Consensus Guidelines Update for Colorectal Neuroendocrine Neoplasms. Neuroendocrinology 2016, 103, 139–143. [Google Scholar] [CrossRef]
- Partelli, S.; Bartsch, D.K.; Capdevila, J.; Chen, J.; Knigge, U.; Niederle, B.; Nieveen van Dijkum, E.J.M.; Pape, U.F.; Pascher, A.; Ramage, J.; et al. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology 2017, 105, 255–265. [Google Scholar] [CrossRef]
- Manguso, N.; Gangi, A.; Johnson, J.; Harit, A.; Nissen, N.; Jamil, L.; Lo, S.; Wachsman, A.; Hendifar, A.; Amersi, F. The Role of Pre-Operative Imaging and Double Balloon Enteroscopy in the Surgical Management of Small Bowel Neuroendocrine Tumors: Is It Necessary? J. Surg. Oncol. 2018, 117, 207–212. [Google Scholar] [CrossRef]
- James, P.D.; Tsolakis, A.V.; Zhang, M.; Belletrutti, P.J.; Mohamed, R.; Roberts, D.J.; Heitman, S.J. Incremental Benefit of Preoperative EUS for the Detection of Pancreatic Neuroendocrine Tumors: A Meta-Analysis. Gastrointest. Endosc. 2015, 81, 848–856.e1. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yamao, K.; Hijioka, S.; Bhatia, V.; Mizuno, N.; Hara, K.; Imaoka, H.; Niwa, Y.; Tajika, M.; Kondo, S.; et al. Evaluation of Ki-67 index in EUS–FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy 2014, 46, 32–38. [Google Scholar] [CrossRef]
- Sundin, A.; Arnold, R.; Baudin, E.; Cwikla, J.B.; Eriksson, B.; Fanti, S.; Fazio, N.; Giammarile, F.; Hicks, R.J.; Kjaer, A.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine & Hybrid Imaging. Neuroendocrinology 2017, 105, 212–244. [Google Scholar] [CrossRef]
- Zamir, M.A.; Hakim, W.; Yusuf, S.; Thomas, R. Imaging of Pancreatic-Neuroendocrine Tumours: An Outline of Conventional Radiological Techniques. Curr. Radiopharm. 2019, 12, 135–155. [Google Scholar] [CrossRef]
- Putzer, D.; Gabriel, M.; Henninger, B.; Kendler, D.; Uprimny, C.; Dobrozemsky, G.; Decristoforo, C.; Bale, R.J.; Jaschke, W.; Virgolini, I.J. Bone Metastases in Patients with Neuroendocrine Tumor: 68Ga-DOTA-Tyr3-Octreotide PET in Comparison to CT and Bone Scintigraphy. J. Nucl. Med. 2009, 50, 1214–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid-Tannwald, C.; Schmid-Tannwald, C.M.; Morelli, J.N.; Neumann, R.; Haug, A.R.; Jansen, N.; Nikolaou, K.; Schramm, N.; Reiser, M.F.; Rist, C. Comparison of Abdominal MRI with Diffusion-Weighted Imaging to 68Ga-DOTATATE PET/CT in Detection of Neuroendocrine Tumors of the Pancreas. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Brenner, R.; Metens, T.; Bali, M.; Demetter, P.; Matos, C. Pancreatic Neuroendocrine Tumor: Added Value of Fusion of T2-weighted Imaging and High b-value Diffusion-Weighted Imaging for Tumor Detection. Eur. J. Radiol. 2012, 81, e746–e749. [Google Scholar] [CrossRef] [PubMed]
- d’Assignies, G.; Fina, P.; Bruno, O.; Vullierme, M.P.; Tubach, F.; Paradis, V.; Sauvanet, A.; Ruszniewski, P.; Vilgrain, V. High Sensitivity of Diffusion-Weighted MR Imaging for the Detection of Liver Metastases from Neuroendocrine Tumors: Comparison with T2-Weighted and Dynamic Gadolinium-Enhanced MR Imaging. Radiology 2013, 268, 390–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronot, M.; Clift, A.K.; Baum, R.P.; Singh, A.; Kulkarni, H.R.; Frilling, A.; Vilgrain, V. Morphological and Functional Imaging for Detecting and Assessing the Resectability of Neuroendocrine Liver Metastases. Neuroendocrinology 2018, 106, 74–88. [Google Scholar] [CrossRef]
