Management of Early-Stage Cervical Cancer: A Literature Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Diagnosis
3. Staging
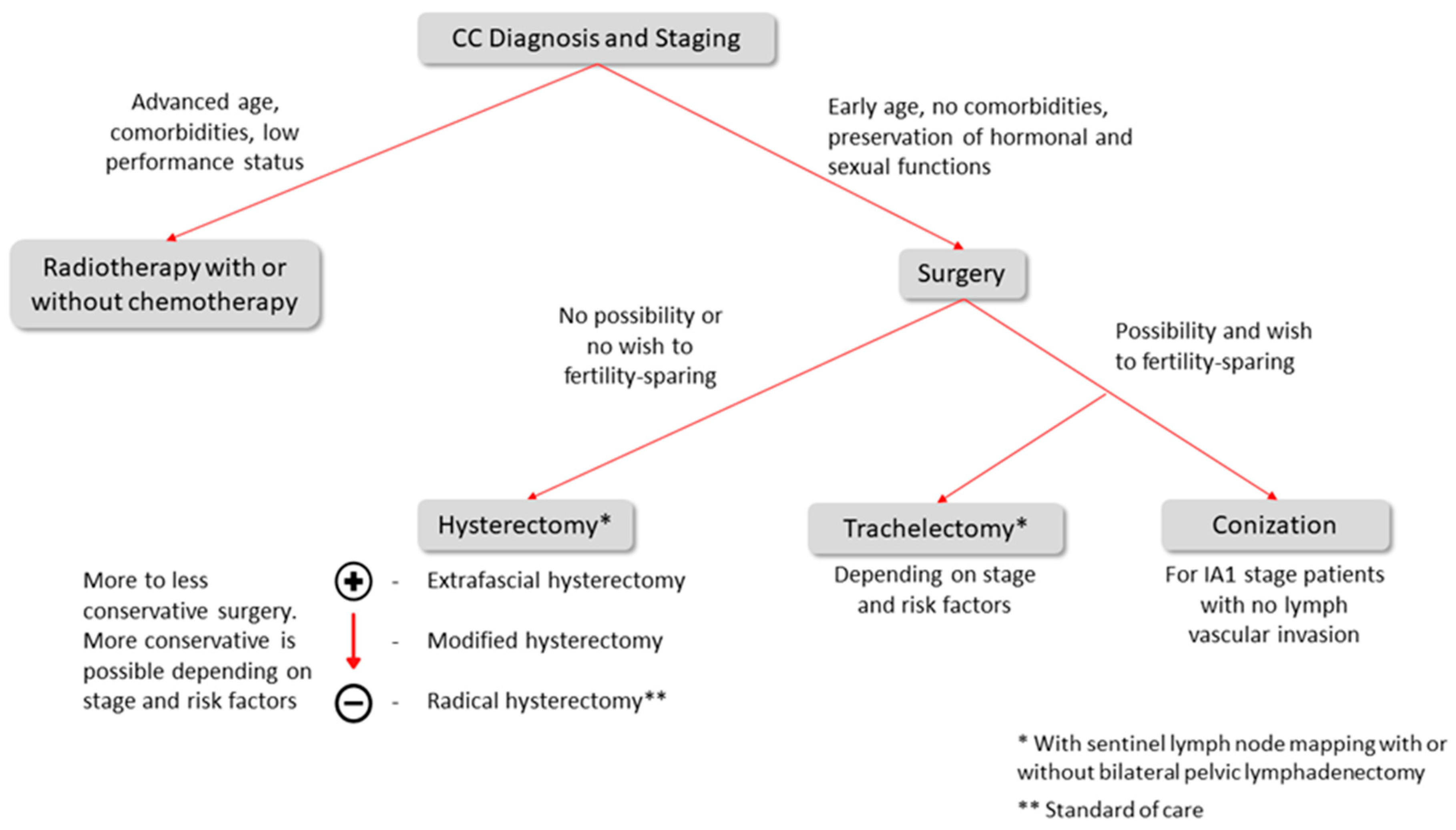
4. Treatment
4.1. Treatment for IA1 Stage
4.2. Treatment for IA2, IB1, IB2, and IIA1 Stages
4.3. Lymph Node Staging
4.4. Fertility Sparing-Surgery
4.5. Adjuvant Treatment
5. Surgical Approach
6. Tumor Size < 2 cm
7. Prognosis
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bedell, S.L.; Goldstein, L.S.; Goldstein, A.R.; Goldstein, A.T. Cervical Cancer Screening: Past, Present, and Future. Sex. Med. Rev. 2020, 8, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.V. The human papillomavirus replication cycle, and its links to cancer progression: A comprehensive review. Clin. Sci. 2017, 131, 2201–2221. [Google Scholar] [CrossRef] [Green Version]
- Pimple, S.; Mishra, G.; Shastri, S. Global strategies for cervical cancer prevention. Curr. Opin. Obstet. Gynecol. 2016, 28, 4–10. [Google Scholar] [CrossRef] [PubMed]
- von Karsa, L.; Arbyn, M.; De Vuyst, H.; Dillner, J.; Dillner, L.; Franceschi, S.; Patnick, J.; Ronco, G.; Segnan, N.; Suonio, E.; et al. European guidelines for quality assurance in cervical cancer screening. Summary of the supplements on HPV screening and vaccination. Papillomavirus Res. 2015, 1, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Gradíssimo, A.; Burk, R.D. Molecular tests potentially improving HPV screening and genotyping for cervical cancer prevention. Expert Rev. Mol. Diagn. 2017, 17, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Fontham, E.T.H.; Wolf, A.M.D.; Church, T.R.; Etzioni, R.; Flowers, C.R.; Herzig, A.; Guerra, C.E.; Oeffinger, K.C.; Shih, Y.T.; Walter, L.C.; et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA A Cancer J. Clin. 2020, 70, 321–346. [Google Scholar] [CrossRef]
- The American College of Obstetricians and Gynecologists. Practice Bulletin No. 168: Cervical Cancer Screening and Prevention. Obstet. Gynecol. 2016, 128, e111–e130. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Viale, P.H. The American Cancer Society’s facts & figures: 2020 edition. J. Adv. Pract. Oncol. 2020, 11, 135. [Google Scholar]
- WHO/ICO. Human Papillomavirus and Related Cancers in World; Information Centre on HPV and Cervical Cancer: Geneva, Switzerland, 2010. [Google Scholar]
- Waggoner, S.E. Cervical cancer. Lancet 2003, 361, 2217–2225. [Google Scholar] [CrossRef]
- Landoni, F.; Maneo, A.; Cormio, G.; Perego, P.; Milani, R.; Caruso, O.; Mangioni, C. Class II versus Class III Radical Hysterectomy in Stage IB–IIA Cervical Cancer: A Prospective Randomized Study. Gynecol. Oncol. 2001, 80, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Dursun, P.; Gultekin, M.; Ayhan, A. The History of Radical Hysterectomy. J. Low. Genit. Tract Dis. 2011, 15, 235–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landoni, F.; Maneo, A.; Colombo, A.; Placa, F.; Milani, R.; Perego, P.; Favini, G.; Ferri, L.; Mangioni, C. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997, 350, 535–540. [Google Scholar] [CrossRef]
