The Role of Bruton’s Kinase Inhibitors in Chronic Lymphocytic Leukemia: Current Status and Future Directions
Abstract
Simple Summary
Abstract
1. Introduction
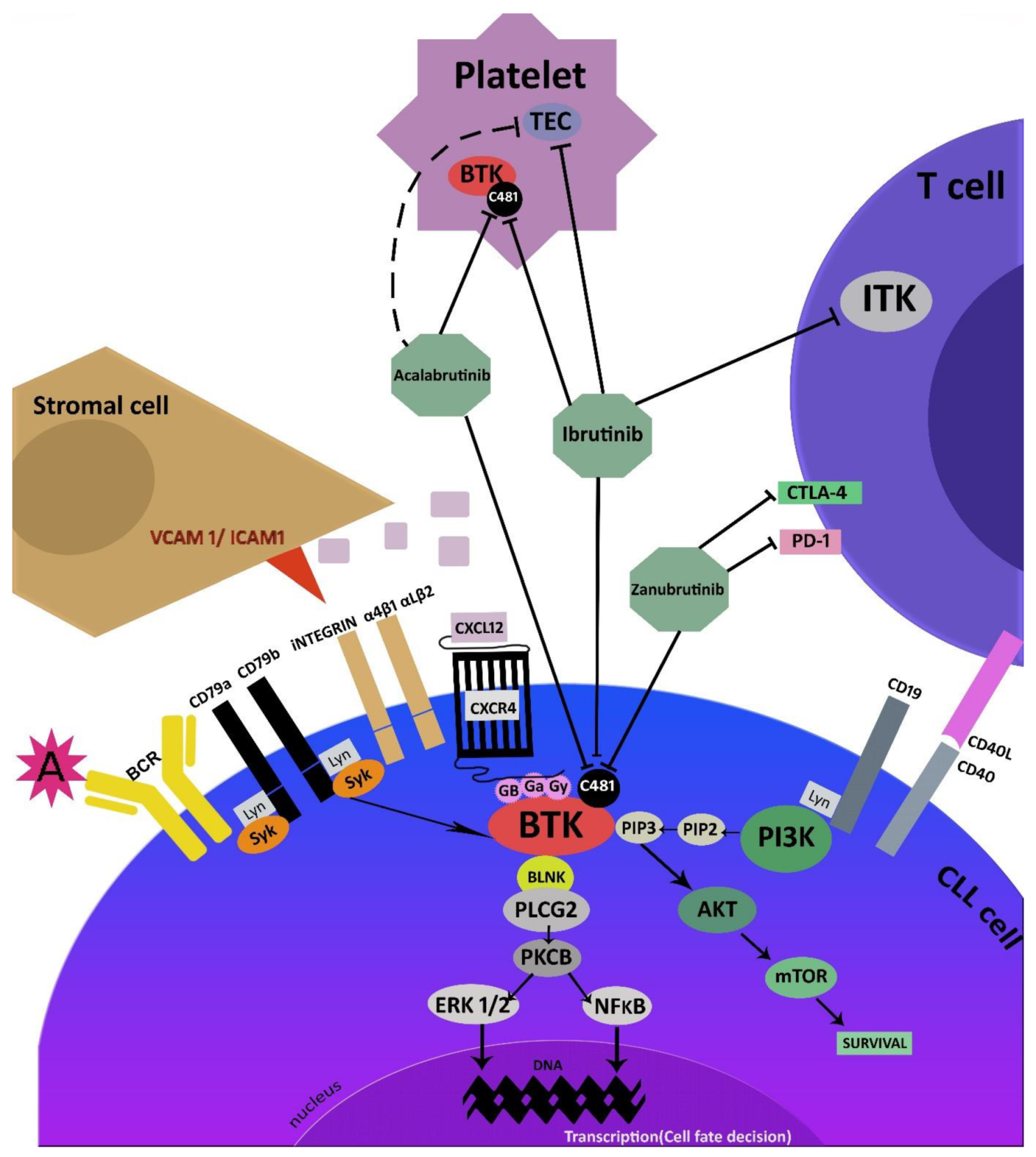
2. Mechanism of Action
3. Irreversible Covalent BTK Inhibitors
3.1. Ibrutnib
3.1.1. Ibrutinib in Relapsed and Refractory CLL
| Study | Treatment | Patients, N | Median Age [years] | Median Follow-Up [months] | ORR (CR) | Median PFS | Median OS | Discontinu Ation Rates |
|---|---|---|---|---|---|---|---|---|
| Byrd et al. [33] Munir et al. [34] (RESONATE) | Ibrutinib vs. Ofatumumab | 195 vs. 196 | 67 vs. 67 | 41 | 91% (21%) vs. 82% (11%) | 44.1 m vs. 8.1 m (p ˂ 0.0001) | 67.7 m vs. 65.1 m HR = 0.810 | 16% for ibrutinib |
| Fraser et al. [39] (HELIOS) | Ibrutinib + BR vs. BR | 289 vs. 289 | 64 vs. 63 | 34.8 | 87% (38%) vs. 66% (8%) | NR vs. 13.3 m, p < 0.0001 | NR vs. NR, p = 0.0598 | 24% vs. 45% |
| Sharman et al. [41] (GENUINE) | Ibrutinib + Ublituximab vs. Ibrutinib | 64 vs. 62 | 66 vs. 67 | 41.6 | 83% (17%) vs. 65% (3%) | NR vs. 35·9 m p = 0.016 | NR vs. NR, p = 0.12 | 15% vs. 12% |
| Byrd et al. [16] (ELEVATE RR) | Acalabrutinib vs. Ibrutinib | 268 vs. 265 | 66 vs. 65 | 31.3 | 81.0% (NA) vs. 77% (NA) | 38.4 vs. 38.4 p ˂ 0.001 | NR vs. NR HR, 0.82 | 14.7% vs. 21.3% |
| Ghia et al. [42] (ASCEND) | Acalabrutinib vs. IR or BR | 155 vs. 155 | 65 vs. 67 | 40.9 | 81% (0%) vs. 75% (1%) | NR vs. 16.5 m p < 0.0001 | 89% vs. 80% at 1 y p < 0.0001). | 14.7% vs. 21% |
| Hillmen et al. [17] ALPINE | Zanubrutinib vs. Ibrutinib | Total 415 | 66 vs. 65 | 15 | 76% (NA) vs. 64%(NA) | 94.9% vs. 84% at 1 y p < 0.0007 | 97% vs. 93% at 1 y | 7.8% vs. 13.0% |
3.1.2. Ibrutinib in Previously Untreated CLL
| Study | Treatment | N | Median Age [years] | Median Follow-Up [months] | ORR (CR) Rate | Median PFS | Median OS | Discontinuation Rates |
|---|---|---|---|---|---|---|---|---|
| Burger at al. [9,34] RESONATE 2 | Ibrutinib vs. Chlorambucil | 136 vs. 133 | 73 vs. 72 | 18.4 | 86% (4%) vs. 35% (2%) | NR vs. 18.9 m; p < 0.001 | HR 0.16 (95% CI, 0.05–0.56) p = 0.001 | 13% for Ibrutinib |
| Fraser et al. [39] (HELIOS) | Ibrutinib + BR vs. BR | 289 vs. 289 | 64 vs. 63 | 63.7 | 87% (38%) vs. 66% (8%) | NR versus 13.3 m p < 0.0001 | NR vs. NR, p = 0.0598 | 47% vs. 51.2% |
| Burger et al. [43] | Ibrutinib vs. Ibrutinib + R | 104 vs. 104 | 65 vs. 65 | 36 | 92.3% (20.2%) vs. 92.3% (26.3%) | 86% vs 86.9% at 3 y p = 0.912) | 92% vs. 89% at 3y p = 0.572 | Ibrutinib 34% at 3 y |
| Moreno et al. [8,44] (iLLUMINATE) | Ibrutinib + O vs. Chlorambucil + O | 113 vs. 116 | 70 vs. 72 | 31.3 | 88% (19%) vs. 73% (9%) | NR vs. 19 m; p < 0.00001 | 86% vs. 85% at 30 m | 9% vs. 13% |
| Woyach et al. [45] (A041202) | BR vs. Ibrutinib vs. Ibrutinib + R. | 183 vs. 182 vs. 182 | 70 vs. 71 vs. 71 | 38 m | 81% vs. 93% vs. 94% at 2 y | 74% vs. 87% vs. 88% at 2 y p < 0.001 | 95% vs. 90% vs. 94% at 2 y p ≥ 0.65 | Ibrutinib 37% Ibrutinib + R 36% at median 38 m |
| Shanefelt et al. [46] (ECOG 1912) | Ibrutinib vs. FCR | 354 vs. 175 | 56.7 vs. 56.7 | 33.6 | 95.8% (17.2%) vs. 81.1% (30.3%) | 89.4 vs. 72.9% at 3-yrs p < 0.001 | 98,8% vs. 91.5% at 3-yrs p < 0.001 | Ibrutinib arm 21.2% |
| Kater et al. [47] (GLOWE) | Ibrutinib + Venetoclax vs. Chlorambucil + O | 106 vs. 105 | 71 vs. 71 | 27.7 | 86.8% (38.7%) vs. 84.8% (11.4%) | NR vs. 21 m p < 0.0001 | 11 deaths vs. 12 deaths HR 1.048 | NA |
| Tam et al. [48] (SEQUOIA) | Zanubrutinib vs. BR | 241 vs. 238 | 70 vs. 70 | 26.2 | 94.6% (6.6%) vs. 85.3% (15.1%) | 85.5% vs. 69.5% at 2 y p < 0.0001 | 94.3 vs. 94.6 at 2 y | 8.3% vs. 13.7% |
| Sharman et al. [49,50] (ELEVATE TN) | Acalalabrutinib vs. Acalalabrutinib + O vs. Chlorambucil + O | 179 vs. 179 vs. 177 | 70 vs. 70 | 46.9 | 89.9% (11.2%) vs. 96.1% (30.7%) vs. 87.4% (13.0%) | NR vs. NR vs. 27.8 m p < 0.0001 | 88% vs. 92.9% vs. 92% at 4 y p = 0.0836 | Acalabrutinib 30.7% vs. Acalabrutinib + O 25.1% vs. Chlorambucil + O 22.6% |
3.2. Acalabrutinib
3.3. Zanubrutinib
3.4. Other Irreversible BTK Inhibitors
3.4.1. Spebrutinib
3.4.2. Orelabrutinib
3.4.3. Tirabrutinib
3.4.4. SHR1459
3.4.5. DTRMWXHS-12
4. Reversible BTK Inhibitors
4.1. Pirtobrutinib
4.2. Vecabrutinib
4.3. Fenebrutinib
4.4. Nemtabrutinib
5. Resistance to BTK Inhibitors
6. Adverse Events
6.1. Bleeding and Bruising
6.2. Cardiovascular Complications
6.3. Cytopenias
6.4. Infections
6.5. Arthralgias and Myalgias
6.6. Dermatologic Complications
6.7. Headaches
6.8. Diarrhea
7. BTK Inhibitors and the COVID-19 Pandemic
8. Future Directions
