Chrysanthemum morifolium Extract Ameliorates Doxorubicin-Induced Cardiotoxicity by Decreasing Apoptosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and CME Treatment
2.3. MTT Cell Viability Assay
2.4. Animal Experiments
2.5. Western Blotting
2.6. TUNEL Staining
2.7. Echocardiography
2.8. Statistics
3. Results
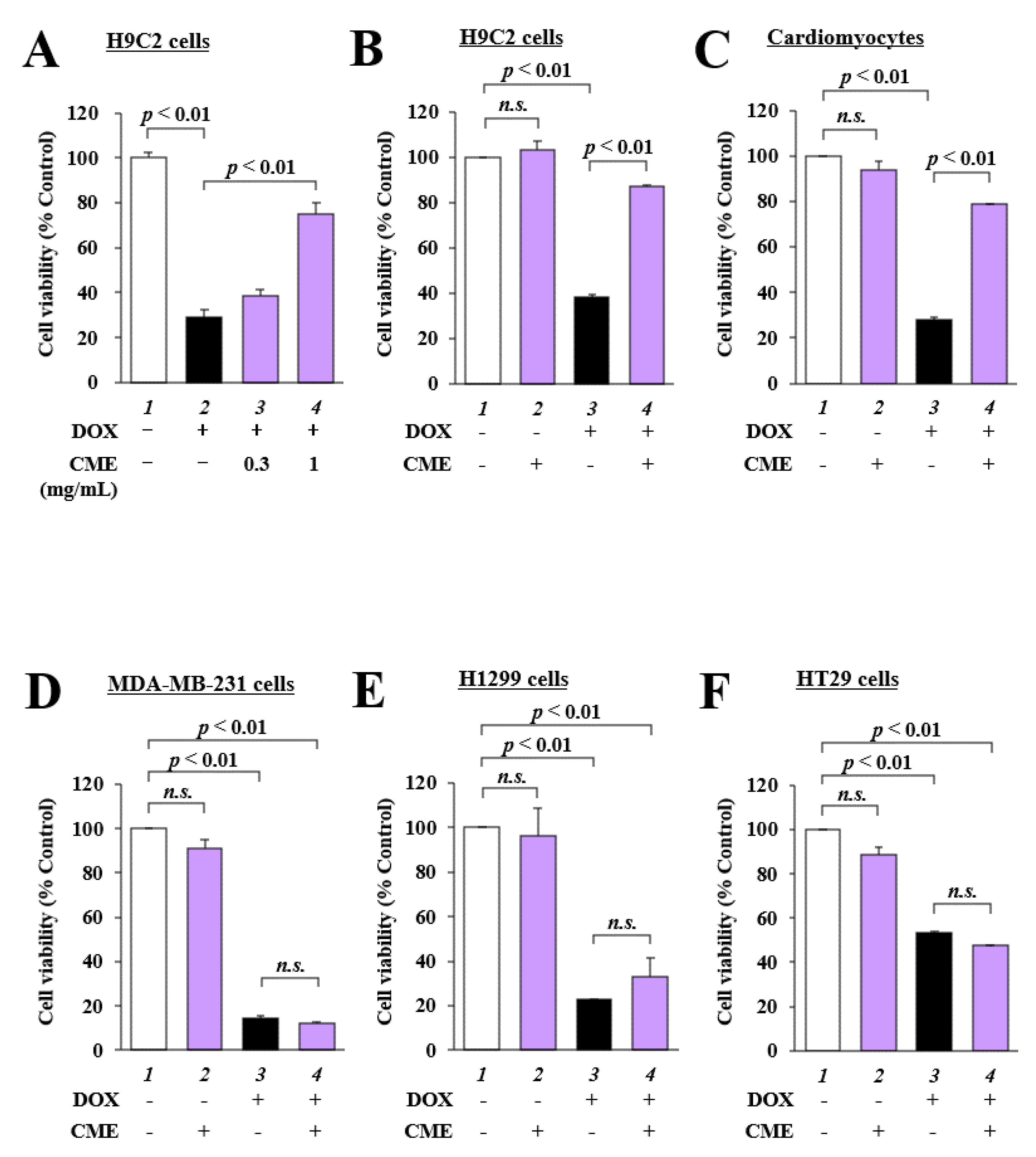
3.1. CME Inhibited DOX-Induced Cytotoxicity in H9C2 Cells and Primary Cultured Cardiomyocytes
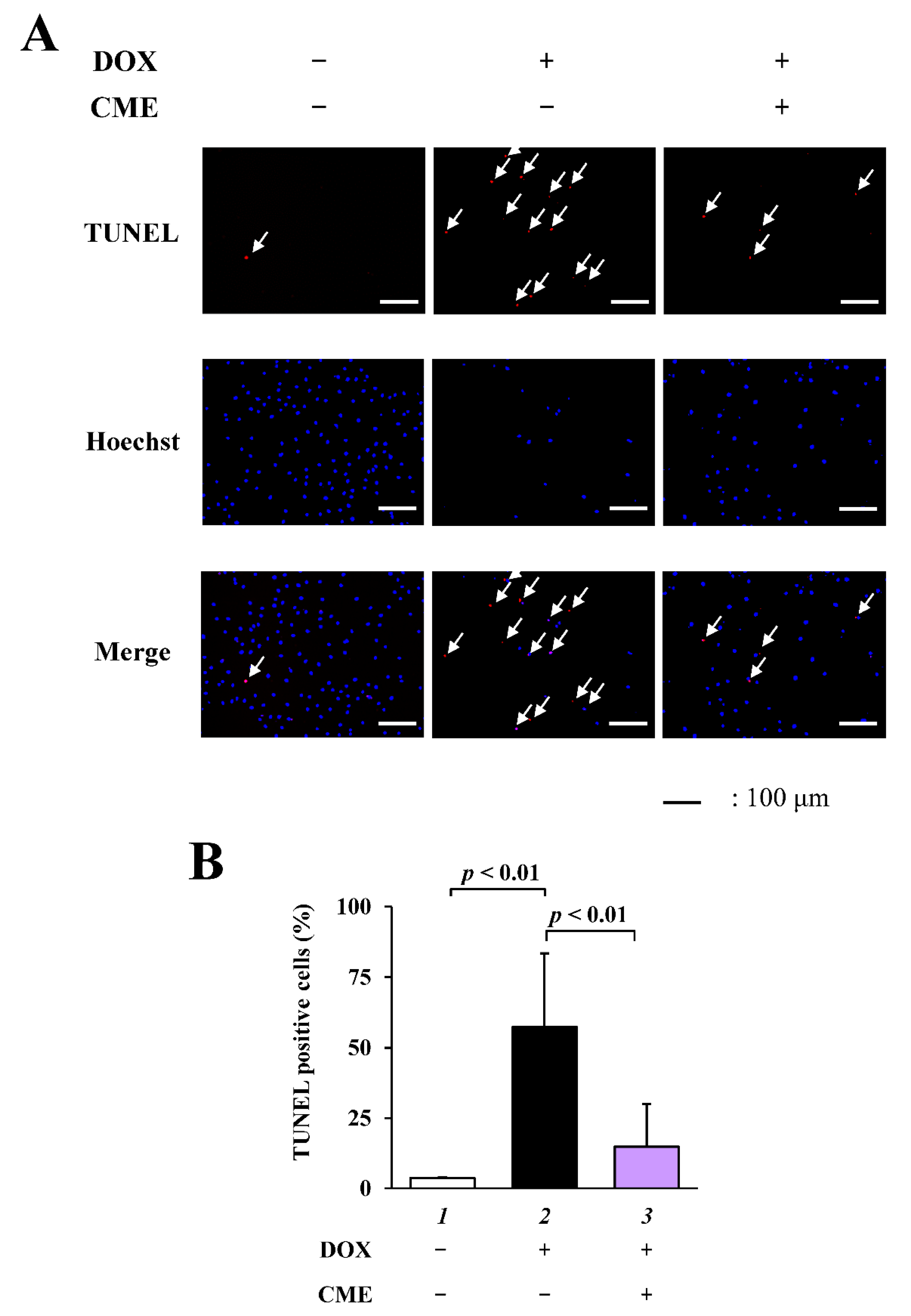
3.2. CME Inhibited DOX-Induced Apoptosis in H9C2 Cells
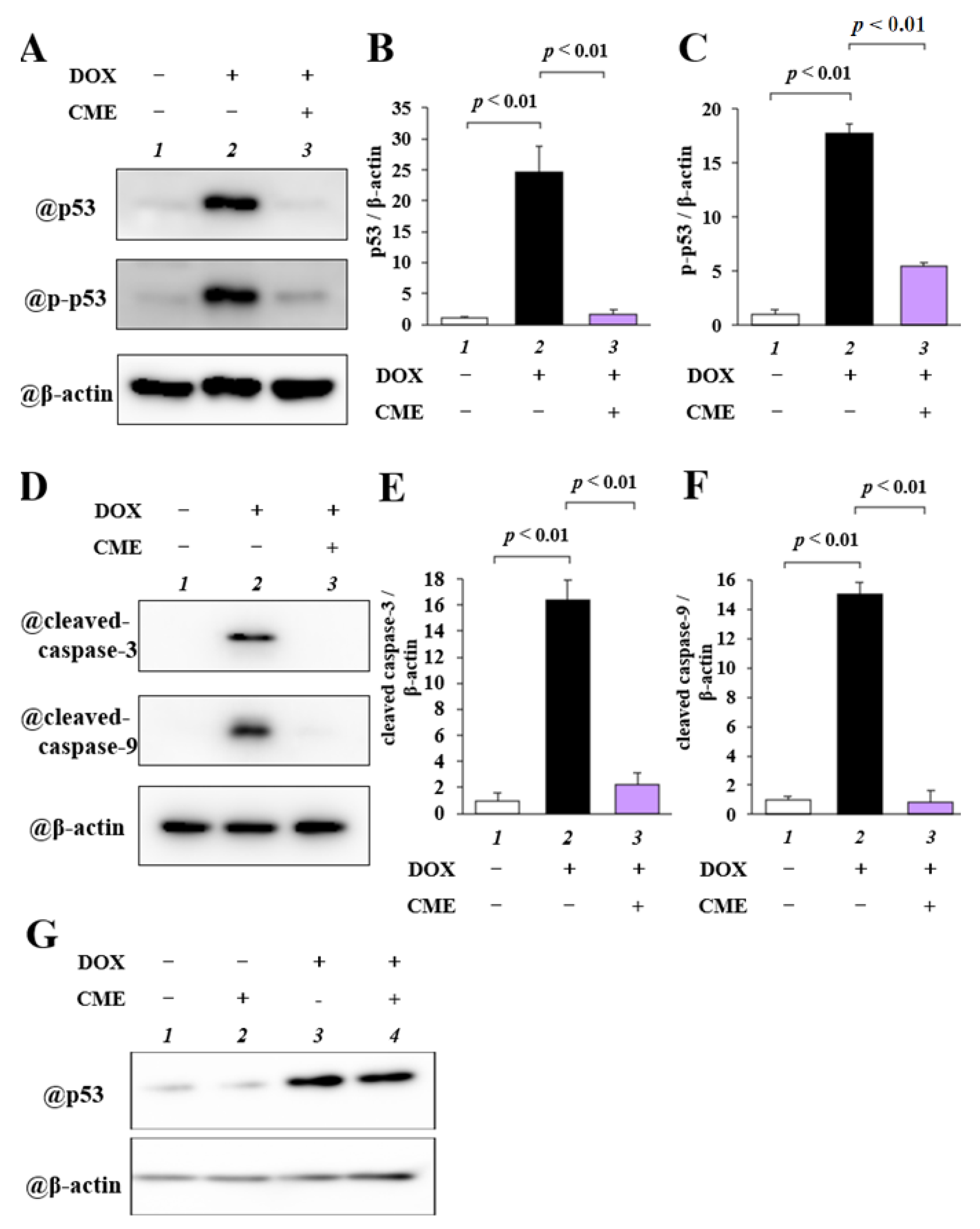
3.3. CME Inhibited DOX-Induced Upregulation of p53, p-p53, Cleaved Caspase-3, and Cleaved Caspase-9
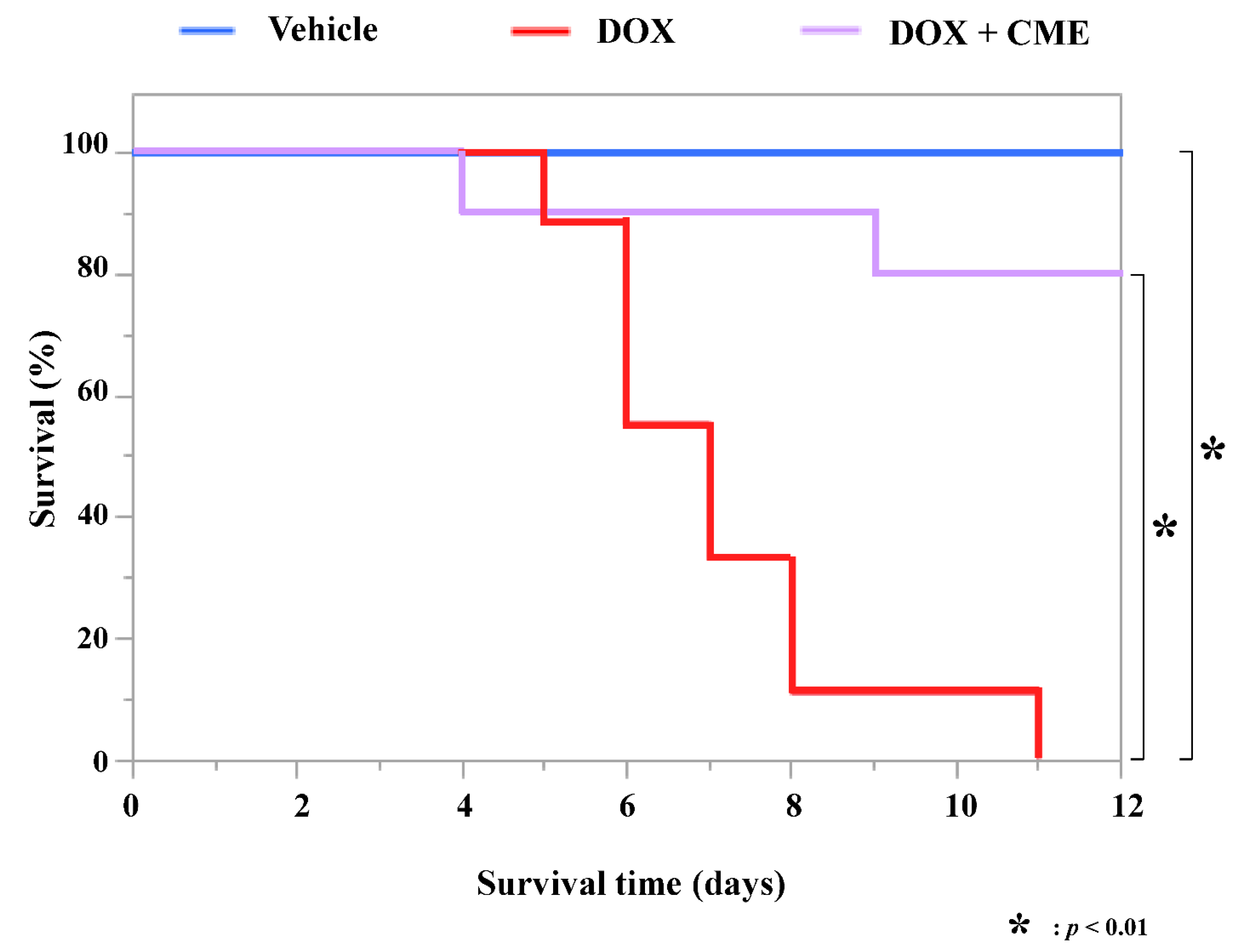
3.4. CME Improved a DOX-Induced Decrease in Survival Rate
3.5. CME Improved DOX-Induced Cardiac Dysfunction
3.6. CME Suppressed DOX-Induced Cardiac Apoptosis in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival: Analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Siegel, R.; DeSantis, C.; Virgo, K.; Stein, K.; Mariotto, A.; Smith, T.; Cooper, D.; Gansler, T.; Lerro, C.; Fedewa, S.; et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J. Clin. 2012, 62, 220–241. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.T.; Bickford, C.L. Cardiovascular Complications of Cancer Therapy: Incidence, Pathogenesis, Diagnosis, and Management. J. Am. Coll. Cardiol. 2009, 53, 2231–2247. [Google Scholar] [CrossRef]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early Detection of Anthracycline Cardiotoxicity and Improvement with Heart Failure Therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef]
- Senkus, E.; Jassem, J. Cardiovascular effects of systemic cancer treatment. Cancer Treat. Rev. 2011, 37, 300–311. [Google Scholar] [CrossRef]