- Stapley, S.; Hamilton, W. Gynaecological symptoms reported by young women: Examining the potential for earlier diagnosis of cervical cancer. Fam. Pract. 2011, 28, 592–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, A.W.; Ramirez, A.J.; Hamilton, W.; Sasieni, P.; Patnick, J.; Forbes, L.J. Delays in diagnosis of young females with symptomatic cervical cancer in England: An interview-based study. Br. J. Gen. Pract. 2014, 64, e602–e610. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Cho, T.; Mogami, T.; Yokota, N.R.; Matsunaga, T.; Asai-Sato, M.; Hirahara, F.; Nojima, M.; Mori, M.; Miyagi, E. Evaluation of endocervical curettage with conization in diagnosis of endocervical lesions. J. Obstet. Gynaecol. Res. 2017, 43, 723–728. [Google Scholar] [CrossRef]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, C.S. World Health Organisation Classification of Tumours of the Female Reproduc-Tive Organs; International Agency for Research on Cancer: Lyon, France, 2014. [Google Scholar]
- Adegoke, O.; Kulasingam, S.; Virnig, B. Cervical Cancer Trends in the United States: A 35-Year Population-Based Analysis. J. Women’s Health 2012, 21, 1031–1037. [Google Scholar] [CrossRef]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynecol. Obstet. 2018, 143, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Olawaiye, A.B.; Baker, T.P.; Washington, M.K.; Mutch, D.G. The new (Version 9) American Joint Committee on Cancer tumor, node, metastasis staging for cervical cancer. CA A Cancer J. Clin. 2021, 71, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Hricak, H.; Gatsonis, C.; Chi, D.S.; Amendola, M.A.; Brandt, K.; Schwartz, L.H.; Koelliker, S.; Siegelman, E.S.; Brown, J.J.; McGhee, R.B., Jr.; et al. Role of Imaging in Pretreatment Evaluation of Early Invasive Cervical Cancer: Results of the Intergroup Study American College of Radiology Imaging Network 6651–Gynecologic Oncology Group 183. J. Clin. Oncol. 2005, 23, 9329–9337. [Google Scholar] [CrossRef] [PubMed]
- Kodama, J.; Mizutani, Y.; Hongo, A.; Yoshinouchi, M.; Kudo, T.; Okuda, H. Optimal surgery and diagnostic approach of stage IA2 squamous cell carcinoma of the cervix. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 101, 192–195. [Google Scholar] [CrossRef]
- Selman, T.J.; Mann, C.; Zamora, J.; Appleyard, T.-L.; Khan, K. Diagnostic accuracy of tests for lymph node status in primary cervical cancer: A systematic review and meta-analysis. Can. Med. Assoc. J. 2008, 178, 855–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vistad, I.; Fosså, S.D.; Dahl, A.A. A critical review of patient-rated quality of life studies of long-term survivors of cervical cancer. Gynecol. Oncol. 2006, 102, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Elghamrawi, K.A.; Haggag, M.H.; Habib, E.E. Treatment complications among long-term survivors of cervical cancer: Treat-ed by surgery or radiotherapy. Oncol. Rev. 2011, 5, 261–266. [Google Scholar] [CrossRef]