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Dohner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive man-agement of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef] [PubMed]
- Eichhorst, B.; Robak, T.; Montserrat, E.; Ghia, P.; Niemann, C.; Kater, A.; Gregor, M.; Cymbalista, F.; Buske, C.; Hillmen, P.; et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 32, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, 1975–2016; National Cancer Institute: Bethesda, MD, USA, 2019. Available online: https://seer.cancer.gov/csr/1975_2016/ (accessed on 9 April 2020).
- Alrawashdh, N.; Sweasy, J.; Erstad, B.; McBride, A.; Persky, D.O.; Abraham, I. Survival trends in chronic lymphocytic leukemia across treatment eras: US SEER database analysis (1985–2017). Ann. Hematol. 2021, 100, 2501–2512. [Google Scholar] [CrossRef] [PubMed]
- Brullo, C.; Villa, C.; Tasso, B.; Russo, E.; Spallarossa, A. Btk Inhibitors: A medicinal chemistry and drug delivery perspective. Int. J. Mol. Sci. 2021, 22, 7641. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Wang, J.; Shi, Y.; Qian, H.; Liu, P. Inhibitors targeting Bruton’s tyrosine kinase in cancers: Drug development advances. Leukemia 2021, 35, 312–332. [Google Scholar] [CrossRef] [PubMed]
- Robak, T.; Burger, J.A.; Tedeschi, A.; Barr, P.M.; Owen, C.; Bairey, O.; Hillmen, P.; Simpson, D.; Grosicki, S.; Devereux, S.; et al. Single-agent ibrutinib versus chemoimmunotherapy regimens for treatment-naïve patients with chronic lymphocytic leu-kemia: A cross-trial comparison of phase 3 studies. Am. J. Hematol. 2018, 93, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Greil, R.; Demirkan, F.; Tedeschi, A.; Anz, B.; Larratt, L.; Simkovic, M.; Novak, J.; Strugov, V.; Gill, D.; et al. First-line treatment of chronic lymphocytic leukemia with ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab: Final analysis of the randomized, phase 3 iLLUMINATE trial. Haematologica 2022, 107. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Kay, N.E.; Reddy, N.M.; Coutre, D.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Ibrutinib versus ofa-tumumab in previously treated chronic lymphoid leukemia. N. Engl. J. Med. 2014, 371, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Ghia, P.; Pluta, A.; Wach, M.; Lysak, D.; Kozak, T.; Simkovic, M.; Kaplan, P.; Kraychok, I.; Illes, A.; De La Serna, J.; et al. Acalabrutinib vs Rituximab plus Idelalisib (IdR) or Bendamustine (BR) by investigator choice in relapsed/refractory (RR) chronic lymphocytic leukemia: Phase 3 ASCEND study. Hematol. Oncol. 2019, 37, 86–87. [Google Scholar] [CrossRef]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): A randomised, controlled, phase 3 trial. Lancet 2020, 395, 1278–1291. [Google Scholar] [PubMed]
- Zain, R.; Vihinen, M. Structure-Function Relationships of Covalent and Non-Covalent BTK Inhibitors. Front. Immunol. 2021, 12, 694853. [Google Scholar] [CrossRef] [PubMed]
- Estupiñán, H.Y.; Wang, Q.; Berglöf, A.; Schaafsma, G.C.P.; Shi, Y.; Zhou, L.; Mohammad, D.K.; Yu, L.; Vihinen, M.; Zain, R.; et al. BTK gatekeeper residue variation combined with cysteine 481 substitution causes super-resistance to irreversible inhibitors acalabrutinib, ibrutinib and zanubrutinib. Leukemia 2021, 35, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.; Furman, R.R.; Coutre, S.E.; Flinn, I.W.; Burger, J.A.; Blum, K.A.; Grant, B.; Sharman, J.P.; Coleman, M.; Wierda, W.G.; et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: A 5-year experience. Blood 2018, 131, 1910–1919. [Google Scholar]
- Byrd, J.C.; Hillmen, P.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; O’Brien, S.; Yenerel, M.N.; Illés, A.; Kay, N.; et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: Results of the first randomized phase III trial. J. Clin. Oncol. 2021, 39, 3441–3452. [Google Scholar] [CrossRef]
- Hillmen., P.; Eichhorst., B.; Brown, J.R.; Lamanna, N.; O’Brien, S.; Tam, C.S.; Qiu, L.; Kazmierczak, M.; Zhou, K.; Šimkovič, M.; et al. First Interim Analysis of Alpine Study: Results of a Phase 3 Randomized Study of Zanubrutinib vs. Ibrutinib in Patients with Re-lapsed/Refractory Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma; EHA Library: Hague, The Netherlands, 2021; 330170; LB1900; Available online: https://library.ehaweb.org/eha/2021/eha2021-virtualcon-gress/330170/peter.hillmen.first.interim.analysis.of.alpine.study.results.of.a.phase.3.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asearch%3Dlb1900 (accessed on 11 June 2021).
