Systematic Review of Single-Fraction Stereotactic Body Radiation Therapy for Early Stage Non-Small-Cell Lung Cancer and Lung Oligometastases: How to Stop Worrying and Love One and Done
Abstract
Simple Summary
Abstract
1. Introduction
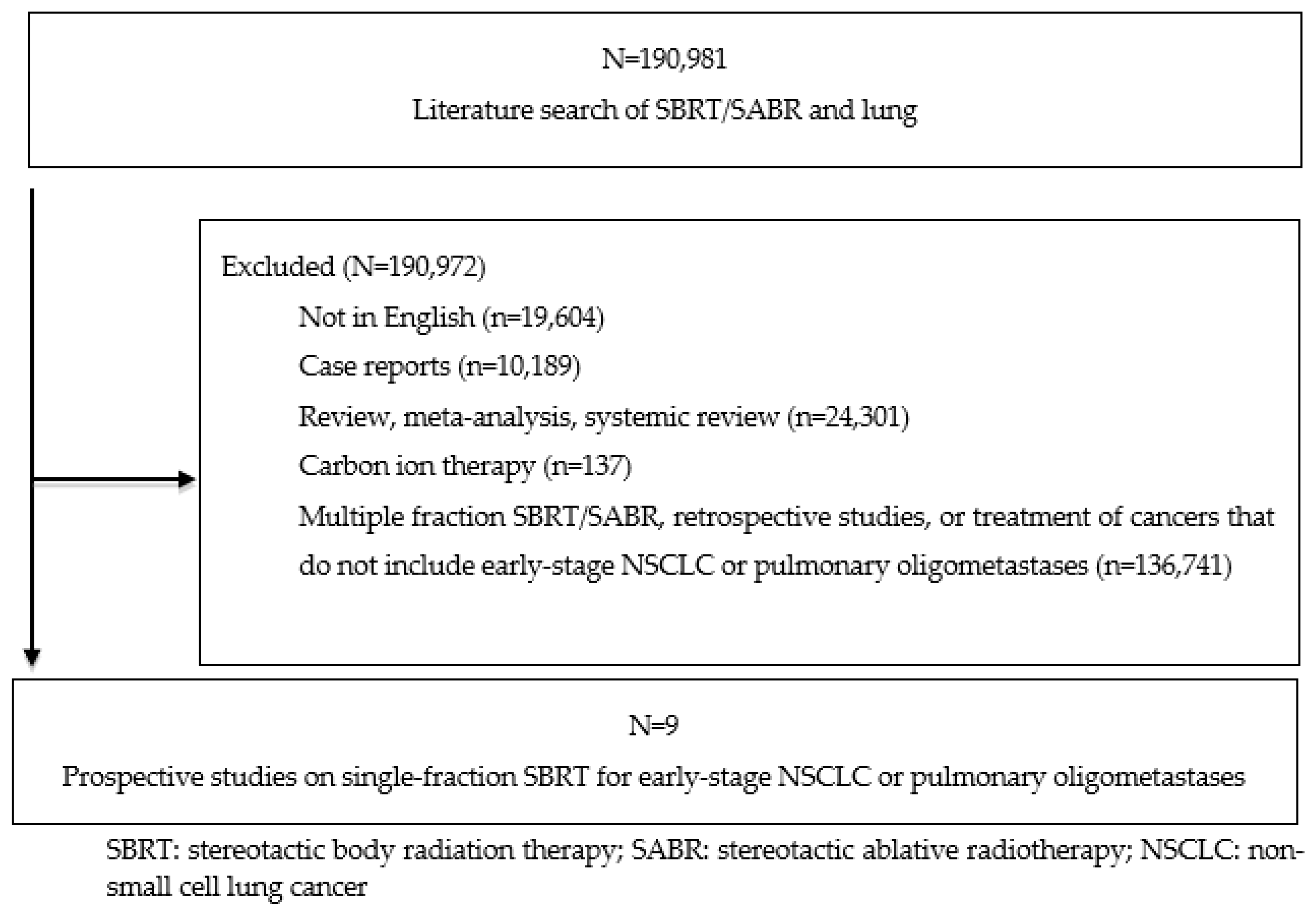
2. Materials and Methods
3. Results
3.1. Medically Inoperable Early Stage NSCLC: Local Control and Survival
3.2. Lung Oligometastases: Local Control and Survival
3.3. Toxicity of SBRT in Primary NSCLC and Lung Oligometastases
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schrag, D.; Hershman, D.L.; Basch, E. Oncology Practice During the COVID-19 Pandemic. JAMA 2020, 323, 2005–2006. [Google Scholar] [CrossRef] [PubMed]
- Couñago, F.; Navarro-Martin, A.; Luna, J.; de Dios, N.R.; Rodríguez, A.; Casas, F.; García, R.; Gómez-Caamaño, A.; Contreras, J.; Serrano, J. GOECP/SEOR clinical recommendations for lung cancer radiotherapy during the COVID-19 pandemic. World J. Clin. Oncol. 2020, 11, 510–527. [Google Scholar] [CrossRef]
- Ng, S.S.W.; Ning, M.S.; Lee, P.; McMahon, R.A.; Siva, S.; Chuong, M.D. Single-Fraction Stereotactic Body Radiation Therapy: A Paradigm During the Coronavirus Disease 2019 (COVID-19) Pandemic and Beyond? Adv. Radiat. Oncol. 2020, 5, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Salama, J.K.; Giuliani, M.E.; Robinson, C.G.; Daly, M.E. Single-fraction SBRT for Early Stage NSCLC-A Viable Option in “These Uncertain Times”? Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Simcock, R.; Thomas, T.V.; Estes, C.; Filippi, A.R.; Katz, M.S.; Pereira, I.J.; Saeed, H. COVID-19: Global radiation oncology’s targeted response for pandemic preparedness. Clin. Transl. Radiat. Oncol. 2020, 22, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.J.; Yom, S.S.; Saeed, H.; El Naqa, I.; Ballas, L.; Bentzen, S.M.; Chao, S.T.; Choudhury, A.; Coles, C.E.; Dover, L.; et al. Radiation Fractionation Schedules Published During the COVID-19 Pandemic: A Systematic Review of the Quality of Evidence and Recommendations for Future Development. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 379–389. [Google Scholar] [CrossRef]