- Kaneyasu, Y.; Fujiwara, H.; Nishimura, T.; Sakurai, H.; Kazumoto, T.; Ikushima, H.; Uno, T.; Tokumaru, S.; Harima, Y.; Gomi, H.; et al. A multi-institutional survey of the quality of life after treatment for uterine cervical cancer: A comparison between radical radiotherapy and surgery in Japan. J. Radiat. Res. 2021, 62, 269–284. [Google Scholar] [CrossRef]
- Plante, M. Evolution in Fertility-Preserving Options for Early-Stage Cervical cancer: Radical trachelectomy, simple trachelectomy, neoadjuvant chemotherapy. Int. J. Gynecol. Cancer 2013, 23, 982–989. [Google Scholar] [CrossRef]
- Peters, W.A., III; Liu, P.Y.; Barrett, R.J., II; Stock, R.J.; Monk, B.J.; Berek, J.S.; Souhami, L.; Grigsby, P.; Gordon, W., Jr.; Alberts, D.S. Concurrent Chemotherapy and Pelvic Radiation Therapy Compared With Pelvic Radiation Therapy Alone as Adjuvant Therapy After Radical Surgery in High-Risk Early-Stage Cancer of the Cervix. J. Clin. Oncol. 2000, 18, 1606–1613. [Google Scholar] [CrossRef]
- Sedlis, A.; Bundy, B.N.; Rotman, M.Z.; Lentz, S.S.; Muderspach, L.I.; Zaino, R.J. A Randomized Trial of Pelvic Radiation Therapy versus No Further Therapy in Selected Patients with Stage IB Carcinoma of the Cervix after Radical Hysterectomy and Pelvic Lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol. Oncol. 1999, 73, 177–183. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Quinn, M.A.; Benedet, J.L.; Odicino, F.; Maisonneuve, P.; Beller, U.; Creasman, W.T.; Heintz, A.P.M.; Ngan, H.Y.S.; Pecorelli, S. Carcinoma of the Cervix Uteri. Int. J. Gynecol. Obstet. 2006, 95, S43–S103. [Google Scholar] [CrossRef]
- Copeland, L.J.; Silva, E.G.; Gershenson, D.M.; Morris, M.; Young, D.C.; Wharton, J. Superficially invasive squamous cell carcinoma of the cervix. Gynecol. Oncol. 1992, 45, 307–312. [Google Scholar] [CrossRef]
- Bekkers, R.L.M.; Keyser, K.G.G.; Bulten, J.; Hanselaar, A.G.J.M.; Schijf, C.P.T.; Boonstra, H.; Massuger, L.F.A.G. The value of loop electrosurgical conization in the treatment of stage IA1 microinvasive carcinoma of the uterine cervix. Int. J. Gynecol. Cancer 2002, 12, 485–489. [Google Scholar] [CrossRef]
- Magrina, J.F.; Goodrich, M.A.; Weaver, A.L.; Podratz, K.C. Modified Radical Hysterectomy: Morbidity and Mortality. Gynecol. Oncol. 1995, 59, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Schmeler, K.M.; Frumovitz, M.; Ramirez, P.T. Conservative management of early stage cervical cancer: Is there a role for less radical surgery? Gynecol. Oncol. 2011, 120, 321–325. [Google Scholar] [CrossRef] [Green Version]
- Frumovitz, M.; Sun, C.C.; Schmeler, K.M.; Deavers, M.T.; dos Reis, R.; Levenback, C.F.; Ramirez, P.T. Parametrial Involvement in Radical Hysterectomy Specimens for Women With Early-Stage Cervical Cancer. Obstet. Gynecol. 2009, 114, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Schmeler, K.M.; Pareja, R.; Blanco, A.L.; Fregnani, J.H.; Lopes, A.; Perrotta, M.; Tsunoda, A.T.; Cantú-De-León, D.F.; Ramondetta, L.M.; Manchana, T.; et al. ConCerv: A prospective trial of conservative surgery for low-risk early-stage cervical cancer. Int. J. Gynecol. Cancer 2021, 31, 1317–1325. [Google Scholar] [CrossRef]