- Dromain, C.; de Baere, T.; Lumbroso, J.; Caillet, H.; Laplanche, A.; Boige, V.; Ducreux, M.; Duvillard, P.; Elias, D.; Schlumberger, M.; et al. Detection of Liver Metastases from Endocrine Tumors: A Prospective Comparison of Somatostatin Receptor Scintigraphy, Computed Tomography, and Magnetic Resonance Imaging. J. Clin. Oncol. 2005, 23, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Cwikła, J.B.; Buscombe, J.R.; Caplin, M.E.; Watkinson, A.F.; Walecki, J.; Gorczyca-Wiśniewska, E.; Hilson, A.J. Diagnostic Imaging of Carcinoid Metastases to the Abdomen and Pelvis. Med. Sci. Monit. 2004, 10, 9–16. [Google Scholar]
- Chambers, A.J.; Pasieka, J.L.; Dixon, E.; Rorstad, O. Role of Imaging in the Preoperative Staging of Small Bowel Neuroendocrine Tumors. J. Am. Coll. Surg. 2010, 211, 620–627. [Google Scholar] [CrossRef]
- Treglia, G.; Castaldi, P.; Rindi, G.; Giordano, A.; Rufini, V. Diagnostic Performance of Gallium-68 Somatostatin Receptor PET and PET/CT in Patients with Thoracic and Gastroenteropancreatic Neuroendocrine Tumours: A Meta-Analysis. Endocrine 2012, 42, 80–87. [Google Scholar] [CrossRef]
- Van Adrichem, R.C.; Kamp, K.; van Deurzen, C.H.; Biermann, K.; Feelders, R.A.; Franssen, G.J.; Kwekkeboom, D.J.; Hofland, L.J.; de Herder, W.W. Is There an Additional Value of Using Somatostatin Receptor Subtype 2a Immunohistochemistry Compared to Somatostatin Receptor Scintigraphy Uptake in Predicting Gastroenteropancreatic Neuroendocrine Tumor Response? Neuroendocrinology 2016, 103, 560–566. [Google Scholar] [CrossRef]
- Sawicki, L.M.; Deuschl, C.; Beiderwellen, K.; Ruhlmann, V.; Poeppel, T.D.; Heusch, P.; Lahner, H.; Führer, D.; Bockisch, A.; Herrmann, K.; et al. Evaluation of 68 Ga-DOTATOC PET/MRI for whole-body staging of neuroendocrine tumours in comparison with 68 Ga-DOTATOC PET/CT. Eur. Radiol. 2017, 27, 4091–4099. [Google Scholar] [CrossRef] [PubMed]
- Johnbeck, C.B.; Knigge, U.; Loft, A.; Berthelsen, A.K.; Mortensen, J.; Oturai, P.; Langer, S.W.; Elema, D.R.; Kjaer, A. Head-to-head comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: A prospective study of 59 patients with neuroendocrine tumors. J. Nucl. Med. 2017, 58, 451–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loft, M.; Carlsen, E.A.; Johnbeck, C.B.; Johannesen, H.H.; Binderup, T.; Pfeifer, A.; Mortensen, J.; Oturai, P.; Loft, A.; Berthelsen, A.K.; et al. 64Cu-DOTATATE PET in Patients with Neuroendocrine Neoplasms: Prospective, Head-to-Head Comparison of Imaging at 1 Hour and 3 Hours After Injection. J. Nucl. Med. 2021, 62, 73–80. [Google Scholar] [CrossRef]
- Jawlakh, H.; Velikyan, I.; Welin, S.; Sundin, A. 68Ga-DOTATOC-PET/MRI and 11C-5-HTP-PET/MRI are superior to 68Ga-DOTATOC-PET/CT for neuroendocrine tumour imaging. J. Neuroendocrinol. 2021, 33, e12981. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Elledge, S.J. Multiple Tumor Suppressor Pathways Negatively Regulate Telomerase. Cell 2003, 113, 881–889. [Google Scholar] [CrossRef] [Green Version]
- Scarpa, A.; Chang, D.K.; Nones, K.; Corbo, V.; Patch, A.M.; Bailey, P.; Lawlor, R.T.; Johns, A.L.; Miller, D.K.; Mafficini, A.; et al. Whole-Genome Landscape of Pancreatic Neuroendocrine Tumours. Nature 2017, 543, 65–71. [Google Scholar] [CrossRef]