- Wouters, K.A.; Kremer, L.C.M.; Miller, T.L.; Herman, E.H.; Lipshultz, S.E. Protecting against anthracycline-induced myocardial damage: A review of the most promising strategies. Br. J. Haematol. 2005, 131, 561–578. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Gerber, M.C.; Weisberg, S.; York, M.; Spicer, D.; Jones, S.E.; Wadler, S.; Desai, A.; Vogel, C.; et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J. Clin. Oncol. 1997, 15, 1318–1332. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Layard, M.W.; Basa, P.; Davis, H.L.; Von Hoff, A.L.; Rozencweig, M.; Muggia, F.M. Risk Factors for Doxorubicin-lnduced Congestive Heart Failure. Ann. Intern. Med. 1979, 91, 710–717. [Google Scholar] [CrossRef]
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.-B.; Ewer, M.; et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef]
- Menna, P.; Paz, O.G.; Chello, M.; Covino, E.; Salvatorelli, E.; Minotti, G. Anthracycline cardiotoxicity. Expert Opin. Drug Saf. 2011, 11, S21–S36. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, M.; Ameri, P.; Marone, G.; Petretta, M.; Abete, P.; Di Lisa, F.; De Placido, S.; Bonaduce, D.; Tocchetti, C.G. Recent Advances on Pathophysiology, Diagnostic and Therapeutic Insights in Cardiac Dysfunction Induced by Antineoplastic Drugs. BioMed Res. Int. 2015, 2015, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Singal, P.K. Adriamycin-Induced Early Changes in Myocardial Antioxidant Enzymes and Their Modulation by Probucol. Circulation 2000, 102, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.-S.; Lyu, Y.L.; Liu, L.; Yeh, E.T.H. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Mercurio, V.; Pirozzi, F.; Lazzarini, E.; Marone, G.; Rizzo, P.; Agnetti, G.; Tocchetti, C.G.; Ghigo, A.; Ameri, P. Models of Heart Failure Based on the Cardiotoxicity of Anticancer Drugs. J. Card. Fail. 2016, 22, 449–458. [Google Scholar] [CrossRef]