- Ahn, I.E.; Brown, J.R. Targeting Bruton’s tyrosine kinase in CLL. Front. Immunology 2021, 12, 687458. [Google Scholar]
- Woyach, J.A.; Bojnik, E.; Ruppert, A.S.; Stefanovski, M.R.; Goettl, V.M.; Smucker, K.A.; Smith, L.L.; Dubovsky, L.S.J.; Towns, W.H.; MacMurray, M.M.; et al. Bruton’s tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL). Blood 2014, 123, 1207–1213. [Google Scholar] [CrossRef]
- Gauld, S.B.; Porto, J.M.D.; Cambier, J.C. B cell antigen receptor signaling: Roles in cell development and disease. Science 2002, 296, 1641–1642. [Google Scholar] [CrossRef]
- Davis, R.E.; Ngo, V.N.; Lenz, G.; Tolar, P.; Young, R.M.; Romesser, P.B.; Kohlhammer, H.; Lamy, L.; Zhao, H.; Yang, Y.; et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 2010, 463, 88–92. [Google Scholar] [CrossRef]
- Kaptein, A.; de Bruin, G.; Emmelot-van Hoek, M.; van de Kar, B.; de Jong, A.; Gulrajani, M.; Demont, D.; Covey, T.; Mittag, D.; Barf, T. Potency and selectivity of BTK inhibitors in clinical development for B-Cell malignancies. Blood 2018, 132, 1871. [Google Scholar] [CrossRef]
- Alsadhan, A.; Cheung, J.; Gulrajani, M.; Gaglione, E.M.; Nierman, P.; Hamdy, A.; Izumi, R.; Bibikova, E.; Patel, P.; Sun, C.; et al. Pharmacodynamic Analysis of btk inhibition in patients with chronic lymphocytic leukemia treated with acalabrutinib. Clin. Cancer Res. 2020, 26, 2800–2809. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.J.; Kim, E.S.; Eaby, B.; Garey, J.; West, D.P.; Lacouture, M.E.; Zhu, A.X.; Fuchs, C.S.; Clark, J.W.; Muzikansky, A.; et al. Epidermal growth factor receptor inhibitor–associated cutaneous toxicities: An evolving paradigm in clinical management. Oncologist 2007, 12, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Barf, T.; Covey, T.; Izumi, R.; Van De Kar, B.; Gulrajani, M.; Van Lith, B.; Van Hoek, M.; De Zwart, E.; Mittag, D.; Demont, D.; et al. Acalabrutinib (ACP-196): A covalent Bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J. Pharmacol. Exp. Ther. 2017, 363, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Pavlasova, G.; Mraz, M. The regulation and function of CD20: An “enigma” of B-cell biology and targeted therapy. Haema-tologica 2020, 105, 1494–1506. [Google Scholar] [CrossRef]
- Cervantes-Gomez, F.; Lamothe, B.; Woyach, J.A.; Wierda, W.G.; Keating, M.J.; Balakrishnan, K.; Gandhi, V. Pharmacological and Protein Profiling Suggests Venetoclax (ABT-199) as optimal partner with ibrutinib in chronic lymphocytic leukemia. Clin. Cancer Res. 2015, 21, 3705–3715. [Google Scholar] [CrossRef] [PubMed]
- Haselager, M.V.; Kielbassa, K.; Ter Burg, J.; Bax, D.J.C.; Fernandes, S.M.; Borst, J.; Tam, C.; Forconi, F.; Chiodin, G.; Brown, J.R.; et al. Changes in Bcl-2 members after ibrutinib or venetoclax uncover functional hierarchy in determining resistance to venetoclax in CLL. Blood 2020, 136, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Scheerens, H.; Li, S.-J.; Schultz, B.E.; Sprengeler, P.A.; Burrill, L.C.; Mendonca, R.V.; Sweeney, M.D.; Scott, K.C.K.; Grothaus, P.G.; et al. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem 2006, 2, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Honigberg, L.A.; Smith, A.M.; Sirisawad, M.; Verner, E.; Loury, D.; Chang, B.; Li, S.; Pan, Z.; Thamm, D.H.; Miller, R.A.; et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. USA 2010, 107, 13075–13080. [Google Scholar] [CrossRef]
- Brown, J.R. How I treat CLL patients with ibrutinib. Blood 2018, 131, 379–386. [Google Scholar] [CrossRef]
- Heerema, N.A.; Luan, Y.; Liu, E.A.; Dean, J.P.; O’Brien, S. Ibrutinib treatment for first-line and relapsed/refractory chronic lymphocytic leukemia: Final analysis of the pivotal phase Ib/II PCYC-1102 study. Clin. Cancer Res. 2020, 26, 3918–3927. [Google Scholar]
- Byrd, J.C.; Hillmen, P.; Brien, O.S.; Barrientos, J.C.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; Barr, P.M.; et al. Long-term follow-up of the RESONATE phase 3 trial of ibrutinib vs. ofatumumab. Blood 2019, 133, 2031–2042. [Google Scholar] [CrossRef] [PubMed]
- Munir, T.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Barr, P.M.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously-treated chronic lym-phocytic leukemia or small lymphocytic lymphoma. Am. J. Hematol. 2019, 94, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.J.; Danilov, A.V. The evolving role of Bruton’s tyrosine kinase inhibitors in chronic lymphocytic leukemia. Ther. Adv. Hematol. 2021, 12, 2040620721989588. [Google Scholar] [CrossRef] [PubMed]
- Jaglowski, S.M.; Jones, J.A.; Nagar, V.; Flynn, J.M.; Andritsos, L.A.; Maddocks, K.J.; Woyach, J.A.; Blum, K.A.; Grever, M.R.; Smucker, K.; et al. Safety and activity of BTK inhibitor ibru- tinib combined with ofatumumab in chronic lymphocytic leu- kemia: A phase 1b/2 study. Blood 2015, 126, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Keating, M.J.; Wierda, W.G.; Sivina, M.; Thompson, P.A.; Ferrajoli, A.; Estrov, Z.; Kantarjian, H.; O’Brien, S.; Burger, J.A. Long-term follow-up of treatment with ibrutinib and rituximab in patients with high-risk chronic lympho- cytic leu-kemia. Clin Cancer Res. 2017, 23, 2154–2158. [Google Scholar] [CrossRef]
- Burger, J.A.; Sivina, M.; Jain, N.; Kim, E.; Kadia, T.; Estrov, Z.; Nogueras-Gonzalez, G.M.; Huang, X.; Jorgensen, J.; Li, J.; et al. Randomized trial of ibrutinib vs ibrutinib plus rituximab in patients with chronic lymphocytic leukemia. Blood 2019, 133, 1011–1019. [Google Scholar] [CrossRef]
- Fraser, G.; Cramer, P.; Demirkan, F.; Silva, R.S.; Grosicki, S.; Pristupa, A.; Janssens, A.; Mayer, J.; Bartlett, N.L.; Dilhuydy, M.S.; et al. Updated results from the phase 3 HELIOS study of ibrutinib, bendamustine, and rituximab in relapsed chronic lym-phocytic leukemia/small lymphocytic lymphoma. Leukemia 2019, 33, 969–980. [Google Scholar] [CrossRef]
- Hillmen, P.; Fraser, G.; Jones, J.; Rule, S.; Brien, S.; Dilhuydy, M.S.; Pristupa, A.; Janssens, A.; Mayer, J.; Bartlett, N.L.; et al. Comparing single-agent ibrutinib, bendamustine plus rituximab (BR) and ibrutinib plus BR in patients with previous-ly-treated chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): An indirect comparison of the RESONATE and HELIOS trials. Blood 2015, 129, 2944. [Google Scholar] [CrossRef]
- Sharman, J.P.; Brander, D.M.; Mato, A.R.; Ghosh, N.; Schuster, S.J.; Kambhampati, S.; Burke, J.M.; Lansigan, F.; Schreeder, M.T.; Lunin, S.D.; et al. Ublituximab plus ibrutinib versus ibrutinib alone for patients with relapsed or refractory high-risk chronic lymphocytic leukaemia (GENUINE): A phase 3, multicentre, open-label, randomised trial. Lancet Haematol. 2021, 8, e254–e266. [Google Scholar] [CrossRef]
- Ghia, P.; Pluta, A.; Wach, M.; Lysak, D.; Kozak, T.; Šimkovič, M.; Kaplan, P.; Kraychok, I.; Illes, A.; De la Serna, J.; et al. ASCEND: Phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J. Clin. Oncol. 2020, 38, 2849–2861. [Google Scholar] [CrossRef]