- Lindberg, K.; Nyman, J.; Riesenfeld Källskog, V.; Hoyer, M.; Lund, J.Å.; Lax, I.; Wersäll, P.; Karlsson, K.; Friesland, S.; Lewensohn, R. Long-term results of a prospective phase II trial of medically inoperable stage I NSCLC treated with SBRT—The Nordic experience. Acta Oncol. 2015, 54, 1096–1104. [Google Scholar] [CrossRef]
- Verstegen, N.E.; Lagerwaard, F.J.; Hashemi, S.M.; Dahele, M.; Slotman, B.J.; Senan, S. Patterns of Disease Recurrence after SABR for Early Stage Non-Small-Cell Lung Cancer: Optimizing Follow-Up Schedules for Salvage Therapy. J. Thorac. Oncol. 2015, 10, 1195–1200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- Singh, A.K.; Gomez-Suescun, J.A.; Stephans, K.L.; Bogart, J.A.; Hermann, G.M.; Tian, L.; Groman, A.; Videtic, G.M. One Versus Three Fractions of Stereotactic Body Radiation Therapy for Peripheral Stage I to II Non-Small Cell Lung Cancer: A Randomized, Multi-Institution, Phase 2 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 752–759. [Google Scholar] [CrossRef]
- Videtic, G.M.; Paulus, R.; Singh, A.K.; Chang, J.Y.; Parker, W.; Olivier, K.R.; Timmerman, R.D.; Komaki, R.R.; Urbanic, J.J.; Stephans, K.L.; et al. Long-term Follow-up on NRG Oncology RTOG 0915 (NCCTG N0927): A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Cummings, M.A.; Ma, S.J.; Hermann, G.; Serra, L.; Syed, Y.; Malhotra, H.K.; Chen, Y.; Milano, M.T.; Gomez-Suescun, J.A.; Singh, D.P.; et al. Comparison of Single- and Five-fraction Regimens of Stereotactic Body Radiation Therapy for Peripheral Early-stage Non-small-cell Lung Cancer: A Two-institution Propensity-matched Analysis. Clin. Lung Cancer 2018, 19, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.J.; Cummings, M.; Serra, L.M.; Syed, Y.A.; Hermann, G.M.; Chen, Y.; Milano, M.T.; Singh, A.K.; Gomez-Suescun, J.A.; Singh, D.P. Three- Versus Five-Fraction Regimens of Stereotactic Body Radiotherapy for Peripheral Early-Stage Non-Small-Cell Lung Cancer: A Two-Institution Propensity Score-Matched Analysis. Clin. Lung Cancer 2018, 19, e297–e302. [Google Scholar] [CrossRef]
- Ma, S.J.; Serra, L.M.; Syed, Y.A.; Hermann, G.M.; Gomez-Suescun, J.A.; Singh, A.K. Comparison of Single- and Three-fraction Schedules of Stereotactic Body Radiation Therapy for Peripheral Early-stage Non-Small-cell Lung Cancer. Clin. Lung Cancer 2018, 19, e235–e240. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 1.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 21 January 2022).