- Li, X.; Liu, M.; Sun, R.; Zeng, Y.; Chen, S.; Zhang, P. Cardiac complications in cancer treatment—A review. Hell. J. Cardiol. 2017, 58, 190–193. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhang, H.; Teh, S.S.; Wang, C.W.; Zhang, Y.; Hayford, F.; Wang, L.; Ma, T.; Dong, Z.; Zhang, Y.; et al. Goji Berries as a Potential Natural Antioxidant Medicine: An Insight into Their Molecular Mechanisms of Action. Oxidative Med. Cell. Longev. 2019, 2019, 2437397. [Google Scholar] [CrossRef]
- Ahamad, J.; Toufeeq, I.; Khan, M.A.; Ameen, M.S.M.; Anwer, E.T.; Uthirapathy, S.; Mir, S.R.; Ahmad, J. Oleuropein: A natural antioxidant molecule in the treatment of metabolic syndrome. Phytother. Res. 2019, 33, 3112–3128. [Google Scholar] [CrossRef]
- Morimoto, T.; Funamoto, M.; Sunagawa, Y.; Katanasaka, Y.; Miyazaki, Y.; Hasegawa, K. Noble Heart Failure Therapy Using Food Compositions. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2018, 138, 1263–1269. [Google Scholar] [CrossRef]
- Morimoto, T.; Sunagawa, Y.; Kawamura, T.; Takaya, T.; Wada, H.; Nagasawa, A.; Komeda, M.; Fujita, M.; Shimatsu, A.; Kita, T.; et al. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J. Clin. Investig. 2008, 118, 868–878. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Kong, Q.; Wu, X.; Lu, J.; Chen, X. Recent progress in doxorubicin-induced cardiotoxicity and protective potential of natural products. Phytomedicine 2018, 40, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Hu, W.; Zhang, D. Resveratrol, a polyphenol phytoalexin, protects against doxorubicin-induced cardiotoxicity. J. Cell. Mol. Med. 2015, 19, 2324–2328. [Google Scholar] [CrossRef] [PubMed]