- Burger, J.A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Tedeschi, A.; Bairey, O.; Hillmen, P.; Coutre, S.E.; Devereux, S.; et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 2020, 34, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Greil, R.; Demirkan, F.; Tedeschi, A.; Anz, B.; Larratt, L.; Šimkovič, M.; Samoilova, O.; Novak, J.; Ben-Yehuda, D.; et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 43–56. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.M.; Ding, W.; Bartlett, N.; Brander, D.M.; Barr, P.M.; Rogers, K.A.; et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N. Engl. J. Med. 2018, 379, 2517–2528. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Wang, X.V.; Kay, N.E.; Hanson, C.A.; O’brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Ibrutinib–Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2019, 381, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Kater, A.; Owen, C.; Moreno, C.; Follows, G.; Munir, T.; Levin, M.D.; Benjamini, O.; Janssens, A.; Osterborg, A.; Robak, T.; et al. Fixed-duration ibrutinib and venetoclax (I+V) versus chlorambucil plus obinutuzumab (Clb+O) for first-line (1L) chroniclymphocytic leukemia (CLL): Primary analysis of the phase 3 glow study. In Proceedings of the 2021 European Hematology Association, Virtual Congress, 9–17 June 2021. Abstract LB1902. [Google Scholar]
- Tam, C.; Giannopoulos, K.; Jurczak, W.; Šimkovič, M.; Shadman, M.; Österborg, A.; Laurenti, L.; Walker, P.; Opat, S.; Chan, H.; et al. SEQUOIA: Results of a phase 3 randomized study of zanubrutinib versus bendamustine + rituximab (BR) in patients with treatment-naïve (TN) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Blood 2021, 138, 396. [Google Scholar] [CrossRef]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Kamdar, M.K.; Munir, T.; Corbett, G.; Fogliatto, L.M.; Herishanu, Y.; et al. Acalabrutinib ± obinutuzumab versus obinutuzumab + chlorambucil in treatment-naïve chronic lymphocytic leukemia: Elevate-TN four-year follow up. J. Clin. Oncol. 2021, 39, 7509. [Google Scholar] [CrossRef]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Hillmen, P.; Rawstron, A.C.; Brock, K.; Muñoz-Vicente, S.; Yates, F.J.; Bishop, R.; Boucher, R.; Macdonald, D.; Fegan, C.; McCaig, A.; et al. Ibrutinib plus venetoclax in relapsed/refractory chronic lymphocytic leukemia: The CLARITY Study. J. Clin. Oncol. 2019, 37, 2722–2729. [Google Scholar] [CrossRef]
- Ghia, P.; Allan, J.N.; Siddiqi, T.; Kipps, T.J.; Jacobs, R.; Opat, S.; Paul, M.; Barr, P.M.; Tedeschi, A.; Trentin, L.; et al. Fixed-duration (FD) first-line treatment (tx) with ibrutinib (I) plus venetoclax (V) for chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL): Primary analysis of the FD cohort of the phase 2 captivate study. J. Clin. Oncol. 2021, 39, 7501. [Google Scholar] [CrossRef]
- Byrd, J.C.; Harrington, B.; O’brien, S.; Jones, J.A.; Schuh, A.; Devereux, S.; Chaves, J.; Wierda, W.G.; Awan, F.T.; Brown, J.R.; et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 323–332. [Google Scholar] [CrossRef]
- Byrd, J.C.; Wierda, W.G.; Schuh, A.; Devereux, S.; Chaves, J.M.; Brown, J.R.; Hillmen, P.; Martin, P.; Awan, F.T.; Stephens, D.M.; et al. Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: Updated phase 2 results. Blood 2020, 135, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Woyach, J.A.; Furman, R.R.; Martin, P.; O’Brien, S.; Brown, J.R.; Stephens, D.M.; Barrientos, J.C.; Devereux, S.; Hillmen, P.; et al. Acalabrutinib in treatment-naive chronic lymphocytic leukemia. Blood 2021, 137, 3327–3338. [Google Scholar] [CrossRef] [PubMed]
- Woyach, J.A.; Blachly, J.S.; Rogers, K.A.; Bhat, S.A.; Jianfar, M.; Lozanski, G.; Weiss, D.M.; Andersen, B.L.; Gulrajani, M.; Frigault, M.M.; et al. Acalabrutinibplus obinutuzumab in treatment-naive and relapsed/refractory chronic lymphocytic leukemia. Cancer Discov. 2020, 10, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.M. Second-generation Bruton’s tyrosine kinase inhibitors: Simply the best treatments for chronic lymphocytic leukemia? J. Clin. Oncol. 2021, 39, 3419–3422. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Lampson, B.L.; Tyekucheva, S.; Wang, Z.; Lowney, J.C.; Pazienza, S.; Montegaard, J.; Patterson, V.; Weinstock, M.; Crombie, J.L.; et al. Acalabrutinib, venetoclax, and obinutuzumab as frontline treatment for chronic lymphocytic leukaemia: A single-arm, open-label, phase 2 study. Lancet Oncol. 2021, 22, 1391–1402. [Google Scholar] [CrossRef]
- Sheng, Z.; Song, S.; Yu, M.; Zhu, H.; Gao, A.; Gao, W.; Ran, X.; Huo, D. Comparison of acalabrutinib plus obinutuzumab, ib-rutinib plus obinutuzumab and venetoclax plus obinutuzumab for untreated CLL: A network meta-analysis. Leuk. Lymphoma 2020, 61, 3432–3439. [Google Scholar] [CrossRef] [PubMed]
- Robak, T.; Robak, P. BCR signaling in chronic lymphocytic leukemia and related inhibitors currently in clinical studies. Int. Rev. Immunol. 2013, 32, 358–376. [Google Scholar] [CrossRef]
- Tam, C.; Grigg, A.P.; Opat, S.; Ku, M.; Gilbertson, M.; Anderson, M.A.; Seymur, J.F.; Ritchie, D.S.; Dircoletto, C.; Dimowski, B.; et al. The BTK inhibitor, BGB-3111, is safe, tolerable, and highly active in patients with relapsed/refractory B-cell malignancies: Initial report of a phase 1 first-in-human trial. Blood 2015, 126, 832. [Google Scholar] [CrossRef]
- Flinsenberg, T.W.; Tromedjo, C.C.; Hu, N.; Liu, Y.; Guo, Y.; Thia, K.Y.; Noori, T.; Song, X.; Yeang, H.X.A.; Tantalo, D.G.; et al. Differential effects of BTK inhibitors ibrutinib and zanubrutinib on NK-cell effector function in patients with mantle cell lymphoma. Haematologica 2019, 105, e76–e79. [Google Scholar] [CrossRef]
- Zou, Y.; Zhu, H.; Li, X.; Xia, Y.; Miao, K.; Zhao, S.; Wu, Y.; Wang, L.; Xu, W.; Li, J. The impacts of zanubrutinib on immune cells in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Hematol. Oncol. 2019, 37, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Trotman, J.; Opat, S.; Burger, J.A.; Cull, G.; Gottlieb, D.; Harrup, R.; Johnston, P.B.; Marlton, P.; Munoz, J.; et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 2019, 134, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Cull, G.; Simpson, D.; Opat, S.; Burger, J.A.; Trotman, J.; Marlton, P.; Gottlieb, D.; Munoz, J.; Seymour, J.F.; Roberts, A.W.; et al. Treatment with the Bruton tyrosine kinase inhibitor zanubrutinib (BGB-3111) demon-strates high overall response rate and durable responses in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): Updated results from a phase 1/2 Trial. Blood 2019, 134, 500. [Google Scholar]
- Tam, C.S.; Robak, T.; Ghia, P.; Kahl, B.S.; Walker, P.; Janowski, W.; Simpson, D.; Shadman, M.; Ganly, P.S.; Laurenti, L.; et al. Zanubrutinib monotherapy for patients with treatment-naïve chronic lymphocytic leukemia and 17p deletion. Haematologica 2020, 106, 2354–2363. [Google Scholar] [CrossRef]
- Hillmen, P.; Brown, J.R.; Eichhorst, B.F.; Lamanna, N.; O’brien, S.M.; Qiu, L.; Salmi, T.; Hilger, J.; Wu, K.; Cohen, A.; et al. ALPINE: Zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Futur. Oncol. 2020, 16, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, A.; Ferrant, E.; Flinn, I.W.; Tam, C.S.; Ghia, P.; Robak, T.; Brown, J.R.; Ramakrishnan, V.; Tian, T.; Kuwahara, S.B.; et al. Zanubrutinib in combination with venetoclax for patients with treatment-naïve (TN) chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) with del(17p): Early results from arm D of the SEQUOIA (BGB-3111-304) trial. Blood 2021, 138, 67. [Google Scholar] [CrossRef]