- Chan, C.S.; Laddha, S.V.; Lewis, P.W.; Koletsky, M.S.; Robzyk, K.; Da Silva, E.; Torres, P.J.; Untch, B.R.; Li, J.; Bose, P.; et al. ATRX, DAXX or MEN1 Mutant Pancreatic Neuroendocrine Tumors Are a Distinct Alpha-Cell Signature Subgroup. Nat. Commun. 2018, 9, 4158. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.H.; Untch, B.R.; Reidy, D.L.; O’Reilly, E.; Dhall, D.; Jih, L.; Basturk, O.; Allen, P.J.; Klimstra, D.S. Well-Differentiated Neuroendocrine Tumors with a Morphologically Apparent High-Grade Component: A Pathway Distinct from Poorly Differentiated Neuroendocrine Carcinomas. Clin. Cancer Res. 2016, 22, 1011–1017. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Shi, C.; Edil, B.H.; de Wilde, R.F.; Klimstra, D.S.; Maitra, A.; Schulick, R.D.; Tang, L.H.; Wolfgang, C.L.; Choti, M.A.; et al. DAXX/ATRX, MEN1, and mTOR Pathway Genes are Frequently Altered in Pancreatic Neuroendocrine Tumors. Science 2011, 331, 1199–1203. [Google Scholar] [CrossRef] [Green Version]
- Van Riet, J.; van de Werken, H.J.G.; Cuppen, E.; Eskens, F.; Tesselaar, M.; van Veenendaal, L.M.; Klümpen, H.J.; Dercksen, M.W.; Valk, G.D.; Lolkema, M.P.; et al. The Genomic Landscape of 85 Advanced Neuroendocrine Neoplasms Reveals Subtype-Heterogeneity and Potential Therapeutic Targets. Nat. Commun. 2021, 12, 4612. [Google Scholar] [CrossRef]
- Francis, J.M.; Kiezun, A.; Ramos, A.H.; Serra, S.; Pedamallu, C.S.; Qian, Z.R.; Banck, M.S.; Kanwar, R.; Kulkarni, A.A.; Karpathakis, A.; et al. Somatic Mutation of CDKN1B in Small Intestine Neuroendocrine Tumors. Nat. Genet. 2013, 45, 1483–1486. [Google Scholar] [CrossRef]
- Park, H.Y.; Kwon, M.J.; Kang, H.S.; Kim, Y.J.; Kim, N.Y.; Kim, M.J.; Min, K.W.; Choi, K.C.; Nam, E.S.; Cho, S.J.; et al. Targeted Next-Generation Sequencing of Well-Differentiated Rectal, Gastric, and Appendiceal Neuroendocrine Tumors to Identify Potential Targets. Hum. Pathol. 2019, 87, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Loree, J.M.; Pereira, A.A.L.; Lam, M.; Willauer, A.N.; Raghav, K.; Dasari, A.; Morris, V.K.; Advani, S.; Menter, D.G.; Eng, C.; et al. Classifying Colorectal Cancer by Tumor Location Rather than Sidedness Highlights a Continuum in Mutation Profiles and Consensus Molecular Subtypes. Clin. Cancer Res. 2018, 24, 1062–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seligmann, J.F.; Fisher, D.; Smith, C.G.; Richman, S.D.; Elliott, F.; Brown, S.; Adams, R.; Maughan, T.; Quirke, P.; Cheadle, J.; et al. Investigating the Poor Outcomes of BRAF-Mutant Advanced Colorectal Cancer: Analysis from 2530 Patients in Randomised Clinical Trials. Ann. Oncol. 2017, 28, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, R.; Chatila, W.K.; Lipsyc, M.D.; Hechtman, J.F.; Cercek, A.; Sanchez-Vega, F.; Jayakumaran, G.; Middha, S.; Zehir, A.; Donoghue, M.T.A.; et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell 2018, 33, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, J.; Lim, J.S.; Jang, S.J.; Cun, Y.; Ozretić, L.; Kong, G.; Leenders, F.; Lu, X.; Fernández-Cuesta, L.; Bosco, G.; et al. Comprehensive Genomic Profiles of Small Cell Lung Cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Gao, J.; Phillips, S.M.; Kundra, R.; Zhang, H.; Wang, J.; Rudolph, J.E.; Yaeger, R.; Soumerai, T.; Nissan, M.H.; et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis. Oncol. 2017, 2017, 1–16. [Google Scholar] [CrossRef]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for Advanced Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Subbiah, V.; Puzanov, I.; Blay, J.Y.; Chau, I.; Lockhart, A.C.; Raje, N.S.; Wolf, J.; Baselga, J.; Meric-Bernstam, F.; Roszik, J.; et al. Pan-Cancer Efficacy of Vemurafenib in BRAF (V600)-Mutant Non-Melanoma Cancers. Cancer Discov. 2020, 10, 657–663. [Google Scholar] [CrossRef] [Green Version]
