A New Tumor Burden Score and Albumin–Bilirubin Grade-Based Prognostic Model for Hepatocellular Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Definition and Diagnosis
2.3. Calculation of TBS
2.4. Calculation of ALBI Score
2.5. Development and Validation of the New Prognostic Model
2.6. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Development of the New TBS–ALBI Prognostic System
3.3. Patient Survival in the Derivation Cohort Based on the TBS–ALBI System
3.4. Validation of the TBS–ALBI System
3.5. Using the TBS–ALBI System to Differentiate Survival in Different Risk Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [Green Version]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Makary, M.S.; Khandpur, U.; Cloyd, J.M.; Mumtaz, K.; Dowell, J.D. Locoregional therapy approaches for hepatocellular carcinoma: Recent advances and management strategies. Cancers 2020, 12, 1914. [Google Scholar] [CrossRef]
- Nakano, S.; Eso, Y.; Okada, H.; Takai, A.; Takahashi, K.; Seno, H. Recent advances in immunotherapy for hepatocellular carcinoma. Cancers 2020, 12, 775. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.H.; Hsu, C.Y.; Hsia, C.Y.; Lee, Y.H.; Su, C.W.; Huang, Y.H.; Lee, F.Y.; Lin, H.C.; Huo, T.I. Prognosis of hepatocellular carcinoma: Assessment of eleven staging systems. J. Hepatol. 2016, 64, 601–608. [Google Scholar] [CrossRef] [Green Version]
- Mazzaferro, V.; Chun, Y.S.; Poon, R.T.; Schwartz, M.E.; Yao, F.Y.; Marsh, J.W.; Bhoori, S.; Lee, S.G. Liver transplantation for hepatocellular carcinoma. Ann. Surg. Oncol. 2008, 15, 1001–1007. [Google Scholar] [CrossRef] [Green Version]
- Yasui, Y.; Tsuchiya, K.; Kurosaki, M.; Takeguchi, T.; Takeguchi, Y.; Okada, M.; Wang, W.; Kubota, Y.; Goto, T.; Komiyama, Y.; et al. Up-to-seven criteria as a useful predictor for tumor downstaging to within Milan criteria and Child-Pugh grade deterioration after initial conventional transarterial chemoembolization. Hepatol. Res. 2018, 48, 442–450. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef]