- Soumerai, J.D.; Mato, A.R.; Dogan, A.; Seshan, V.E.; Joffe, E.; Flaherty, K.; Carter, J.; Hochberg, E.; Barnes, J.A.; Hamilton, A.M.; et al. Zanubrutinib, obinutuzumab, and venetoclax with minimal residual disease-driven discontinuation in previously un-treated patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: A multicentre, single-arm, phase 2 trial. Lancet Haematol. 2021, 8, e879–e890. [Google Scholar] [CrossRef]
- Chen, W.; Loury, D.J.; Mody, T.D. Preparation of N-{3-[2-(phenylamino) pyrimidin-4-ylamino] phenyl} Amides as Inhibitors of Bruton’s Tyrosine Kinase. U.S. Patent WO 2013173518 A1, 21 November 2013. [Google Scholar]
- Schafer, P.H.; Kivitz, A.J.; Ma, J.; Korish, S.; Sutherland, D.; Li, L.; Azaryan, A.; Kosek, J.; Adams, M.; Capone, L.; et al. Spebrutinib (CC-292) affects markers of B cell activation, chemotaxis, and osteoclasts in patients with rheumatoid arthritis: Results from a mechanistic study. Rheumatol. Ther. 2019, 7, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.K.; Tester, R.; Aslanian, S.; Karp, R.; Sheets, M.; Labenski, M.T.; Witowski, S.R.; Lounsbury, H.; Chaturvedi,, P.; Mazdiyasni,, H.; et al. Inhibition of Btk with CC-292provides early pharmacodynamic assessment ofactivity in mice and humans. J. Pharmacoll. Exp. Ther. 2013, 346, 219–228. [Google Scholar] [CrossRef]
- Brown, J.R.; Harb, W.A.; Hill, B.T.; Gabrilove, J.; Sharman, J.P.; Schreeder, M.T.; Barr, P.M.; Foran, J.M.; Miller, T.P.; Burger, J.A.; et al. Phase I study of single-agent CC-292, a highly selective Brutons tyrosine kinase inhibitor, in relapsed/refractory chronic lymphocytic leukemia. Haematologica 2016, 101, e295–e298. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, R.; Liang, R.; Gao, Y.; Liu, R.; Chen, X.; Lu, Z.; Wang, Z.; Yu, L.; Shakib, S.; et al. Abstract CT132: Orelabrutinib, a potent and selective Bruton’s tyrosine kinase inhibitor with superior safety profile and excellent PK/PD properties. Tumor Biol. 2020, 80, CT132. [Google Scholar] [CrossRef]
- Xu, W.; Song, Y.; Li, Z.; Yang, S.; Liu, L.; Hu, Y.; Zhang, W.; Zhou, J.; Gao, S.; Ding, K.; et al. Safety, Tolerability and efficacy of orelabrutinib, once a day, to treat chinese patients with relapsed or refractory chronic lymphocytic leukemia/small cell leukemia. Blood 2019, 134, 4319. [Google Scholar] [CrossRef]
- Dhillon, S. Orelabrutinib: First Approval. Drugs 2021, 81, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Walter, H.S.; Rule, S.A.; Dyer, M.J.S.; Karlin, L.; Jones, C.; Cazin, B.; Quittet, P.; Shah, N.; Hutchinson, C.V.; Honda, H.; et al. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood 2016, 127, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yao, Q.; Zhang, F. Diagnosis, prognosis and treatment of primary central nervous system lymphoma in the elderly population (Review). Int. J. Oncol. 2021, 58, 371–387. [Google Scholar] [CrossRef]
- Walter, H.S.; Jayne, S.; Rule, S.A.; Cartron, G.; Morschhauser, F.; Macip, S.; Karlin, L.; Jones, C.; Herbaux, C.; Quittet, P.; et al. Long-term follow-up of patients with CLL treated with the selective Bruton’s tyrosine kinase inhibitor ONO/GS-4059. Blood 2017, 129, 2808–2810. [Google Scholar] [CrossRef]
- Ribeiro, M.L.; Reyes-Garau, D.; Vinyoles, M.; Profitós Pelejà, N.; Santos, J.; Armengol, M.; Fernández-Serrano, M.; Sedó Mor, A.; Bech-Serra, J.J.; Blecua, P.; et al. Antitumor activity of the novel BTK inhibitor TG-1701 is associated with disruption of Ikaros signaling in patients with B-cell Non-Hodgkin lymphoma. Clin. Cancer. Res. 2021, 27, 6591–6601. [Google Scholar] [CrossRef] [PubMed]
- Cheah, C.Y.; Wickham, N.; Jurczak, W.; Lasica, M.; Wróbel, T.; Walewski, J.; Yannakou, C.K.; Lewis, K.L.; Dlugosz-Danecka, M.; Giannopoulos, K.; et al. Clinical activity of TG-1701, as monotherapy and in combination with ublituximab and umbralisib (U2), in patients with B-cell malignancies. Blood 2020, 136, 1130. [Google Scholar]
- Gill, J.; He, W.; Schuster, S.J.; Brander, D.M.; Chatburn, E.; Kennard, K.; Anderson, B.D.; Nasta, S.; Landsburg, D.J.; Porter, D.L.; et al. A phase Ia/Ib study of a novel BTK inhibitor, DTRMWXHS-12 (DTRM-12), and combination products, with everolimus and pomalidomide, in pts with CLL or other B-cell lymphomas. J. Clin. Oncol. 2017, 35, TPS7570. [Google Scholar] [CrossRef]
- Mato, A.R.; Schuster, S.J.; Foss, F.M.; Isufi, I.; Ding, W.; Brander, D.M.; Sitlinger, A.; Tun, H.W.; Moustafa, M.A.; Kennard, K.; et al. A Phase Ia/Ib study exploring the synthetic lethality of the orally administered novel BTK inhibitor, Dtrmwxhs-12 (DTRM-12), in combination with everolimus and pomalidomide in patients with relapsed/refractory CLL, DLBCL or other B-cell lymphomas. Blood 2019, 134, 810. [Google Scholar] [CrossRef]
- Tambaro, F.P.; De Novellis, D.; Wierda, W.G. The Role of BTK Inhibition in the Treatment of Chronic Lymphocytic Leukemia: A Clinical View. J. Exp. Pharmacol. 2021, 13, 923–935. [Google Scholar] [CrossRef]
- Lewis, K.L.; Cheah, C.Y.J. Non-covalent BTK inhibitors-the new BTKids on the block for B-cell malignancies. Pers. Med. 2021, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.-M.; Ribrag, V. Pirtobrutinib shows evidence to inaugurate a third generation of BTK inhibitors. Lancet 2021, 397, 855–857. [Google Scholar] [CrossRef]
- Mato, A.R.; Shah, N.N.; Jurczak, W.; Cheah, C.Y.; Pagel, J.M.; Woyach, J.A.; Fakhri, B.; Eyre, T.A.; Lamanna, N.; Patel, M.R.; et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): A phase 1/2 study. Lancet 2021, 397, 892–901. [Google Scholar] [CrossRef]
- Binnerts, M.E.; Otipoby, K.L.; Hopkins, B.T. SNS-062 is a potent noncovalent BTK inhibitor with comparable activity against wide type BTK and BTK with an acquired resistance mutation. Mol. Cancer Ther. 2015, 14, C186. [Google Scholar]
- Burger, J.A. Bruton tyrosine kinase inhibitors: Present and future. Cancer J. 2019, 25, 386–393. [Google Scholar] [CrossRef]
- Crawford, J.J.; Johnson, A.R.; Misner, D.L.; Belmont, L.D.; Castanedo, G.; Choy, R.; Coraggio, M.; Dong, L.; Eigenbrot, C.; Erickson, R.; et al. Discovery of GDC-0853: A potent, selective, and noncovalent bruton’s tyrosine kinase inhibitor in early clinical development. J. Med. Chem. 2018, 61, 2227–2245. [Google Scholar] [CrossRef] [PubMed]
- Reiff, S.D.; Muhowski, E.M.; Guinn, D.; Lehman, A.; Fabian, C.A.; Cheney, C.; Mantel, R.; Smith, L.; Johnson, A.J.; Young, W.B.; et al. Noncovalent inhibition of C481S Bruton tyrosine kinase by GDC-0853: A new treatment strategy for ibrutinib-resistant CLL. Blood 2018, 132, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Tuckwell, K.; Katsumoto, T.R.; Zhao, R.; Galanter, J.; Lee, C.K.; Rae, J.; Toth, B.; Ramamoorthi, N.; Hackney, J.A.; et al. Fenebrutinib versus placebo or adalimumab in rheumatoid arthritis: A randomized, double-blind, phase II trial (ANDES Study). Arthritis Rheumatol. 2020, 72, 1435–1446. [Google Scholar] [CrossRef]
- Byrd, J.C.; Smith, S.; Wagner-Johnston, N.; Sharman, J.; Chen, A.I.; Advani, R.; Augustson, B.; Marlton, P.; Commerford, S.R.; Okrah, K.; et al. First-in-human phase 1 study of the BTK inhibitor GDC-0853 in relapsed or refractory B-cell NHL and CLL. Oncotarget 2018, 9, 13023–13035. [Google Scholar] [CrossRef]