- Pongjit, K.; Ninsontia, C.; Choatham, C.; Chanvorachote, P. Protective effect of Glycine max and Chrysanthemum indicum extracts against cisplatin-induced renal epithelial cell death. Hum. Exp. Toxicol. 2011, 30, 1931–1944. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mou, X.; Zhou, D.; Zhou, D.; Shou, C. Extraction of flavonoids from Chrysanthemum morifolium and antitumor activity in vitro. Exp. Ther. Med. 2017, 15, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Li, J.; You, T.; Hu, C. Anti-inflammatory and immunomodulatory activities of the extracts from the inflorescence of Chrysanthemum indicum Linné. J. Ethnopharmacol. 2005, 101, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.N.; Sun, C.; Shan, W.G.; Shen, S.R. Semi-bionic extraction of active components against stomach cancer from chrysanthemum. J. Zhejiang Univ. Technol. 2012, 40, 630–633. [Google Scholar]
- Suzuki, H.; Katanasaka, Y.; Sunagawa, Y.; Miyazaki, Y.; Funamoto, M.; Wada, H.; Hasegawa, K.; Morimoto, T. Tyrosine phosphorylation of RACK1 triggers cardiomyocyte hypertrophy by regulating the interaction between p300 and GATA4. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2016, 1862, 1544–1557. [Google Scholar] [CrossRef]
- Shimizu, K.; Sunagawa, Y.; Funamoto, M.; Wakabayashi, H.; Genpei, M.; Miyazaki, Y.; Katanasaka, Y.; Sari, N.; Shimizu, S.; Katayama, A.; et al. The Synthetic Curcumin Analogue GO-Y030 Effectively Suppresses the Development of Pressure Overload-induced Heart Failure in Mice. Sci. Rep. 2020, 10, 7172. [Google Scholar] [CrossRef]