- Lai, Q.; Avolio, A.W.; Manzia, T.M.; Sorge, R.; Agnes, S.; Tisone, G.; Berloco, P.B.; Rossi, M. Combination of biological and morphological parameters for the selection of patients with hepatocellular carcinoma waiting for liver transplantation. Clin. Transpl. 2012, 26, E125–E131. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Huang, Y.H.; Hsia, C.Y.; Su, C.W.; Lin, H.C.; Loong, C.C.; Chiou, Y.Y.; Chiang, J.H.; Lee, P.C.; Huo, T.I.; et al. A new prognostic model for hepatocellular carcinoma based on total tumor volume: The Taipei Integrated Scoring System. J. Hepatol. 2010, 53, 108–117. [Google Scholar] [CrossRef]
- Sasaki, K.; Morioka, D.; Conci, S.; Margonis, G.A.; Sawada, Y.; Ruzzenente, A.; Kumamoto, T.; Iacono, C.; Andreatos, N.; Guglielmi, A.; et al. The Tumor Burden Score: A new “metro-ticket” prognostic tool for colorectal liver metastases based on tumor size and number of tumors. Ann. Surg. 2018, 267, 132–141. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Moris, D.; Hyer, J.M.; Bagante, F.; Sahara, K.; Moro, A.; Paredes, A.Z.; Mehta, R.; Ratti, F.; Marques, H.P.; et al. Hepatocellular carcinoma tumour burden score to stratify prognosis after resection. Br. J. Surg. 2020, 107, 854–864. [Google Scholar] [CrossRef]
- Moris, D.; Shaw, B.I.; McElroy, L.; Barbas, A.S. Using Hepatocellular carcinoma tumor burden score to stratify prognosis after liver transplantation. Cancers 2020, 12, 3372. [Google Scholar] [CrossRef]
- Vitale, A.; Lai, Q.; Farinati, F.; Bucci, L.; Giannini, E.G.; Napoli, L.; Ciccarese, F.; Rapaccini, G.L.; Di Marco, M.; Caturelli, E.; et al. Utility of tumor burden score to stratify prognosis of patients with hepatocellular cancer: Results of 4759 Cases from ITA.LI.CA Study Group. J. Gastrointest. Surg. 2018, 22, 859–871. [Google Scholar] [CrossRef]
- Kumada, T.; Toyoda, H.; Tada, T.; Yasuda, S.; Tanaka, J. Changes in background liver function in patients with hepatocellular carcinoma over 30 years: Comparison of Child-Pugh classification and albumin bilirubin grade. Liver Cancer 2020, 9, 518–528. [Google Scholar] [CrossRef]
- Huo, T.I.; Lin, H.C.; Hsia, C.Y.; Wu, J.C.; Lee, P.C.; Chi, C.W.; Lee, S.D. The model for end-stage liver disease based cancer staging systems are better prognostic models for hepatocellular carcinoma: A prospective sequential survey. Am. J. Gastroenterol. 2007, 102, 1920–1930. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Huo, T.I. ALBI grade as a new player in hepatocellular carcinoma. J. Chin. Med. Assoc. 2019, 82, 112. [Google Scholar] [CrossRef]