- Shirley, M. Bruton tyrosine kinase inhibitors in B-cell malignancies: Their use and differential features. Target Oncol. 2022, 17, 69–84. [Google Scholar] [CrossRef]
- Reiff, S.D.; Mantel, R.; Smith, L.L.; Greene, J.; Muhowski, E.M.; Fabian, C.A.; Goettl, V.M.; Tran, M.; Harrington, B.; Rogers, K.A.; et al. The BTK inhibitor ARQ 531 targets ibrutinib-resistant CLL and Richter transformation. Cancer Discov. 2018, 8, 1300–1315. [Google Scholar] [CrossRef] [PubMed]
- Quinquenel, A.; Fornecker, L.M.; Letestu, R.; Ysebaert, L.; Fleury, C.; Lazarian, G.; Dilhuydy, M.S.; Nollet, D.; Guieze, R.; Feugier, P.; et al. Prevalence of BTK and PLCG2 mutations in a real-life CLL cohort still on ibrutinib after 3 years: A FILO group study. Blood 2019, 134, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.; Liu, Y.; Wang, C.; Xu, Z.; Zhang, Y.; Liu, Y.; Zhao, G.; Ling, Y. Review of the development of BTK inhibitors in overcoming the clinical limitations of ibrutinib. Eur. J Med. Chem. 2022, 229, 114009. [Google Scholar] [CrossRef] [PubMed]
- Woyach, J.A.; Furman, R.R.; Liu, T.M.; Ozer, H.G.; Zapatka, M.; Ruppert, A.S.; Xue, L.; Li, D.H.; Steggerda, S.M.; Versele, M.; et al. Re-sistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N. Engl. J. Med. 2014, 370, 2286–2294. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Guinn, D.; Lehman, A.; Blachly, J.S.; Lozanski, A.; Heerema, N.A.; Zhao, W.; Coleman, J.; Jones, D.; et al. BTK C481S-mediated resistance to ibrutinib in chronic lymphocytic leukemia. J. Clin. Oncol. 2017, 35, 1437–1443. [Google Scholar] [CrossRef]
- Woyach, J.; Huang, Y.; Rogers, K.; Bhat, S.A.; Grever, M.R.; Lozanski, A.; Doong, T.-J.; Blachly, J.S.; Lozanski, G.; Jones, D.; et al. Resistance to acalabrutinib in CLL is mediated primarily by BTK mutations. Blood 2019, 134, 504. [Google Scholar] [CrossRef]
- George, B.; Chowdhury, S.M.; Hart, A.; Sircar, A.; Singh, S.K.; Nath, U.K.; Mamgain, M.; Singhal, N.K.; Sehgal, L.; Jain, N. Ibrutinib resistance mechanisms and treatment strategies for B-cell lymphomas. Cancers 2020, 12, 1328. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Yang, J.; Zhou, K. The resistance mechanisms and treatment strategies of BTK inhibitors in B-cell lymphoma. Hematol. Oncol. 2021, 39, 605–615. [Google Scholar] [CrossRef]
- Bond, D.A.; Woyach, J.A. Targeting BTK in CLL: Beyond ibrutinib. Curr. Hematol. Malign. Rep. 2019, 14, 197–205. [Google Scholar] [CrossRef]
- Jones, J.A.; Mato, A.R.; Wierda, W.G.; Davids, M.S.; Choi, M.; Cheson, B.D.; Furman, R.R.; Lamanna, N.; Barr, P.M.; Zhou, L.; et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: An interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018, 19, 65–75. [Google Scholar] [CrossRef]
- Skånland, S.S.; Mato, A.R. Overcoming resistance to targeted therapies in chronic lymphocytic leukemia. Blood Adv. 2021, 5, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.R.; Qi, J.; Cook, E.M.; Nichols, C.; Dadashian, E.L.; Underbayev, C.; Herman, S.E.M.; Saba, N.S.; Keyvanfar, K.; Sun, C.; et al. A CD19/CD3 bispecific antibody for effective immunotherapy of chronic lymphocytic leukemia in the ibrutinib era. Blood 2018, 132, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Turtle, C.J.; Hay, K.; Hanafi, L.-A.; Li, D.; Cherian, S.; Chen, X.; Wood, B.; Lozanski, A.; Byrd, J.C.; Heimfeld, S.; et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor–modified T cells after failure of ibrutinib. J. Clin. Oncol. 2017, 35, 3010–3020. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, T.; Soumerai, J.D.; Dorritie, K.A.; Kathleen, A.; Dorritie, M.D.; Deborah, M.; Stephens, P.A.; Riedell, J.E.; Arnason, J.E.; Kipps, T.J.; et al. Rapid undetectable MRD (uMRD) responses in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) treated with lisocabtagene maraleucel (liso-cel), a CD19-directed CAR T cell product: Updated results from Transcend CLL 004, a phase 1/2 study including patients with high-risk disease previously treated with ibrutinib. Blood 2019, 134, 503. [Google Scholar]
- Sun, Y.; Zhao, X.; Ding, N.; Gao, H.; Wu, Y.; Yang, Y.; Zhao, M.; Hwang, J.; Song, Y.; Liu, W.; et al. PROTAC-induced BTK degradation as a novel therapy for mutated BTK C481S induced ibrutinib-resistant B-cell malignancies. Cell Res. 2018, 28, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Al-Sawaf, O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am. J. Hematol. 2021, 96, 1679–1705. [Google Scholar] [CrossRef]
- Smolewski, P.; Robak, T. Current treatment of refractory/relapsed chronic lymphocytic leukemia: A focus on novel drugs. Acta Haematol. 2020, 144, 365–379. [Google Scholar] [CrossRef]
- O’Brien, S.M.; Brown, J.R.; Byrd, J.C.; Furman, R.R.; Ghia, P.; Sharman, J.P.; Wierda, W.G. Monitoring and managing BTK inhibitor treatment-related adverse events in clinical practice. Front. Oncol. 2021, 11, 720704. [Google Scholar] [CrossRef]
- Von Hundelshausen, P.; Siess, W. Bleeding by Bruton tyrosine kinase-inhibitors: Dependency on drug type and disease. Cancers 2021, 13, 1103. [Google Scholar] [CrossRef]
- Shatzel, J.J.; Olson, S.R.; Tao, D.L.; McCarty, O.J.T.; Danilov, A.V.; DeLoughery, T.G. Ibrutinib-associated bleeding: Patho-genesis, management and risk reduction strategies. J. Thromb. Haemost. 2017, 15, 835–847. [Google Scholar] [CrossRef]
- Caron, F.; Leong, D.P.; Hillis, C.; Fraser, G.; Siegal, D. Current understanding of bleeding with ibrutinib use: A systematic review and meta-analysis. Blood Adv. 2017, 1, 772–778. [Google Scholar] [CrossRef]
- Bye, A.P.; Unsworth, A.; Desborough, M.; Hildyard, C.; Appleby, N.; Bruce, D.; Kriek, N.; Nock, S.H.; Sage, T.; Hughes, C.; et al. Severe platelet dysfunction in NHL patients receiving ibrutinib is absent in patients receiving acalabrutinib. Blood Adv. 2017, 1, 2610–2623. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Dimopoulos, M.A.; Garcia-Sanz, R.; Trotman, J.; Opat, S.; Roberts, A.W.; Owen, R.G.; Song, Y.; Xu, W.; Zhu, J.; et al. Pooled safety analysis of zanubrutinib monotherapy in patients with B-cell malignancies. Blood Adv. 2021, 5, 2473–9529. [Google Scholar] [CrossRef] [PubMed]
- Langerbeins, P.; Zhang, C.; Robrecht, S.; Cramer, P.; Fürstenau, M.; Al-Sawaf, O.; von Tresckow, J.; Fink, A.M.; Kreuzer, K.A.; Vehling-Kaiser, U.; et al. The CLL12 trial: Ibrutinib versus placebo in treatment-naïve, early stage chronic lymphocytic leu-kemia. Blood 2021, 10, 2021010845. [Google Scholar]
- Yun, S.; Vincelette, N.D.; Acharya, U.; Abraham, I. Risk of Atrial Fibrillation and Bleeding Diathesis Associated with Ibrutinib Treatment: A Systematic Review and Pooled Analysis of Four Randomized Controlled Trials. Clin. Lymphoma Myeloma Leuk. 2017, 17, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Mato, A.R.; Nabhan, C.; Barr, P.M.; Ujjani, C.S.; Hill, B.T.; Lamanna, N.; Skarbnik, A.P.; Howlett, C.; Pu, J.J.; Sehgal, A.R.; et al. Outcomes of CLL patients treated with sequential kinase inhibitor therapy: A real world experience. Blood 2016, 128, 199–2205. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Caron, F.; Hillis, C.; Duan, A.; Healey, J.S.; Fraser, G.; Siegal, D. The risk of atrial fibrillation with ibrutinib use: A systematic review and meta-analysis. Blood 2016, 128, 138–140. [Google Scholar] [CrossRef]