- Siva, S.; Bressel, M.; Mai, T.; Le, H.; Vinod, S.; de Silva, H.; Macdonald, S.; Skala, M.; Hardcastle, N.; Rezo, A.; et al. Single-Fraction vs Multifraction Stereotactic Ablative Body Radiotherapy for Pulmonary Oligometastases (SAFRON II): The Trans Tasman Radiation Oncology Group 13.01 Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1476–1485. [Google Scholar] [CrossRef]
- Lewis, S.L.; Porceddu, S.; Nakamura, N.; Palma, D.A.; Lo, S.S.; Hoskin, P.; Moghanaki, D.; Chmura, S.J.; Salama, J.K. Definitive Stereotactic Body Radiotherapy (SBRT) for Extracranial Oligometastases: An International Survey of >1000 Radiation Oncologists. Am. J. Clin. Oncol. 2017, 40, 418–422. [Google Scholar] [CrossRef]
- Mou, B.; Hyde, D.; Araujo, C.; Bartha, L.; Bergman, A.; Liu, M. Implementation of Single-Fraction Lung Stereotactic Ablative Radiotherapy in a Multicenter Provincial Cancer Program During the COVID-19 Pandemic. Cureus 2021, 13, e15598. [Google Scholar] [CrossRef]
- Nagata, Y.; Ozawa, S.; Nakao, M.; Kawahara, D.; Kimura, T.; Murakami, Y. Survey of stereotactic body radiation therapy in Japan. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, E449. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Filippi, A.R.; Badellino, S.; Guarneri, A.; Levis, M.; Botticella, A.; Mantovani, C.; Ragona, R.; Racca, P.; Buffoni, L.; Novello, S.; et al. Outcomes of single fraction stereotactic ablative radiotherapy for lung metastases. Technol. Cancer Res. Treat. 2014, 13, 37–45. [Google Scholar] [CrossRef]
- Fritz, P.; Kraus, H.J.; Mühlnickel, W.; Hammer, U.; Dölken, W.; Engel-Riedel, W.; Chemaissani, A.; Stoelben, E. Stereotactic, single-dose irradiation of stage I non-small cell lung cancer and lung metastases. Radiat. Oncol. 2006, 1, 30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hof, H.; Herfarth, K.K.; Münter, M.; Hoess, A.; Motsch, J.; Wannenmacher, M.; ürgen Debus, J. Stereotactic single-dose radiotherapy of stage I non-small-cell lung cancer (NSCLC). Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 335–341. [Google Scholar] [CrossRef]
- Hof, H.; Hoess, A.; Oetzel, D.; Debus, J.; Herfarth, K. Stereotactic single-dose radiotherapy of lung metastases. Strahlenther. Onkol. 2007, 183, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.T.; Loo, B.W.; Ho, A.; Cotrutz, C.; Koong, A.C.; Wakelee, H.; Kee, S.T.; Constantinescu, D.; Whyte, R.I.; Donington, J. Results of a phase I dose-escalation study using single-fraction stereotactic radiotherapy for lung tumors. J. Thorac. Oncol. 2006, 1, 802–809. [Google Scholar] [CrossRef]
- Whyte, R.I.; Crownover, R.; Murphy, M.J.; Martin, D.P.; Rice, T.W.; DeCamp, M.M., Jr.; Rodebaugh, R.; Weinhous, M.S.; Le, Q.T. Stereotactic radiosurgery for lung tumors: Preliminary report of a phase I trial. Ann. Thorac. Surg. 2003, 75, 1097–1101. [Google Scholar] [CrossRef]
- Faivre-Finn, C.; Fenwick, J.D.; Franks, K.N.; Harrow, S.; Hatton, M.Q.; Hiley, C.; McAleese, J.J.; McDonald, F.; O’Hare, J.; Peedell, C.; et al. Reduced Fractionation in Lung Cancer Patients Treated with Curative-intent Radiotherapy during the COVID-19 Pandemic. Clin. Oncol. 2020, 32, 481–489. [Google Scholar] [CrossRef]