- Ho, S.Y.; Hsu, C.Y.; Liu, P.H.; Hsia, C.Y.; Lei, H.J.; Huang, Y.H.; Ko, C.C.; Su, C.W.; Lee, R.C.; Hou, M.C.; et al. Albumin-bilirubin grade-based nomogram of the BCLC system for personalized prognostic prediction in hepatocellular carcinoma. Liver Int. 2020, 40, 205–214. [Google Scholar] [CrossRef]
- Ho, S.Y.; Hsu, C.Y.; Liu, P.H.; Ko, C.C.; Huang, Y.H.; Su, C.W.; Lee, R.C.; Hou, M.C.; Huo, T.I. Survival of patients with hepatocellular carcinoma in renal insufficiency: Prognostic role of albumin-bilirubin grade. Cancers 2020, 12, 1130. [Google Scholar] [CrossRef]
- Lescure, C.; Estrade, F.; Pedrono, M.; Campillo-Gimenez, B.; Le Sourd, S.; Pracht, M.; Palard, X.; Bourien, H.; Muzellec, L.; Uguen, T.; et al. ALBI score is a strong predictor of toxicity following SIRT for hepatocellular carcinoma. Cancers 2021, 13, 3794. [Google Scholar] [CrossRef]
- Liu, P.H.; Lee, Y.H.; Hsia, C.Y.; Hsu, C.Y.; Huang, Y.H.; Chiou, Y.Y.; Lin, H.C.; Huo, T.I. Surgical resection versus transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: A propensity score analysis. Ann. Surg. Oncol. 2014, 21, 1825–1833. [Google Scholar] [CrossRef]
- Huo, T.; Huang, Y.H.; Wu, J.C.; Chiang, J.H.; Lee, P.C.; Chang, F.Y.; Lee, S.D. Comparison of transarterial chemoembolization and percutaneous acetic acid injection as the primary loco-regional therapy for unresectable hepatocellular carcinoma: A prospective survey. Aliment. Pharmacol. 2004, 19, 1301–1308. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Hsia, C.Y.; Huang, Y.H.; Su, C.W.; Lin, H.C.; Pai, J.T.; Loong, C.C.; Chiou, Y.Y.; Lee, R.C.; Lee, F.Y.; et al. Comparison of surgical resection and transarterial chemoembolization for hepatocellular carcinoma beyond the Milan criteria: A propensity score analysis. Ann. Surg. Oncol. 2012, 19, 842–849. [Google Scholar] [CrossRef]
- Liu, P.H.; Huo, T.I.; Miksad, R.A. Hepatocellular carcinoma with portal vein tumor involvement: Best management strategies. Semin. Liver Dis. 2018, 38, 242–251. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Liu, P.H.; Ho, S.Y.; Huang, Y.H.; Lee, Y.H.; Lee, R.C.; Nagaria, T.S.; Hou, M.C.; Huo, T.I. Metastasis in patients with hepatocellular carcinoma: Prevalence, determinants, prognostic impact and ability to improve the Barcelona Clinic Liver Cancer system. Liver Int. 2018, 38, 1803–1811. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Lee, Y.H.; Hsia, C.Y.; Huang, Y.H.; Su, C.W.; Lin, H.C.; Lee, R.C.; Chiou, Y.Y.; Lee, F.Y.; Huo, T.I. Performance status in patients with hepatocellular carcinoma: Determinants, prognostic impact, and ability to improve the Barcelona Clinic Liver Cancer system. Hepatology 2013, 57, 112–119. [Google Scholar] [CrossRef]
- Pourhoseingholi, M.A.; Hajizadeh, E.; Moghimi Dehkordi, B.; Safaee, A.; Abadi, A.; Zali, M.R. Comparing Cox regression and parametric models for survival of patients with gastric carcinoma. Asian Pac. J. Cancer Prev. 2007, 8, 412–416. [Google Scholar]
- Fang, K.C.; Kao, W.Y.; Su, C.W.; Chen, P.C.; Lee, P.C.; Huang, Y.H.; Huo, T.I.; Chang, C.C.; Hou, M.C.; Lin, H.C.; et al. The prognosis of single large hepatocellular carcinoma was distinct from Barcelona Clinic Liver Cancer Stage A or B: The role of albumin-bilirubin grade. Liver Cancer 2018, 7, 335–358. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Mehta, R.; Paredes, A.Z.; Moris, D.; Sahara, K.; Bagante, F.; Ratti, F.; Marques, H.P.; Silva, S.; Soubrane, O.; et al. Overall tumor burden dictates outcomes for patients undergoing resection of multinodular hepatocellular carcinoma beyond the Milan criteria. Ann. Surg. 2020, 272, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.Y.; Liu, P.H.; Hsu, C.Y.; Ko, C.C.; Huang, Y.H.; Su, C.W.; Lee, R.C.; Tsai, P.H.; Hou, M.C.; Huo, T.I. Tumor burden score as a new prognostic marker for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. J. Gastroenterol. Hepatol. 2021, 36, 3196–3203. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Hsu, C.Y.; Huang, Y.H.; Hsia, C.Y.; Chiou, Y.Y.; Su, C.W.; Lin, H.C.; Huo, T.I. Vascular invasion in hepatocellular carcinoma: Prevalence, determinants and prognostic impact. J. Clin. Gastroenterol. 2014, 48, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Aithal, G.P.; Palaniyappan, N.; China, L.; Härmälä, S.; Macken, L.; Ryan, J.M.; Wilkes, E.A.; Moore, K.; Leithead, J.A.; Hayes, P.C.; et al. Guidelines on the management of ascites in cirrhosis. Gut 2021, 70, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Lee, Y.H.; Huang, Y.H.; Hsia, C.Y.; Su, C.W.; Lin, H.C.; Lee, R.C.; Chiou, Y.Y.; Lee, F.Y.; Huo, T.I.; et al. Ascites in patients with hepatocellular carcinoma: Prevalence, associated factors, prognostic impact, and staging strategy. Hepatol. Int. 2013, 7, 188–198. [Google Scholar] [CrossRef]