- Guha, A.; Derbala, M.H.; Zhao, Q.; Wiczer, T.E.; Woyach, J.A.; Byrd, J.C.; Awan, F.T.; Addison, D. Ventricular arrhythmias following ibrutinib initiation for lymphoid malignancies. J. Am. Coll. Cardiol. 2018, 72, 697–698. [Google Scholar] [CrossRef]
- Grewal, U.S.; Thotamgari, S.R.; Sheth, A.R.; Gaddam, S.J.; Ahmad, J.; Beedupalli, K.; Dominic, P. Cardiovascular complications associated with novel agents in the chronic lymphocytic leukemia armamentarium: A pharmacovigilance analysis. Int. J. Cardiol. 2021, 344, 186–189. [Google Scholar] [CrossRef]
- Caldeira, D.; Alves, D.; Costa, J.; Ferreira, J.J.; Pinto, F.J. Ibrutinib increases the risk of hypertension and atrial fibrillation: Systematic review and meta-analysis. PLoS ONE 2019, 14, e0211228. [Google Scholar] [CrossRef]
- Binsah, G.; Philip, T.A.; Ferrajoli, A.; Jan, B.; Jain, N.; Wierda, W.G.; O’Brien, S.; Durand, J.B.; Keating, M.J. An observational study of the occurrence of atrial fibrillation and hypertension in patientstreated with ibrutinib. Blood 2014, 124, 5657. [Google Scholar] [CrossRef]
- O’Brien, S.; Hillmen, P.; Coutre, S.; Barr, P.M.; Fraser, G.; Tedeschi, A.; Burger, J.A.; Dilhuydy, M.S.; Hess, G.; Moreno, C.; et al. Safety analysis of four randomized controlled studies of ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma or mantle cell lymphoma. Clin. Lymphoma Myeloma Leuk. 2018, 18, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, T.; Wiczer, T.; Waller, A.; Philippon, J.; Porter, K.; Haddad, D.; Guha, A.; Rogers, K.A.; Bhat, S.; Byrd, J.C.; et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood 2019, 134, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Vitale, C.; Salvetti, C.; Griggio, V.; Porrazzo, M.; Schiattone, L.; Zamprogna, G.; Visentin, A.; Vassallo, F.; Cassin, R.; Rigolin, G.M.; et al. Preexisting and treatment-emergent autoimmune cytopenias in patients with CLL treated with targeted drugs. Blood 2021, 137, 3507–3517. [Google Scholar] [CrossRef] [PubMed]
- Noto, A.; Cassin, R.; Mattiello, V.; Reda, G. The role of novel agents in treating CLL-associated autoimmune hemolytic anemia. J. Clin. Med. 2021, 10, 2064. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Zhou, C.; Guo, H.; Zheng, M. Effects of BTK signalling in pathogenic microorganism infections. J. Cell. Mol. Med. 2019, 23, 6522. [Google Scholar] [CrossRef]
- McDonald, C.; Xanthopoulos, C.; Kostareli, E. The role of Bruton’s tyrosine kinase in the immune system and disease. Immunology 2021, 164, 722–736. [Google Scholar] [CrossRef]
- Pleyer, C.; Sun, C.; Desai, S.; Ahn, I.E.; Tian, X.; Nierman, P.; Soto, S.; Superata, J.; Valdez, J.; Lotter, J.; et al. Reconsti-tution of humoral immunity and decreased risk of infections in patients with chronic lymphocytic leukemia treated with Bruton tyrosine kinase inhibitors. Leuk. Lymphoma 2020, 61, 2375–2382. [Google Scholar] [CrossRef]
- Varughese, T.; Taur, Y.; Cohen, N.; Palomba, M.L.; Seo, S.K.; Hohl, T.M.; Redelman-Sidi, G. Serious Infections in Patients Receiving Ibrutinib for Treatment of Lymphoid Cancer. Clin. Infect. Dis. 2018, 67, 687–692. [Google Scholar] [CrossRef]
- Holowka, T.; Cheung, H.; Malinis, M.; Gan, G.; Deng, Y.; Perreault, S.; Isufi, I.; Azar, M.M. Incidence and associated risk factors for invasive fungal infections and other serious infections in patients on ibrutinib. J. Infect. Chemother. 2021, 27, 1700–1705. [Google Scholar] [CrossRef]
- Frei, M.; Aitken, S.L.; Jain, N.; Thompson, P.; Wierda, W.; Kontoyiannis, D.P.; DiPippo, A.J. Incidence and characterization of fungal infections in chronic lymphocytic leukemia patients receiving ibrutinib. Leuk. Lymphoma 2020, 61, 2488–2491. [Google Scholar] [CrossRef]
- Mauro, F.; Giannarelli, D.; Visentin, A.; Reda, G.; Sportoletti, P.; Frustaci, A.; Chiarenza, A.; Ciolli, S.; Vitale, C.; Laurenti, L.; et al. Prognostic impact and risk factors of infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Cancers 2021, 13, 3240. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, A.; Lamanna, N. Managing toxicities of Bruton tyrosine kinase inhibitors. Hematology 2020, 2020, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Pleyer, C.; Ali, M.A.; Cohen, J.I.; Tian, X.; Soto, S.; Ahn, I.E.; Gaglione, E.M.; Nierman, P.; Marti, G.E.; Hesdorffer, C.; et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood 2021, 137, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.M.; LoRe, V.A., 3rd; Mato, A.R.; Chong, E.A.; Barrientos, J.C.; Gerson, J.N.; Barta, S.K.; Landsburg, D.J.; Nasta, S.D.; Svoboda, J.; et al. Ibrutinib-associated arthralgias/myalgias in patients with chronic lymphocytic leu-kemia: Incidence and impact on clinical outcomes. Clin. Lymphoma Myeloma Leukemia 2020, 20, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Roeker, L.E.; Eyre, T.A.; Thompson, M.C.; Lamanna, N.; Coltoff, A.R.; Davids, M.S.; Baker, P.O.; Leslie, L.A.; Rogers, K.A.; Allan, J.N.; et al. Outcomes of front-line ibrutinib treated CLL patients excluded from landmark clinical trial. Am. J. Hematol. 2021, 138, 1768–1773. [Google Scholar]
- Mato, A.; Jahnke, J.; Li, P.; Mehra, M.; Ladage, V.P.; Mahler, M.; Huntington, S.; Doshi, J.A. Real-world treatment and outcomes among older adults with chronic lymphocytic leukemia before the novel agents era. Haematologica 2018, 103, e462–e465. [Google Scholar] [CrossRef]
- Forum, U.C. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: A UK and Ireland analysis of outcomes in 315 patients. Haematologica 2016, 101, 1563–1572. [Google Scholar] [CrossRef]
- Stephens, D.M.; Byrd, J.C. How I manage ibrutinib intolerance and complications in patients with chronic lymphocytic leu-kemia. Blood 2019, 133, 1298–1307. [Google Scholar] [CrossRef]
- Awan, F.T.; Schuh, A.; Brown, J.R.; Furman, R.R.; Pagel, J.M.; Hillmen, P.; Stephens, D.M.; Woyach, J.; Bibikova, E.; Charuworn, P.; et al. Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv. 2019, 3, 1553–1562. [Google Scholar] [CrossRef]
- Iberri, D.J.; Kwong, B.Y.; Stevens, L.A.; Coutre, S.E.; Kim, J.; Sabile, J.M.; Advani, R.H. Ibrutinib-associated rash: A single-centre experience of clinicopathological features and management. Br. J. Haematol. 2016, 180, 164–166. [Google Scholar] [CrossRef]
- Sibaud, V.; Beylot-Barry, M.; Protin, C.; Vigarios, E.; Recher, C.; Ysebaert, L. Dermatological Toxicities of Bruton’s Tyrosine Kinase Inhibitors. Am. J. Clin. Dermatol. 2020, 21, 799–812. [Google Scholar] [CrossRef]
- Bitar, C.; Farooqui, M.Z.H.; Valdez, J.; Saba, N.S.; Soto, S.; Bray, A.; Marti, G.; Wiestner, A.; Cowen, E.W. Hair and nail changes during long-term therapy with ibrutinib for chronic lymphocytic leukemia. JAMA Dermatol. 2016, 152, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.A.; Wierda, W.G. Acalabrutinib: A Selective Bruton Tyrosine Kinase Inhibitor for the Treatment of B-Cell Malignancies. Front. Oncol. 2021, 11, 668162. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.C.L.; Nierman, P.K.; Kendall, E.K.; Cheung, J.; Gulrajani, M.; Herman, S.E.M.; Pleyer, C.; Ahn, I.E.; Stetler-Stevenson, M.; Yuan, C.M.; et al. Clinical and biological implications of target occupancy in CLL treated with the BTK inhibitor acalabrutinib. Blood 2020, 136, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Treon, S.P.; Castillo, J.J.; Skarbnik, A.P.; Soumerai, J.D.; Ghobrial, I.M.; Guerrera, M.L.; Meid, K.; Yang, G. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19 infected patients. Blood 2020, 135, 1912–1915. [Google Scholar] [CrossRef]