- Guckenberger, M.; Belka, C.; Bezjak, A.; Bradley, J.; Daly, M.E.; DeRuysscher, D.; Dziadziuszko, R.; Faivre-Finn, C.; Flentje, M.; Gore, E.; et al. Practice recommendations for lung cancer radiotherapy during the COVID-19 pandemic: An ESTRO-ASTRO consensus statement. Radiother. Oncol. 2020, 146, 223–229. [Google Scholar] [CrossRef]
- Daly, M.E.; Perks, J.R.; Chen, A.M. Patterns-of-care for thoracic stereotactic body radiotherapy among practicing radiation oncologists in the United States. J. Thorac. Oncol. 2013, 8, 202–207. [Google Scholar] [CrossRef]
- Bongers, E.M.; Haasbeek, C.J.; Lagerwaard, F.J.; Slotman, B.J.; Senan, S. Incidence and risk factors for chest wall toxicity after risk-adapted stereotactic radiotherapy for early-stage lung cancer. J. Thorac. Oncol. 2011, 6, 2052–2057. [Google Scholar] [CrossRef]
- Manyam, B.V.; Videtic, G.M.M.; Verdecchia, K.; Reddy, C.A.; Woody, N.M.; Stephans, K.L. Effect of Tumor Location and Dosimetric Predictors for Chest Wall Toxicity in Single-Fraction Stereotactic Body Radiation Therapy for Stage I Non-Small Cell Lung Cancer. Pract. Radiat. Oncol. 2019, 9, e187–e195. [Google Scholar] [CrossRef]
- Nambu, A.; Onishi, H.; Aoki, S.; Tominaga, L.; Kuriyama, K.; Araya, M.; Saito, R.; Maehata, Y.; Komiyama, T.; Marino, K.; et al. Rib fracture after stereotactic radiotherapy for primary lung cancer: Prevalence, degree of clinical symptoms, and risk factors. BMC Cancer 2013, 13, 68. [Google Scholar] [CrossRef]
- Stephans, K.L.; Djemil, T.; Tendulkar, R.D.; Robinson, C.G.; Reddy, C.A.; Videtic, G.M. Prediction of chest wall toxicity from lung stereotactic body radiotherapy (SBRT). Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Sato, M.; Hirose, K.; Akimoto, H.; Kawaguchi, H.; Hatayama, Y.; Ono, S.; Takai, Y. Radiation-induced rib fracture after stereotactic body radiotherapy with a total dose of 54–56 Gy given in 9–7 fractions for patients with peripheral lung tumor: Impact of maximum dose and fraction size. Radiat. Oncol. 2015, 10, 99. [Google Scholar] [CrossRef][Green Version]
- Asai, K.; Shioyama, Y.; Nakamura, K.; Sasaki, T.; Ohga, S.; Nonoshita, T.; Yoshitake, T.; Ohnishi, K.; Terashima, K.; Matsumoto, K.; et al. Radiation-induced rib fractures after hypofractionated stereotactic body radiation therapy: Risk factors and dose-volume relationship. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, H.J.; Chang, A.R. Predictors of chest wall toxicity after stereotactic ablative radiotherapy using real-time tumor tracking for lung tumors. Radiat. Oncol. 2017, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Taremi, M.; Hope, A.; Lindsay, P.; Dahele, M.; Fung, S.; Purdie, T.G.; Jaffray, D.; Dawson, L.; Bezjak, A. Predictors of radiotherapy induced bone injury (RIBI) after stereotactic lung radiotherapy. Radiat. Oncol. 2012, 7, 159. [Google Scholar] [CrossRef]
- Bayman, E.O.; Parekh, K.R.; Keech, J.; Selte, A.; Brennan, T.J. A Prospective Study of Chronic Pain after Thoracic Surgery. Anesthesiology 2017, 126, 938–951. [Google Scholar] [CrossRef]
- Steegers, M.A.; Snik, D.M.; Verhagen, A.F.; van der Drift, M.A.; Wilder-Smith, O.H. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J. Pain 2008, 9, 955–961. [Google Scholar] [CrossRef]