- Ho, S.Y.; Liu, P.H.; Hsu, C.Y.; Hsia, C.Y.; Huang, Y.H.; Su, C.W.; Lei, H.J.; Lee, R.C.; Hou, M.C.; Huo, T.I. A new prognostic model based on albumin-bilirubin grade for hepatocellular carcinoma beyond the Milan criteria. Dig. Dis. Sci. 2020, 65, 658–667. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Liu, P.H.; Lee, Y.H.; Hsia, C.Y.; Huang, Y.H.; Lin, H.C.; Chiou, Y.Y.; Lee, F.Y.; Huo, T.I. Using serum alpha-fetoprotein for prognostic prediction in patients with hepatocellular carcinoma: What is the most optimal cutoff? PLoS ONE 2015, 10, e0118825. [Google Scholar]
- Galle, P.R.; Foerster, F.; Kudo, M.; Chan, S.L.; Llovet, J.M.; Qin, S.; Schelman, W.R.; Chintharlapalli, S.; Abada, P.B.; Sherman, M.; et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019, 39, 2214–2229. [Google Scholar] [CrossRef] [Green Version]
- Cassim, S.; Raymond, V.A.; Dehbidi-Assadzadeh, L.; Lapierre, P.; Bilodeau, M. Metabolic reprogramming enables hepatocar-cinoma cells to efficiently adapt and survive to a nutrient-restricted microenvironment. Cell Cycle 2018, 17, 903–916. [Google Scholar] [CrossRef]
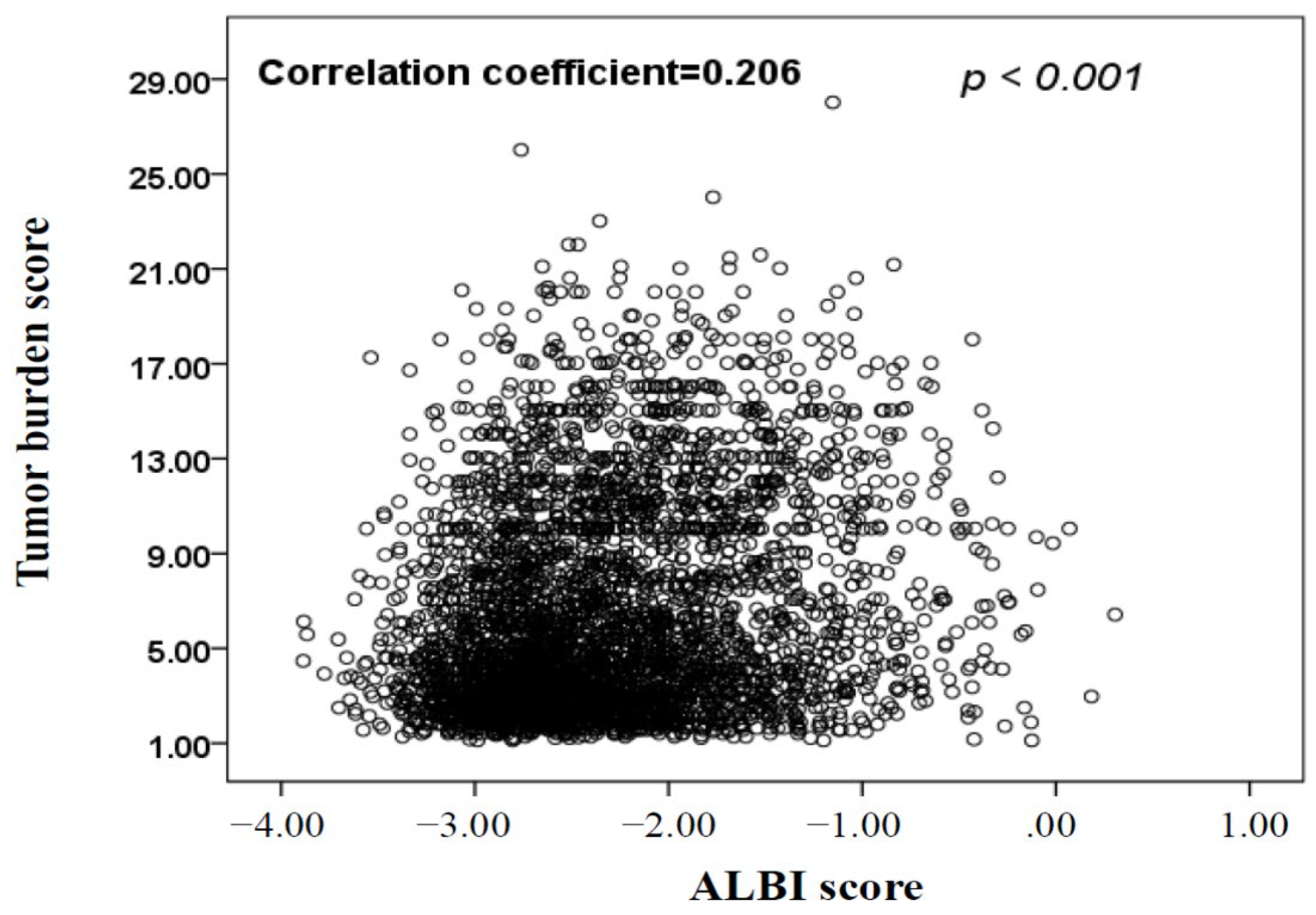
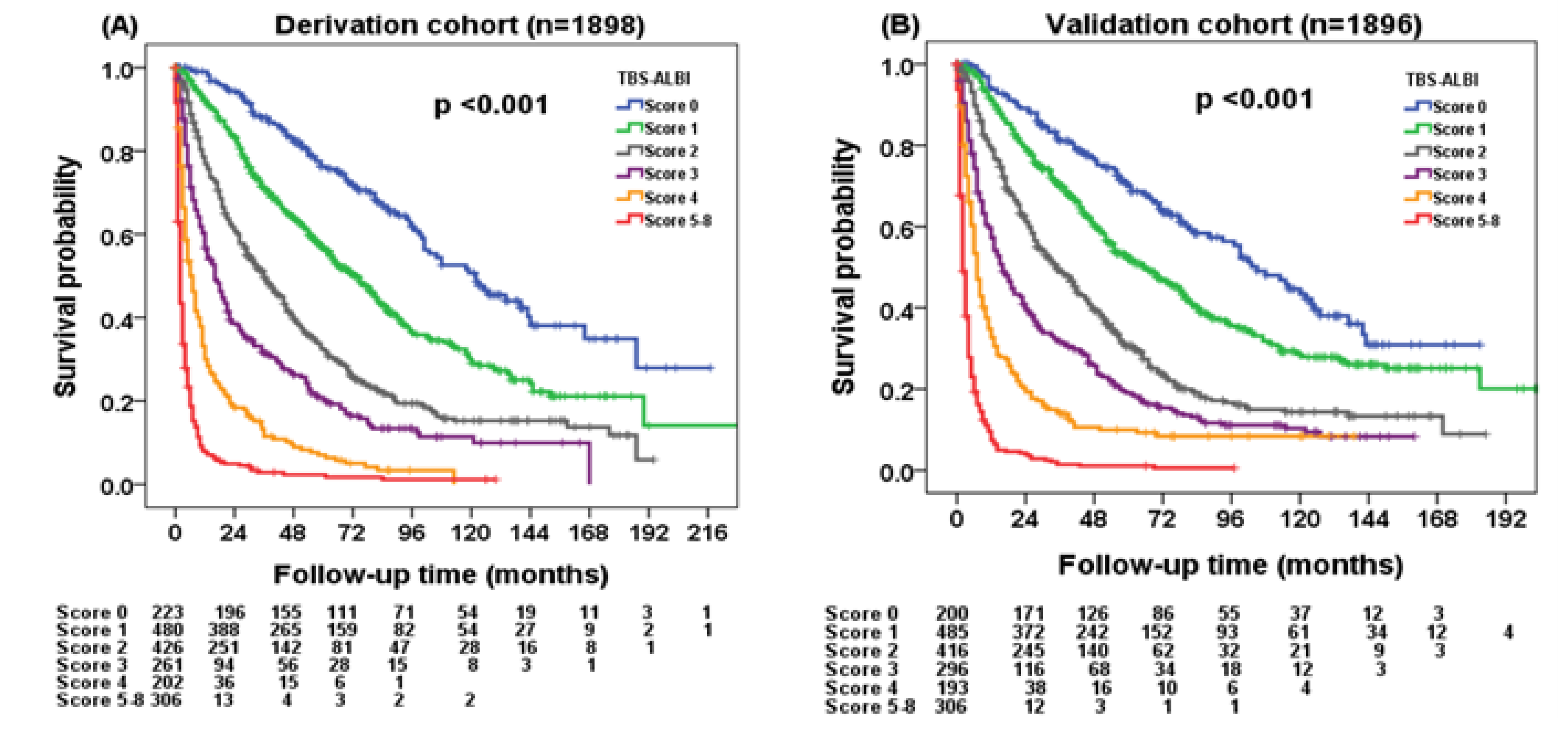

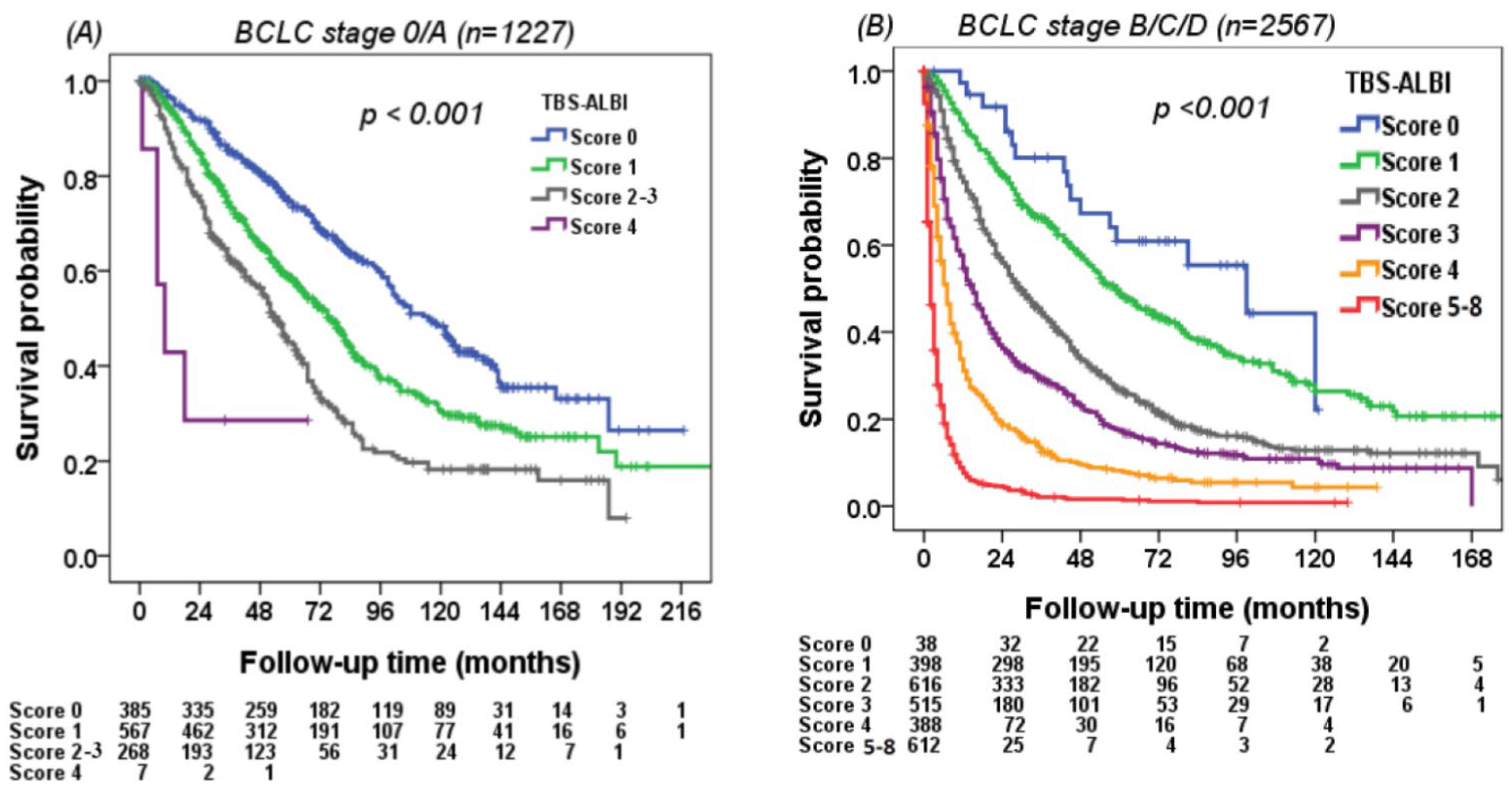
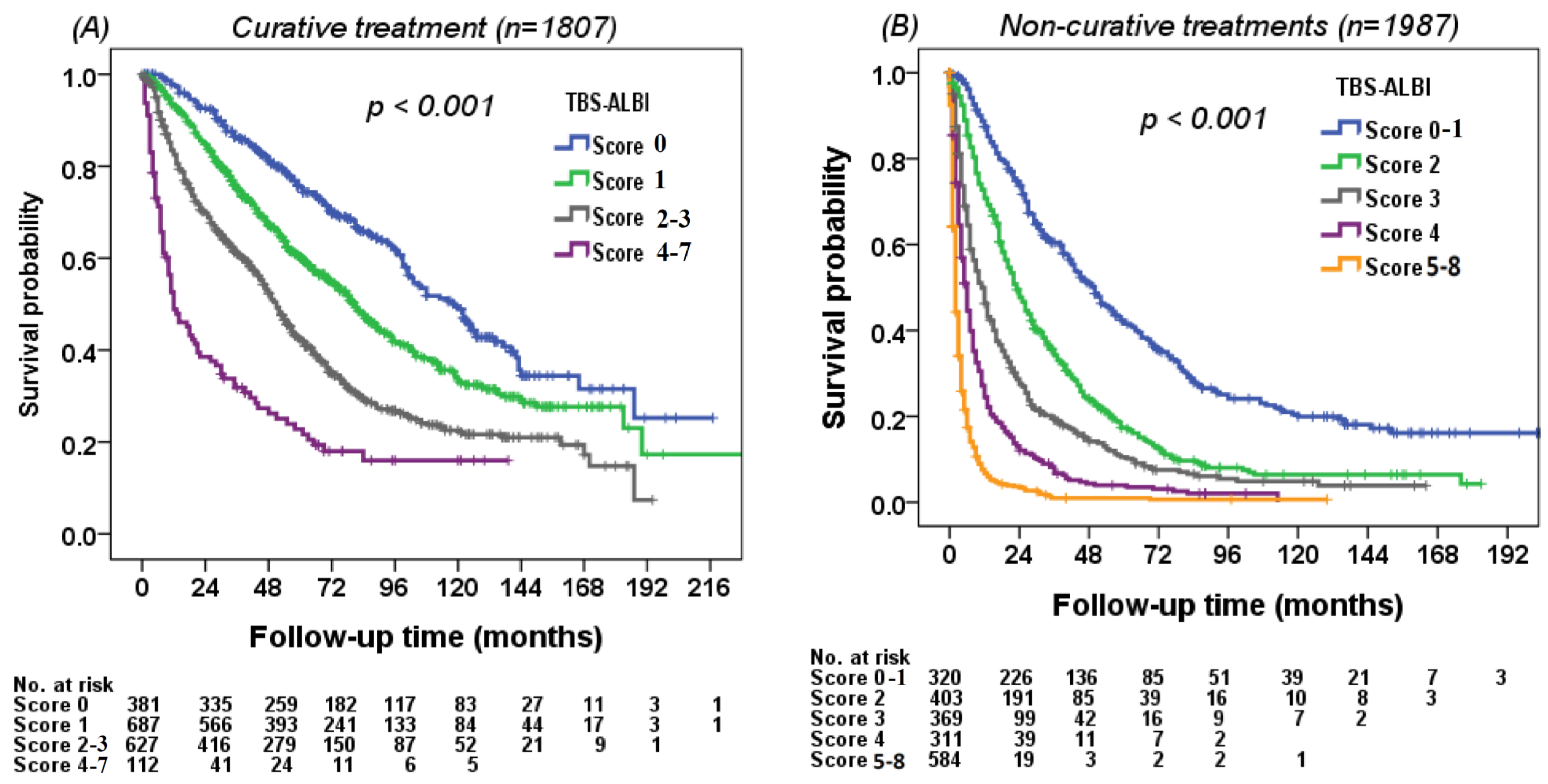
| Variables | All Patients | Derivation Cohort (n = 1898) | Validation Cohort (n = 1896) | p |
|---|---|---|---|---|
| Age (years, mean ± SD) | 66 ± 13 | 64 ± 13 | 65 ± 13 | 0.715 |
| Male, n (%) | 2895 (76) | 1450 (76) | 1445 (75) | 0.909 |
| Etiologies of liver disease | 0.894 | |||
| HBV, n (%) | 1513 (40) | 737 (39) | 776 (41) | |
| HCV, n (%) | 824 (22) | 450 (24) | 374 (20) | |
| HBV + HCV, n (%) | 135 (3) | 61 (3) | 74 (4) | |
| Others, n (%) | 1322 (35) | 650 (34) | 672 (35) | |
| Performance status (0/1/2/3–4), n (%) | 2226/780/431/357 (59/21/11/9) | 1119/376/218/185 (59/20/12/9) | 1107/404/213/172 (59/21/11/9) | 0.758 |
| Diabetes mellitus, n (%) | 972 (26) | 459(25) | 503 (27) | 0.206 |