- Morimoto, T.; Fujita, M.; Kawamura, T.; Sunagawa, Y.; Takaya, T.; Wada, H.; Shimatsu, A.; Kita, T.; Hasegawa, K. Myocardial Regulation of p300 and p53 by Doxorubicin Involves Ubiquitin Pathways. Circ. J. 2008, 72, 1506–1511. [Google Scholar] [CrossRef]
- Kawamura, T.; Hasegawa, K.; Morimoto, T.; Iwai-Kanai, E.; Miyamoto, S.; Kawase, Y.; Ono, K.; Wada, H.; Akao, M.; Kita, T. Expression of p300 protects cardiac myocytes from apoptosis in vivo. Biochem. Biophys. Res. Commun. 2004, 315, 733–738. [Google Scholar] [CrossRef]
- Communal, C.; Singh, K.; Pimentel, D.R.; Colucci, W.S. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the β-adrenergic pathway. Circulation 1999, 100, 2210–2212. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, Y.; Wada, H.; Suzuki, H.; Sasaki, H.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Katanasaka, Y.; Shimatsu, A.; Kimura, T.; et al. A novel drug delivery system of oral curcumin markedly improves efcacy of treatment for heart failure afer myocardial infarction in rats. Biol. Pharm. Bull. 2012, 35, 139–144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Funamoto, M.; Sunagawa, Y.; Katanasaka, Y.; Shimizu, K.; Miyazaki, Y.; Sari, N.; Shimizu, S.; Mori, K.; Wada, H.; Hasegawa, K.; et al. Histone Acetylation Domains Are Differentially Induced during Development of Heart Failure in Dahl Salt-Sensitive Rats. Int. J. Mol. Sci. 2021, 22, 1771. [Google Scholar] [CrossRef] [PubMed]
- Singal, P.; Li, T.; Kumar, D.; Danelisen, I.; Iliskovic, N. Adriamycin-induced heart failure: Mechanisms and modulation. Mol. Cell. Biochem. 