- Videtic, G.M.M.; Reddy, C.A.; Woody, N.M.; Stephans, K.L. Ten-Year Experience in Implementing Single-Fraction Lung SBRT for Medically Inoperable Early-Stage Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 436–442. [Google Scholar] [CrossRef]
- Ma, S.J.; Syed, Y.A.; Rivers, C.I.; Suescun, J.A.G.; Singh, A.K. Comparison of single- and five-fraction schedules of stereotactic body radiation therapy for central lung tumours: A single institution experience. J. Radiother. Pract. 2017, 16, 148–154. [Google Scholar] [CrossRef]
- Siva, S.; Slotman, B.J. Stereotactic Ablative Body Radiotherapy for Lung Metastases: Where is the Evidence and What are We Doing With It? Semin. Radiat. Oncol. 2017, 27, 229–239. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.A.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.I.; Rodrigues, G.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.; Mathews, L.; Liu, M.; Schellenberg, D.; Mou, B.; Berrang, T.; Harrow, S.; Correa, R.J.; Bhat, V.; Pai, H.; et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 1-3 Oligometastatic tumors (SABR-COMET-3): Study protocol for a randomized phase III trial. BMC Cancer 2020, 20, 380. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Correa, R.J.; Schneiders, F.; Haasbeek, C.J.; Rodrigues, G.B.; Lock, M.; Yaremko, B.P.; Bauman, G.S.; et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 4-10 oligometastatic tumors (SABR-COMET-10): Study protocol for a randomized phase III trial. BMC Cancer 2019, 19, 816. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, V.; Zhao, J.; Milano, M.T.; Li, L.; Rimner, A.; Das, S.; Li, X.A.; Miften, M.; Liao, Z.; Martel, M.; et al. Organs at Risk Considerations for Thoracic Stereotactic Body Radiation Therapy: What Is Safe for Lung Parenchyma? Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 172–187. [Google Scholar]
- Lee, P.; Loo, B.W., Jr.; Biswas, T.; Ding, G.X.; El Naqa, I.M.; Jackson, A.; Kong, F.M.; LaCouture, T.; Miften, M.; Solberg, T.; et al. Local Control After Stereotactic Body Radiation Therapy for Stage I Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.; Mathew, A.S.; Bahig, H.; Bratman, S.V.; Filion, E.; Glick, D.; Louie, A.V.; Raman, S.; Swaminath, A.; Warner, A.; et al. SUNSET: Stereotactic Radiation for Ultracentral Non-Small-Cell Lung Cancer-A Safety and Efficacy Trial. Clin. Lung Cancer 2018, 19, e529–e532. [Google Scholar] [CrossRef] [PubMed]
- Fakiris, A.J.; McGarry, R.C.; Yiannoutsos, C.T.; Papiez, L.; Williams, M.; Henderson, M.A.; Timmerman, R. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: Four-year results of a prospective phase II study. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 677–682. [Google Scholar] [CrossRef]
- Timmerman, R.; Paulus, R.; Galvin, J.; Michalski, J.; Straube, W.; Bradley, J.; Fakiris, A.; Bezjak, A.; Videtic, G.; Johnstone, D.; et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010, 303, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
| Study | No. | F/u (Median) | Age (Median) | Location | Stage | Dose/Fx | LC | RC | PFS | DFS | OS | Distant Failure |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hof et al., 2003 [23] | 10 | 14.9 | 71 | NA | T1-2N0M0 | 19–26 Gy/1 fx | 80% at follow-up, 50% remained locally controlled | NA | LRFS 89% at 12 months, 71.1% at 24 months | NA | 80% after 12 months, 64% at 24 months | Systemic metastases in 5 patients; time until diagnosis was median of 7.2 months. |