| Tumor nodules (single/multiple), (%) | 2437/1357 (64/36) | 1227/671 (65/35) | 1210/686 (64/36) | 0.611 |
| Maximal tumor diameter ≥5 cm, n (%) | 1668 (44) | 838 (44) | 830 (44) | 0.819 |
| Tumor burden score (mean ± SD) | 5.0 ± 4.4 | 6.5 ± 4.3 | 6.5 ± 4.4 | 0.550 |
| Tumor burden score (low/medium/high) | 1160/2299/335 (31/61/8) | 578/1157/163 (30/61/9) | 582/1142/172 (31/60/9) | 0.900 |
| Vascular invasion or distant metastasis, n (%) | 1038 (27) | 494 (26) | 544 (28) | 0.069 |
| Serum AFP ≥ 400 ng/mL, n (%) | 1117 (29) | 546 (29) | 571 (30) | 0.373 |
| Ascites, n (%) | 861 (23) | 432 (23) | 429 (23) | 0.938 |
| Laboratory values (mean ± SD) | ||||
| Albumin (g/L) | 37 ± 6 | 36 ± 6 | 36 ± 6 | 0.600 |
| Total bilirubin (μmol/L) | 15 ± 48 | 26 ± 51 | 26 ± 44 | 0.685 |
| Platelets (1000/μL) | 153 ± 96 | 170 ± 95 | 170 ± 97 | 0.938 |
| INR of prothrombin time | 1.06 ± 0.2 | 1.1 ± 0.3 | 1.1 ± 0.2 | 0.187 |
| Creatinine (mg/dL) | 1.0 ± 1.0 | 1.2 ± 1.0 | 1.2 ± 1.0 | 0.944 |
| CTP class (A/B/C) | 2787/831/176 (73/22/5) | 1480/386/104 (74/20/6) | 1379/445/72 (72/24/4) | 0.006 |
| CTP score, mean ± SD | 5.0 ± 1.5 | 6.0 ± 1.5 | 6.0 ± 1.5 | 0.644 |
| ALBI grade (1/2/3), n (%) | 1444/1970/380 (38/52/10) | 745/943/210 (39/50/11) | 699/1027/170 (37/54/9) | 0.899 |
| ALBI score, mean ± SD | −2.40 ± 0.65 | −2.29 ± 0.66 | −2.30 ± 0.63 | 0.810 |
| Tumor staging, (%) | ||||
| BCLC stage (0/A/B/C/D) | 8/24/17/40/11 | 8/24/18/38/11 | 8/25/16/41/11 | 0.754 |
| HKLC (I/II/III/IV/V) | 32/27/10/8/22 | 32/27/10/8/23 | 32/27/10/9/22 | 0.885 |
| TIS (0/1/2/3/4/5/6) | 36/22/21/12/12/11/6/1 | 37/21/13/11/12/5/1 | 36/22/12/12/11/6/1 | 0.378 |
| JIS (0/1/2/3/4/5) | 9/33/30/17/9/2 | 9/33/30/17/9/2 | 9/33/30/17/9/1 | 0.827 |
| CLIP (0/1/2/3/4/5/6) | 32/26/15/12/9/5/1 | 33/26/15/12/9/4/1 | 31/27/15/11/10/5/1 | 0.346 |
| Okuda (1/2/3) | 53/38/9 | 53/38/9 | 53/39/8 | 0.616 |
| Tokyo (0/1/2/3/4/5/6/7/8) | 6/22/26/19/12/8/4/2/1 | 6/23/25/19/12/8/4/2/1 | 6/21/27/19/13/8/3/2/1 | 0.833 |
| TNM (I/II/III/IV) | 33/25/36/6 | 34/24/36/6 | 33/24/36/6 | 0.681 |
| Treatments, n (%) | 0.775 | |||
| Surgical resection | 1107 (29) | 569 (30) | 538 (28) | |
| Local ablation therapy | 680 (18) | 327 (17) | 353 (19) | |
| TACE | 1034 (27) | 521 (27) | 513 (27) | |
| Liver transplantation | 20 (1) | 9 (1) | 11 (1) | |