- Sevin, B.-U.; Lu, Y.; Bloch, D.A.; Nadji, M.; Koechli, O.R.; Averette, H.E. Surgically defined prognostic parameters in patients with early cervical carcinoma: A multivariate survival tree analysis. Cancer 1996, 78, 1438–1446. [Google Scholar] [CrossRef]
- Sironi, S.; Buda, A.; Picchio, M.; Perego, P.; Moreni, R.; Pellegrino, A.; Colombo, M.; Mangioni, C.; Messa, C.; Fazio, F. Lymph Node Metastasis in Patients with Clinical Early-Stage Cervical Cancer: Detection with Integrated FDG PET/CT. Radiology 2006, 238, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Lukas, R.; Helena, R.; Jiri, H.M.; Martin, H.; Petr, S. Current status of sentinel lymph node mapping in the management of cervical cancer. Expert Rev. Anticancer Ther. 2013, 13, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Atri, M.; Zhang, Z.; Dehdashti, F.; Lee, S.I.; Ali, S.; Marques, H.; Koh, W.-J.; Moore, K.; Landrum, L.; Kim, J.-W.; et al. Utility of PET-CT to evaluate retroperitoneal lymph node metastasis in advanced cervical cancer: Results of ACRIN6671/GOG0233 trial. Gynecol. Oncol. 2016, 142, 413–419. [Google Scholar] [CrossRef] [Green Version]
- Hauspy, J.; Beiner, M.; Harley, I.; Ehrlich, L.; Rasty, G.; Covens, A. Sentinel lymph nodes in early stage cervical cancer. Gynecol. Oncol. 2007, 105, 285–290. [Google Scholar] [CrossRef]
- Balaya, V.; Guani, B.; Pache, B.; Durand, Y.-G.; Bonsang-Kitzis, H.; Ngô, C.; Mathevet, P.; Lécuru, F. Sentinel lymph node in cervical cancer: Time to move forward. Chin. Clin. Oncol. 2021, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Levenback, C.; Coleman, R.L.; Burke, T.W.; Lin, W.M.; Erdman, W.; Deavers, M.; Delpassand, E.S. Lymphatic Mapping and Sentinel Node Identification in Patients With Cervix Cancer Undergoing Radical Hysterectomy and Pelvic Lymphadenectomy. J. Clin. Oncol. 2002, 20, 688–693. [Google Scholar] [CrossRef]
- Bats, A.-S.; Buénerd, A.; Querleu, D.; Leblanc, E.; Daraï, E.; Morice, P.; Marret, H.; Gillaizeau, F.; Mathevet, P.; Lécuru, F. Diagnostic value of intraoperative examination of sentinel lymph node in early cervical cancer: A prospective, multicenter study. Gynecol. Oncol. 2011, 123, 230–235. [Google Scholar] [CrossRef]
- Slama, J.; Dundr, P.; Dusek, L.; Cibula, D. High false negative rate of frozen section examination of sentinel lymph nodes in patients with cervical cancer. Gynecol. Oncol. 2013, 129, 384–388. [Google Scholar] [CrossRef]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Meder, C.H.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients with Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 641–655. [Google Scholar] [CrossRef]
- Tax, C.; Rovers, M.; de Graaf, C.; Zusterzeel, P.L.; Bekkers, R.L. The sentinel node procedure in early stage cervical cancer, taking the next step; a diagnostic review. Gynecol. Oncol. 2015, 139, 559–567. [Google Scholar] [CrossRef]