| Fritz et al., 2006 [22] | 33 | 18 | 72 | P | Stage 1 | 30 Gy/1 fx | 94%; probability at 4 years, 83% | NA | NA | NA | Median 20.4 months. 83% at 1 year, 63% at 2 years, 53% at 3 years, 39% at 4 years. | NA |
| Le et al., 2006 [25] | 20 | 18 | 73 | C and P | T1-2N0M0 | 15–30 Gy/1 fx | Overall 1-year FFLR, 67%. 1-year FFLR 100% T1 tumors, 83% T2 tumors >20 Gy, 51% T2 tumors <20 Gy; 1-year FFLR all NSCLC tumors 91% >20 Gy and 54% <20 Gy | NA | NA | NA | 85% at 1 year | NA |
| Videtic et al., 2018 (RTOG 0915) [11] | 84 | 4 years | 75 | P | T1-2N0M0 | 34 Gy/1 fx or 48 Gy/4 fx | 97% arm 1, 93% arm 2 at 1 year; 2-year primary failure rate, 2.6% arm 1, 2.2% arm 2. 5-year primary failure rate, 11% arm 1, 7% arm 2 | NA | Median 2.6 years arm 1, 2.8 years arm 2. 19% arm 1, 33% arm 2 at 5 years. | 77% arm 1, 84% arm 2 at 1 year. 56% arm 1, 71% in arm 2 at 2 years | 85% arm 1, 91% arm 2 at 1 year. 61% arm 1, 78% in arm 2 at 2 years. Median 4.1 years arm 1, 4.6 years arm 2. 30% arm 1, 41% arm 2 at 5 years | 38% arm 1, 41% arm 2. |
| Singh et al., 2019 [10] | 98 | 54 | 71 | P | T1-2N0M0 | 30 Gy/1 fx or 60 Gy/3 fx | 95% arm 1, 97% in arm 2 at 2 years | 2-year regional nodal failure rate, 8% arm 1, 16% arm 2 | 65% arm 1, 50% arm 2 at 2 years | NA | 73% arm 1, 62% arm 2 at 2 years | 13% arm 1, 19% arm 2 at 2 years |
| Study | No. | F/u (Median) | Age (Median) | Location | Dose/Fx | LC | RC | PFS | DFS | OS | Distant Failure |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fritz et al., 2006 [22] | 25 | 22 | 65 | P | 30 Gy/1 fx | 87%; probability at 5 years, 80% | NA | NA | NA | Median 26 months. 97% at 1 year, 73% at 2 years, 42% at 3 years, 42% at 2 years, 42% at 5 years | NA |
| Le et al., 2006 [25] | 12 | 18 | 73 | C and P | 15–30 Gy/1 fx | Overall 1-year FFLR, 25%. 1-year FFLR 44% > 20 Gy | NA | NA | NA | 56% at 1 year | NA |
| Hof et al., 2007 [24] | 61 | 14 | NA | NA | 12–30 Gy/1 fx | LPFR 89% at 1 year, 74% at 2 years, 63% at 3 years | NA | NA | NA | 78.4% at 1 year, 65.1% at 2 years, 47.8% at 3 years | NA |
| Filippi et al., 2014 [21] | 67 | 24 | NA | P | 26 Gy/1 fx | 93% at 1 year, 88% at 2 years; local failures, 11% of patients. | NA | 72% at 1 year, 55% at 2 years | NA | 85% at 1 year, 71% at 2 years. CSS 90% at 1 year, 76% at 2 years | 55% of patients, at median of 8 months post-radiation |
| Siva et al., 2021 [16] | 87 | 36.5 | 66.6 (mean) | P | 28 Gy/1 fx vs 48 Gy/4 fx | 1-year FFLR: 93% vs 95% at 1 year, 64% vs 80% at 3 years | NA | NA | Median 14.3 months vs. 13.2 months | 95% vs 93% at 1 year, 81% vs. 67% at 3 years | Median time to distant failure: 16.0 months vs 14.5 months |
| Study | Grade 1–2 Toxicity | Grade ≥3 Toxicity | Toxicity Notes |
|---|---|---|---|
| Whyte et al., 2003 [26] | NA | 0% | 1 COPD exacerbation, 4 pneumothoraxes s/p fiducial placement |
| Hof et al., 2003 [23] | NA; normal-perifocal tissue reaction, 70% | Grade >2, 0% | Some dyspnea reported, no PFT performed |