| Targeted therapy | 303 (8) | 154 (8) | 149 (8) | |
| Others | 650 (17) | 318 (17) | 332 (17) |
| Variables | Number | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| 3-Year Survival (%) | 5-Year Survival (%) | p | Hazard Ratio | 95% CI | p | ||
| Age (<55/≥55 years) | 955/943 | 44/47 | 37/34 | 0.936 | |||
| Sex (male/female) | 2895/899 | 64/73 | 45/52 | 0.004 | |||
| HBV (negative/positive) | 905/993 | 45/48 | 31/38 | 0.041 | |||
| HCV (negative/positive) | 1311/587 | 46/48 | 35/35 | 0.462 | |||
| Platelet (<150,000/≥150,000/μL) | 986/912 | 43/50 | 17/13 | 0.034 | |||
| Ascites (absent/present) | 1466/432 | 55/18 | 41/13 | <0.001 | 1.343 | 1.169–1.542 | <0.001 |
| Serum AFP (<400/≥400 ng/mL) | 1352/546 | 56/22 | 43/15 | <0.001 | 1.523 | 1.345–1.726 | <0.001 |
| Vascular invasion or distant metastasis (no/yes) | 1404/494 | 57/9 | 43/6 | <0.001 | 2.312 | 2.010–2.658 | <0.001 |
| Diabetes mellitus (no/yes) | 1429/469 | 48/43 | 36/31 | 0.025 | |||
| Performance status 0–1/2–4 | 1119/709 | 54/18 | 42/9 | <0.001 | 1.853 | 1.612–2.129 | <0.001 |
| Tumor burden score | |||||||
| Low | 578 | 71 | 56 | ||||
| Medium | 1157 | 39 | 28 | <0.001 | 1.655 | 1.446–1.893 | <0.001 |
| High | 163 | 9 | 6 | <0.001 | 2.395 | 1.919–2.990 | <0.001 |
| ALBI | |||||||
| Grade 1 | 745 | 68 | 55 | ||||
| Grade 2 | 943 | 37 | 25 | <0.001 | 1.879 | 1.661–2.126 | <0.001 |
| Grade 3 | 210 | 11 | 7 | <0.001 | 3.090 | 2.540–3.761 | <0.001 |
| Prognostic Factors | Score | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Tumor burden score | Low | Medium | High |
| ALBI grade | 1 | 2 | 3 |
| Vascular invasion or distant metastasis | Absent | Present | |
| Ascites | Absent | Present | |
| Serum AFP (ng/mL) | <400 | ≥400 | |
| Performance status | 0–1 | 2–4 | |
| Models | Homogeneity (Wald χ2) | Corrected Akaike Information Criterion |
|---|---|---|
| Validation cohort (n = 1896) | ||
| BCLC | 477.462 | 18,747.588 |
| HKLC | 543.712 | 18,681.338 |
| TIS | 676.704 | 18,544.279 |
| JIS | 503.527 | 18,721.523 |
| CLIP | 756.433 | 18,468.617 |
| Okuda | 466.310 | 18,758.439 |
| Tokyo | 517.941 | 18,707.108 |
| TNM | 294.309 | 18,930.740 |
| TBS–ALBI | 871.542 | 18,353.503 |
| Curative treatment (n = 902) | ||
| BCLC | 35.453 | 6429.229 |
| HKLC | 36.586 | 6428.089 |
| TIS | 35.420 | 6427.088 |