- Malczewska, A.; Oberg, K.; Kos-Kudla, B. NETest is Superior to Chromogranin A in Neuroendocrine Neoplasia: A Prospective ENETS CoE Analysis. Endocr. Connect 2021, 10, 110–123. [Google Scholar] [CrossRef]
- Oberg, K.; Modlin, I.M.; de Herder, W.; Pavel, M.; Klimstra, D.; Frilling, A.; Metz, D.C.; Heaney, A.; Kwekkeboom, D.; Strosberg, J.; et al. Consensus on Biomarkers for Neuroendocrine Tumour Disease. Lancet Oncol. 2015, 16, e435–e446. [Google Scholar] [CrossRef] [Green Version]
- Kidd, M.; Drozdov, I.A.; Matar, S.; Gurunlian, N.; Ferranti, N.J.; Malczewska, A.; Bennett, P.; Bodei, L.; Modlin, I.M. Utility of a Ready-to-Use PCR System for Neuroendocrine Tumor Diagnosis. PLoS ONE 2019, 14, e0218592. [Google Scholar] [CrossRef] [PubMed]
- Van Treijen, M.J.C.; Korse, C.M.; van Leeuwaarde, R.S.; Saveur, L.J.; Vriens, M.R.; Verbeek, W.H.M.; Tesselaar, M.E.T.; Valk, G.D. Blood Transcript Profiling for the Detection of Neuroendocrine Tumors: Results of a Large Independent Validation Study. Front. Endocrinol. 2018, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Malczewska, A.; Witkowska, M.; Makulik, K.; Bocian, A.; Walter, A.; Pilch-Kowalczyk, J.; Zajęcki, W.; Bodei, L.; Oberg, K.E.; Kos-Kudła, B. NETest Liquid Biopsy is Diagnostic of Small Intestine and Pancreatic Neuroendocrine Tumors and Correlates with Imaging. Endocr. Connect. 2019, 8, 442–453. [Google Scholar] [CrossRef] [Green Version]
- Liu, E.; Paulson, S.; Gulati, A.; Freudman, J.; Grosh, W.; Kafer, S.; Wickremesinghe, P.C.; Salem, R.R.; Bodei, L. Assessment of NETest Clinical Utility in a U.S. Registry-Based Study. Oncologist 2019, 24, 783–790. [Google Scholar] [CrossRef] [Green Version]
- Malczewska, A.; Procner, A.; Walter, A.; Kusnierz, K.; Zajecki, W.; Aslanian, H.; Kos-Kudla, B. The NETest Liquid Biopsy is Diagnostic for Gastric Neuroendocrine Tumors: Observations on the Blood-Based Identification of Microscopic and Macroscopic Residual Disease. BMC Gastroenterol. 2020, 20, 235. [Google Scholar] [CrossRef]
- Modlin, I.M.; Kidd, M.; Oberg, K.; Falconi, M.; Filosso, P.L.; Frilling, A.; Malczewska, A.; Salem, R.; Toumpanakis, C.; Laskaratos, F.M.; et al. Early Identification of Residual Disease After Neuroendocrine Tumor Resection Using a Liquid Biopsy Multigenomic mRNA Signature (NETest). Ann. Surg. Oncol. 2021, 28, 7506–7517. [Google Scholar] [CrossRef]
- Partelli, S.; Andreasi, V.; Muffatti, F.; Schiavo Lena, M.; Falconi, M. Circulating Neuroendocrine Gene Transcripts (NETest): A Postoperative Strategy for Early Identification of the Efficacy of Radical Surgery for Pancreatic Neuroendocrine Tumors. Ann. Surg. Oncol. 2020, 27, 3928–3936. [Google Scholar] [CrossRef]
- Bodei, L.; Kidd, M.S.; Singh, A.; van der Zwan, W.A.; Severi, S.; Drozdov, I.A.; Malczewska, A.; Baum, R.P.; Kwekkeboom, D.J.; Paganelli, G.; et al. PRRT Neuroendocrine Tumor Response Monitored Using Circulating Transcript Analysis: The NETest. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 895–906. [Google Scholar] [CrossRef]