| Fritz et al., 2006 [22] | Grade 1 radiation dermatitis, 7%; asymptomatic radiation pneumonitis via CT at 6 months, 73% | 0% | NSCLC patients pneumonitic alterations with asymptomatic, temporary pleural effusions, 24%; no changes in respiratory function |
| Le et al., 2006 [25] | Grade 2 pleural effusions, pneumonitis, radiation-induced atrial fibrillation, 12.5% | Grade 3 pneumonitis, 3%; Grade 4, 0%; Grade 5 pneumonitis, pleural effusions, tracheoesophageal fistula, 9% | 3 post-treatment deaths; all received prior chemotherapy, 2 prior radiation therapy |
| Videtic et al., 2019 (RTOG 0915) [11] | 1 additional Grade 1 AE arm 1, 1 Grade 2 AE (previous Grade 1) arm 2 | Current rates: Grade ≥3 2.6% arm 1, 11.1% arm 2 | Reported toxicities: DLCO changes, pneumonitis, PFT changes; 1 treatment-related deaths (arm 2) |
| Singh et al., 2019 [10] | Grade 1–2, 22% arm 1, 20% arm 2 | Grade 3, 17% arm 1, 15% arm 2; no Grade 4 or 5 | Better social functioning, fewer constitutional symptoms, less dyspnea arm 1. |
| Hof et al., 2007 [24] | Grade 1–2, 0%; normal perifocal tissue changes, 70% | Grade 3 pneumonitis requiring treatment and supplemental oxygen, 5%. Grade 4 or higher, 0% | None |
| Filippi et al., 2014 [21] | Grade 1, 10%. | Grade 2–3 late, 12% | Peripheral lesions with late chest wall toxicity, 9% (2 rib fractures, 4 chronic chest pain) |
| Siva et al., 2021 [16] | NA | 3–5%; 1 patient with interstitial lung disease received multi-fraction SBRT and had grade 5 treatment-related hypoxia and radiation pneumonitis | Radiation dermatitis and esophagitis were more common in multi-fraction SBRT arm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartl, A.J.; Mahoney, M.; Hennon, M.W.; Yendamuri, S.; Videtic, G.M.M.; Stephans, K.L.; Siva, S.; Farrugia, M.K.; Ma, S.J.; Singh, A.K. Systematic Review of Single-Fraction Stereotactic Body Radiation Therapy for Early Stage Non-Small-Cell Lung Cancer and Lung Oligometastases: How to Stop Worrying and Love One and Done. Cancers 2022, 14, 790. https://doi.org/10.3390/cancers14030790
Bartl AJ, Mahoney M, Hennon MW, Yendamuri S, Videtic GMM, Stephans KL, Siva S, Farrugia MK, Ma SJ, Singh AK. Systematic Review of Single-Fraction Stereotactic Body Radiation Therapy for Early Stage Non-Small-Cell Lung Cancer and Lung Oligometastases: How to Stop Worrying and Love One and Done. Cancers. 2022; 14(3):790. https://doi.org/10.3390/cancers14030790
Chicago/Turabian StyleBartl, Austin J., Mary Mahoney, Mark W. Hennon, Sai Yendamuri, Gregory M. M. Videtic, Kevin L. Stephans, Shankar Siva, Mark K. Farrugia, Sung Jun Ma, and Anurag K. Singh. 2022. "Systematic Review of Single-Fraction Stereotactic Body Radiation Therapy for Early Stage Non-Small-Cell Lung Cancer and Lung Oligometastases: How to Stop Worrying and Love One and Done" Cancers 14, no. 3: 790. https://doi.org/10.3390/cancers14030790
APA StyleBartl, A. J., Mahoney, M., Hennon, M. W., Yendamuri, S., Videtic, G. M. M., Stephans, K. L., Siva, S., Farrugia, M. K., Ma, S. J., & Singh, A. K. (2022). Systematic Review of Single-Fraction Stereotactic Body Radiation Therapy for Early Stage Non-Small-Cell Lung Cancer and Lung Oligometastases: How to Stop Worrying and Love One and Done. Cancers, 14(3), 790. https://doi.org/10.3390/cancers14030790