2000, 207, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.; Santos, R.X.; Carvalho, C.; Correia, S.; Pereira, G.C.; Pereira, S.S.; Oliveira, P.J.; Santos, M.S.; Proença, T.; Moreira, P.I. Doxorubicin increases the susceptibility of brain mitochondria to Ca2+-induced permeability transition and oxidative damage. Free Radic. Biol. Med. 2008, 45, 1395–1402. [Google Scholar] [CrossRef]
- Outomuro, D.; Grana, D.R.; Azzato, F.; Milei, J. Adriamycin-induced myocardial toxicity: New solutions for an old problem? Int. J. Cardiol. 2007, 117, 6–15. [Google Scholar] [CrossRef]
- Wu, X.N.; Yu, C.H.; Bao, Q.N. Anti-inflammatory effect and mechanism study of total flavonoids in Chrysanthemum morifolium Ramat. Chinese J. Clin. Pharmacol. Ther. 2009, 14, 1000–1004. [Google Scholar]
- Wencker, D.; Chandra, M.; Nguyen, K.; Miao, W.; Garantziotis, S.; Factor, S.M.; Shirani, J.; Armstrong, R.C.; Kitsis, R.N. A mechanistic role for cardiac myocyte apoptosis in heart failure. J. Clin. Investig. 2003, 111, 1497–1504. [Google Scholar] [CrossRef]
- Kumar, S.; Enjamoori, R.; Jaiswal, A.; Ray, R.; Seth, S.; Maulik, S.K. Catecholamine-induced myocardial fibrosis and oxidative stress is attenuated by Terminalia arjuna (Roxb.). J. Pharm. Pharmacol. 2009, 61, 1529–1536. [Google Scholar] [CrossRef]
- Ning, B.-B.; Zhang, Y.; Wu, D.-D.; Cui, J.-G.; Liu, L.; Wang, P.-W.; Wang, W.-J.; Zhu, W.-L.; Chen, Y.; Zhang, T. Luteolin-7-diglucuronide attenuates isoproterenol-induced myocardial injury and fibrosis in mice. Acta Pharmacol. Sin. 2017, 38, 331–341. [Google Scholar] [CrossRef]
- Gao, T.; Zhu, Z.-Y.; Zhou, X.; Xie, M.-L. Chrysanthemum morifolium extract improves hypertension-induced cardiac hypertrophy in rats by reduction of blood pressure and inhibition of myocardial hypoxia inducible factor-1alpha expression. Pharm. Biol. 2016, 54, 2895–2900. [Google Scholar] [CrossRef]