- Roschewski, M.; Lionakis, M.S.; Sharman, J.P.; Roswarski, J.; Goy, A.; Monticelli, M.A.; Roshon, M.; Wrzesinski, S.H.; Desai, J.V.; Zarakas, M.A.; et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci. Immunol. 2020, 5, 0110. [Google Scholar] [CrossRef]
- Chong, E.A.; Roeker, L.E.; Shadman, M.; Davids, M.S.; Schuster, S.J.; Mato, A.R. BTK inhibitors in cancer patients with COVID-19: “The winner will be the one who controls that chaos” (Napoleon Bonaparte). Clin Cancer Res. 2020, 26, 3514–3516. [Google Scholar] [CrossRef]
- Thibaud, S.; Tremblay, D.; Bhalla, S.; Zimmerman, B.; Sigel, K.; Gabrilove, J. Protective role of Bruton tyrosine kinase inhibitors in patients with chronic lymphocytic leukaemia and COVID-19. Br. J. Haematol. 2020, 190, 73–76. [Google Scholar] [CrossRef]
- Robak, P.; Robak, T. Novel synthetic drugs currently in clinical development for chronic lymphocytic leukemia. Expert Opin. Investig. Drugs 2017, 26, 1249–1265. [Google Scholar] [CrossRef]
- Solimando, A.G.; Ribatti, D.; Vacca, A.; Einsele, H. Targeting B-cell non Hodgkin lymphoma: New and old tricks. Leuk. Res. 2016, 42, 93–104. [Google Scholar] [CrossRef]
- Bottoni, A.; Rizzotto, L.; Lai, T.-H.; Liu, C.; Smith, L.L.; Mantel, R.; Reiff, S.; El-Gamal, D.; Larkin, K.; Johnson, A.J.; et al. Targeting BTK through microRNA in chronic lymphocytic leukemia. Blood 2016, 128, 3101–3112. [Google Scholar] [CrossRef]
- Lamothe, B.; Cervantes-Gomez, F.; Sivina, M.; Wierda, W.G.; Keating, M.J.; Gandhi, V. Proteasome inhibitor carfilzomib complements ibrutinib’s action in chronic lymphocytic leukemia. Blood 2015, 125, 407–410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- López-Oreja, I.; Playa-Albinyana, H.; Arenas, F.; López-Guerra, M.; Colomer, D. Challenges with approved targeted therapies against recurrent mutations in CLL: A place for new actionable targets. Cancers 2021, 13, 3150. [Google Scholar] [CrossRef] [PubMed]
- Giménez, N.; Schulz, R.; Higashi, M.; Aymerich, M.; Villamor, N.; Delgado, J.; Juan, M.; López-Guerra, M.; Campo, E.; Rosich, L.; et al. Targeting IRAK4 disrupts inflammatory pathways and delays tumor development in chronic lymphocytic leukemia. Leukemia 2020, 34, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Dadashian, E.L.; McAuley, E.M.; Liu, D.; Shaffer, A.L.; Young, R.M.; Iyer, J.R.; Kruhlak, M.J.; Staudt, L.M.; Wiestner, A.; Herman, S.E.M. TLR signaling is activated in lymph node–resident CLL cells and is only partially inhibited by ibrutinib. Cancer Res. 2019, 79, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Crassini, K.; Shen, Y.; Stevenson, W.S.; Christopherson, R.; Ward, C.; Mulligan, S.P.; Best, O.G. MEK1/2 inhibition by binimetinib is effective as a single agent and potentiates the actions of Venetoclax and ABT-737 under conditions that mimic the chronic lymphocytic leukaemia (CLL) tumour microenvironment. Br. J. Haematol. 2018, 182, 360–372. [Google Scholar] [CrossRef]
- Awan, F.T.; Thirman, M.J.; Patel-Donnelly, D.; Assouline, S.; Rao, A.V.; Ye, W.; Hill, B.; Sharman, J.P. Entospletinib mono-therapy inpatients with relapsed or refractory chronic lymphocytic leukemia previously treated with B-cell receptor inhib-itors: Results of aphase 2 study. Leuk. Lymphoma 2019, 60, 1972–1977. [Google Scholar] [CrossRef] [PubMed]
- Fürstenau, M.; Eichhorst, B. Novel agents in chronic lymphocytic leukemia: New combination therapies and strategies to overcome resistance. Cancers 2021, 13, 1336. [Google Scholar] [CrossRef]
- Giménez, N.; Martínez-Trillos, A.; Montraveta, A.; Lopez-Guerra, M.; Rosich, L.; Nadeu, F.; Valero, J.G.; Aymerich, M.; Magnano, L.; Rozman, M.; et al. Mutations in the RAS-BRAF-MAPK-ERK pathway define a specific subgroup of patients with adverse clinical features and provide new therapeutic options in chronic lymphocytic leukemia. Haematologica 2018, 104, 576–586. [Google Scholar] [CrossRef]
- Gill, S.I.; Vides, V.; Frey, N.V.; Metzger, S.; O’Brien, M.; Hexner, E.; Mato, A.R.; Lacey, S.F.; Melenhorst, J.; Pequignot, E.; et al. Prospective clinical trial of anti-CD19 CAR T cells in combination with ibrutinib for the treatment of chronic lymphocytic leukemia shows a high response rate. Blood. 2018, 132 (Suppl. 1), 298. [Google Scholar] [CrossRef]
| BTKi | Binding | T1/2 [hours] | IC50 [nM] | Dosing | Clinical Trials in CLL |
|---|---|---|---|---|---|
| Ibrutinib (PCYC-1102) | Covalent irreversible C481 | 4–8 | 0.5 | 420 mg | NCT04771507 NCT03513562 NCT02912754 |
| Acalabrutinib (ACP-196) | Covalent irreversible C481 | 0.9 | 5.1 | 100 mg twice a day | NCT04008706 NCT04930536 NCT04722172 |
| Zanubrutinib (BGB-3111) | Covalent irreversible C481 | 2–4 | 0.5 | 160 or 320 mg twice a day | NCT04116437 NCT04458610 NCT03824483 NCT04282018 NCT04515238 NCT03336333 |
| Spebrutinib (CC-292) | Covalent irreversible C481 | 8–24 | <0.5 | 1000 mg | NCT02031419 |
| Tirabrutinib (ONO/GS-4059) | Covalent irreversible C481 | NA | 5.6 | 80 mg | NCT03740529 NCT03162536 |
| Orelabrutinib (ICP-022) | Covalent irreversible C481 | ~1.5–4 h | 1.6 | 150 mg | NCT03493217 NCT04014205 |
| SHR1459 (TG-1701) | Covalent irreversible C481 | NA | 3 | 300 mg | NCT03671590; NCT04806035 |
| DTRMWXHS-12 (DTRM-12) | Covalent irreversible C481 | ~4 | NA | 200 mg | NCT02900716 NCT04305444 |
| Pirtobrutinib (LOXO-305) | Non-covalent reversible | NA | 0.85 | 200 mg | NCT05023980 NCT04965493 NCT05024045 NCT04666038 |
| Vecabrutinib (SNS-062) | Non-covalent reversible | 6.6-8 | 24 | 25 mg escalated to 500 mg | NCT03037645 |
| Fenebrutinib (GDC-0853) | Non-covalent reversible | 2.2 | 0.91 | 200 mg twice a day | NCT01991184 |
| Nemta brutinib (ARQ 531) | Non-covalent reversible | NA | 0.85 | 65-100 mg | NCT04728893 NCT03162536 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robak, T.; Witkowska, M.; Smolewski, P. The Role of Bruton’s Kinase Inhibitors in Chronic Lymphocytic Leukemia: Current Status and Future Directions. Cancers 2022, 14, 771. https://doi.org/10.3390/cancers14030771
Robak T, Witkowska M, Smolewski P. The Role of Bruton’s Kinase Inhibitors in Chronic Lymphocytic Leukemia: Current Status and Future Directions. Cancers. 2022; 14(3):771. https://doi.org/10.3390/cancers14030771
Chicago/Turabian StyleRobak, Tadeusz, Magda Witkowska, and Piotr Smolewski. 2022. "The Role of Bruton’s Kinase Inhibitors in Chronic Lymphocytic Leukemia: Current Status and Future Directions" Cancers 14, no. 3: 771. https://doi.org/10.3390/cancers14030771
APA StyleRobak, T., Witkowska, M., & Smolewski, P. (2022). The Role of Bruton’s Kinase Inhibitors in Chronic Lymphocytic Leukemia: Current Status and Future Directions. Cancers, 14(3), 771. https://doi.org/10.3390/cancers14030771