| JIS | 39.044 | 6425.638 |
| CLIP | 51.544 | 6413.138 |
| Okuda | 30.110 | 6434.517 |
| Tokyo | 36.884 | 6427.798 |
| TNM | 12.795 | 6451.887 |
| TBS–ALBI | 76.604 | 6388.087 |
| Non-curative treatment (n = 994) | ||
| BCLC | 250.074 | 10,420.458 |
| HKLC | 268.381 | 10,402.051 |
| TIS | 380.781 | 10,289.651 |
| JIS | 241.444 | 10,428.988 |
| CLIP | 372.307 | 10,298.125 |
| Okuda | 205.407 | 10,465.025 |
| Tokyo | 198.510 | 10,471.922 |
| TNM | 162.579 | 10,507.853 |
| TBS–ALBI | 432.154 | 10,238.278 |
| HBV-related HCC (n = 776) | ||
| BCLC | 201.496 | 6260.271 |
| HKLC | 225.089 | 6235.670 |
| TIS | 257.214 | 6202.857 |
| JIS | 204.453 | 6257.314 |
| CLIP | 278.184 | 6183.584 |
| Okuda | 181.108 | 6280.659 |
| Tokyo | 203.245 | 6258.522 |
| TNM | 130.840 | 6330.928 |
| TBS–ALBI | 359.468 | 6102.300 |
| HCV-related HCC (n = 374) | ||
| BCLC | 115.183 | 2894.521 |
| HKLC | 135.930 | 2873.744 |
| TIS | 144.346 | 2863.157 |
| JIS | 105.382 | 2904.322 |
| CLIP | 185.719 | 2823.985 |
| Okuda | 85.960 | 2923.744 |
| Tokyo | 97.339 | 2682.659 |
| TNM | 47.638 | 2962.066 |
| TBS-ALB | 189.422 | 2820.261 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, S.-Y.; Liu, P.-H.; Hsu, C.-Y.; Huang, Y.-H.; Liao, J.-I.; Su, C.-W.; Hou, M.-C.; Huo, T.-I. A New Tumor Burden Score and Albumin–Bilirubin Grade-Based Prognostic Model for Hepatocellular Carcinoma. Cancers 2022, 14, 649. https://doi.org/10.3390/cancers14030649
Ho S-Y, Liu P-H, Hsu C-Y, Huang Y-H, Liao J-I, Su C-W, Hou M-C, Huo T-I. A New Tumor Burden Score and Albumin–Bilirubin Grade-Based Prognostic Model for Hepatocellular Carcinoma. Cancers. 2022; 14(3):649. https://doi.org/10.3390/cancers14030649
Chicago/Turabian StyleHo, Shu-Yein, Po-Hong Liu, Chia-Yang Hsu, Yi-Hsiang Huang, Jia-I Liao, Chien-Wei Su, Ming-Chih Hou, and Teh-Ia Huo. 2022. "A New Tumor Burden Score and Albumin–Bilirubin Grade-Based Prognostic Model for Hepatocellular Carcinoma" Cancers 14, no. 3: 649. https://doi.org/10.3390/cancers14030649
APA StyleHo, S.-Y., Liu, P.-H., Hsu, C.-Y., Huang, Y.-H., Liao, J.-I., Su, C.-W., Hou, M.-C., & Huo, T.-I. (2022). A New Tumor Burden Score and Albumin–Bilirubin Grade-Based Prognostic Model for Hepatocellular Carcinoma. Cancers, 14(3), 649. https://doi.org/10.3390/cancers14030649