- Chen, M.; Wang, K.; Zhang, Y.; Zhang, M.; Ma, Y.; Sun, H.; Jin, Z.; Zheng, H.; Jiang, H.; Yu, P.; et al. New insights into the biological activities of Chrysanthemum morifolium: Natural flavonoids alleviate diabetes by targeting α-glucosidase and the PTP-1B signaling pathway. Eur. J. Med. Chem. 2019, 178, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Ukiya, M.; Akihisa, T.; Tokuda, H.; Suzuki, H.; Mukainaka, T.; Ichiishi, E.; Yasukawa, K.; Kasahara, Y.; Nishino, H. Constituents of Compositae plants: III. Anti-tumor promoting effects and cytotoxic activity against human cancer cell lines of triterpene diols and triols from edible chrysanthemum flowers. Cancer Lett. 2002, 177, 7–12. [Google Scholar] [CrossRef]
- Xie, Y.-Y.; Yuan, D.; Yang, J.-Y.; Wang, L.-H.; Wu, C.-F. Cytotoxic activity of flavonoids from the flowers of Chrysanthemum morifolium on human colon cancer Colon205 cells. J. Asian Nat. Prod. Res. 2009, 11, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-Q.; Chen, K.; Shi, Q.; Kilkuskie, R.E.; Cheng, Y.-C.; Lee, K.-H. Anti-AIDS Agents, 10. Acacetin-7-O-β-D-galactopyranoside, an Anti-HIV Principle from Chrysanthemum morifolium and a Structure-Activity Correlation with Some Related Flavonoids. J. Nat. Prod. 1994, 57, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, H.J.; Lee, Y.S. A new Anti-HIV Flavonoid Glucuronide fromChrysanthemum morifolium. Planta Medica 2003, 69, 859–861. [Google Scholar] [CrossRef]