- Modlin, I.M.; Kidd, M.; Frilling, A.; Falconi, M.; Filosso, P.L.; Malczewska, A.; Kitz, A. Molecular Genomic Assessment Using a Blood-based mRNA Signature (NETest) is Cost-effective and Predicts Neuroendocrine Tumor Recurrence With 94% Accuracy. Ann. Surg. 2021, 274, 481–490. [Google Scholar] [CrossRef]
- Hijioka, S.; Morizane, C.; Ikeda, M.; Ishii, H.; Okusaka, T.; Furuse, J. Current Status of Medical Treatment for Gastroenteropancreatic Neuroendocrine Neoplasms and Future Perspectives. Jpn. J. Clin. Oncol. 2021, 51, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Borga, C.; Businello, G.; Murgioni, S.; Bergamo, F.; Martini, C.; de Carlo, E.; Trevellin, E.; Vettor, R.; Fassan, M. Treatment Personalization in Gastrointestinal Neuroendocrine Tumors. Curr. Treat. Options Oncol. 2021, 22, 29. [Google Scholar] [CrossRef] [PubMed]

| Country | Reference | GEP-NEN Incidence (Cases Per 100,000) * | Data Time Period |
|---|---|---|---|
| Netherlands | [12] | 2.12 | 2001–2010 |
| Germany | [19] | 2.2 | 2006 |
| Taiwan | [22] | 2.31 | 2015 |
| Japan | [21] | 3.53 | 2016 |
| United States of America | [2] | 3.56 | 2012 |
| Iceland | [17] | 3.85 | 2000–2014 |
| Australia | [24] | 4.46 | 2006–2015 |
| United Kingdom | [16] | 4.6 | 2015 |
| Norway | [18] | 6.62 | 2009 |
| Definition | Cell Morphology | Ki67 Proliferative Index a | Mitotic Count b |
|---|---|---|---|
| NET G1 | Well-differentiated | <3% | <2 |
| NET G2 | 3–20% | 2–20 | |
| NET G3 | >20% | >20 | |
| NEC | Poorly differentiated | >20% | >20 |
| Small-cell type | |||
| Large-cell type | |||
| MiNEN | Well- or poorly differentiated | Variable | Variable |
| Author | Sites of NET | Sensitivity (%) | Specificity (%) | Accuracy (%) |
|---|---|---|---|---|
| van Treijen et al., 2018 [75] | GEP | 89 | 72 | nd |
| Malczewska et al., 2019 [76] | P, SI | 99 | 95 | 97 |
| Liu et al., 2019 [77] | GEP, BP, U | nd | nd | 96 |
| Malczewska et al., 2020 [78] | G | 100 | 87 | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takayanagi, D.; Cho, H.; Machida, E.; Kawamura, A.; Takashima, A.; Wada, S.; Tsunoda, T.; Kohno, T.; Shiraishi, K. Update on Epidemiology, Diagnosis, and Biomarkers in Gastroenteropancreatic Neuroendocrine Neoplasms. Cancers 2022, 14, 1119. https://doi.org/10.3390/cancers14051119
Takayanagi D, Cho H, Machida E, Kawamura A, Takashima A, Wada S, Tsunoda T, Kohno T, Shiraishi K. Update on Epidemiology, Diagnosis, and Biomarkers in Gastroenteropancreatic Neuroendocrine Neoplasms. Cancers. 2022; 14(5):1119. https://doi.org/10.3390/cancers14051119
Chicago/Turabian StyleTakayanagi, Daisuke, Hourin Cho, Erika Machida, Atsushi Kawamura, Atsuo Takashima, Satoshi Wada, Takuya Tsunoda, Takashi Kohno, and Kouya Shiraishi. 2022. "Update on Epidemiology, Diagnosis, and Biomarkers in Gastroenteropancreatic Neuroendocrine Neoplasms" Cancers 14, no. 5: 1119. https://doi.org/10.3390/cancers14051119
APA StyleTakayanagi, D., Cho, H., Machida, E., Kawamura, A., Takashima, A., Wada, S., Tsunoda, T., Kohno, T., & Shiraishi, K. (2022). Update on Epidemiology, Diagnosis, and Biomarkers in Gastroenteropancreatic Neuroendocrine Neoplasms. Cancers, 14(5), 1119. https://doi.org/10.3390/cancers14051119