- Li, L.; Gu, L.; Chen, Z.; Wang, R.; Ye, J.; Jiang, H. Toxicity Study of Ethanolic Extract of Chrysanthemum morifolium in Rats. J. Food Sci. 2010, 75, T105–T109. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Yang, Z.-Z.; Ye, L.-Q.; Han, X.-L.; Zhao, D.-D.; Ding, F.-Y.; Ding, N.; Wu, H.-C.; Yu, M.; Xu, G.-Y.; et al. Establishment of an in vitro safety assessment model for lipid-lowering drugs using same-origin human pluripotent stem cell-derived cardiomyocytes and endothelial cells. Acta Pharmacol. Sin. 2021, 43, 240–250. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono, M.; Sunagawa, Y.; Mochizuki, S.; Katagiri, T.; Takai, H.; Iwashimizu, S.; Inai, K.; Funamoto, M.; Shimizu, K.; Shimizu, S.; et al. Chrysanthemum morifolium Extract Ameliorates Doxorubicin-Induced Cardiotoxicity by Decreasing Apoptosis. Cancers 2022, 14, 683. https://doi.org/10.3390/cancers14030683
Ono M, Sunagawa Y, Mochizuki S, Katagiri T, Takai H, Iwashimizu S, Inai K, Funamoto M, Shimizu K, Shimizu S, et al. Chrysanthemum morifolium Extract Ameliorates Doxorubicin-Induced Cardiotoxicity by Decreasing Apoptosis. Cancers. 2022; 14(3):683. https://doi.org/10.3390/cancers14030683
Chicago/Turabian StyleOno, Masaya, Yoichi Sunagawa, Saho Mochizuki, Takahiro Katagiri, Hidemichi Takai, Sonoka Iwashimizu, Kyoko Inai, Masafumi Funamoto, Kana Shimizu, Satoshi Shimizu, and et al. 2022. "Chrysanthemum morifolium Extract Ameliorates Doxorubicin-Induced Cardiotoxicity by Decreasing Apoptosis" Cancers 14, no. 3: 683. https://doi.org/10.3390/cancers14030683
APA StyleOno, M., Sunagawa, Y., Mochizuki, S., Katagiri, T., Takai, H., Iwashimizu, S., Inai, K., Funamoto, M., Shimizu, K., Shimizu, S., Katanasaka, Y., Komiyama, M., Hawke, P., Hara, H., Arakawa, Y., Mori, K., Asai, A., Hasegawa, K., & Morimoto, T. (2022). Chrysanthemum morifolium Extract Ameliorates Doxorubicin-Induced Cardiotoxicity by Decreasing Apoptosis. Cancers, 14(3), 683. https://doi.org/10.3390/cancers14030683