- Delomenie, M.; Bonsang-Kitzis, H.; Bats, A.-S.; Ngo, C.; Balaya, V.; Xuan, H.T.N.; Koual, M.; Mathevet, P.; Lecuru, F. The clinical implication of lymph nodes micrometastases and isolated tumor cells in patients with cervical cancer: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 241, 71–76. [Google Scholar] [CrossRef]
- Cibula, D.; Abu-Rustum, N.; Dušek, L.; Zikan, M.; Zaal, A.; Sevcik, L.; Kenter, G.; Querleu, D.; Jach, R.; Bats, A.-S.; et al. Prognostic significance of low volume sentinel lymph node disease in early-stage cervical cancer. Gynecol. Oncol. 2012, 124, 496–501. [Google Scholar] [CrossRef]
- Cibula, D.; McCluggage, W.G. Sentinel lymph node (SLN) concept in cervical cancer: Current limitations and unanswered questions. Gynecol. Oncol. 2018, 152, 202–207. [Google Scholar] [CrossRef]
- Marchiolè, P.; Buénerd, A.; Benchaib, M.; Nezhat, K.; Dargent, D.; Mathevet, P. Clinical significance of lympho vascular space involvement and lymph node micrometastases in early-stage cervical cancer: A retrospective case-control surgico-pathological study. Gynecol. Oncol. 2005, 97, 727–732. [Google Scholar] [CrossRef]
- Zaal, A.; Zweemer, R.P.; Zikan, M.; Dušek, L.; Querleu, D.; Lécuru, F.; Bats, A.-S.; Jach, R.; Sevcik, L.; Graf, P.; et al. Pelvic Lymphadenectomy Improves Survival in Patients with Cervical Cancer With Low-Volume Disease in the Sentinel Node: A Retrospective Multicenter Cohort Study. Int. J. Gynecol. Cancer 2014, 24, 303–311. [Google Scholar] [CrossRef]
- Cibula, D.; Zikan, M.; Slama, J.; Fischerova, D.; Kocian, R.; Germanova, A.; Burgetova, A.; Dusek, L.; Dundr, P.; Gregova, M.; et al. Risk of micrometastases in non-sentinel pelvic lymph nodes in cervical cancer. Gynecol. Oncol. 2016, 143, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Guani, B.; Dorez, M.; Magaud, L.; Buenerd, A.; Lecuru, F.; Mathevet, P. Impact of micrometastasis or isolated tumor cells on recurrence and survival in patients with early cervical cancer: SENTICOL Trial. Int. J. Gynecol. Cancer 2019, 29, 447–452. [Google Scholar] [CrossRef]
- Dostalek, L.; Åvall-Lundqvist, E.; Creutzberg, C.L.; Kurdiani, D.; Ponce, J.; Dostalkova, I.; Cibula, D. ESGO Survey on Current Practice in the Management of Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 1226–1231. [Google Scholar] [CrossRef]
- SEER Data: Surveillance, Epidemiology and End Results. Available online: https://seer.cancer.gov/statfacts/html/cervix.html (accessed on 18 October 2021).
- Dargent, D.; Martin, X.; Sacchetoni, A.; Mathevet, P. Laparoscopic vaginal radical trachelectomy: A treatment to preserve the fertility of cervical carcinoma patients. Cancer 2000, 88, 1877–1882. [Google Scholar] [CrossRef]
- Plante, M.; Gregoire, J.; Renaud, M.-C.; Roy, M. The vaginal radical trachelectomy: An update of a series of 125 cases and 106 pregnancies. Gynecol. Oncol. 2011, 121, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.P.; Sonoda, Y.; Leitao, M.M.; Zivanovic, O.; Brown, C.L.; Chi, D.S.; Barakat, R.R.; Abu-Rustum, N.R. Oncologic outcome of fertility-sparing radical trachelectomy versus radical hysterectomy for stage IB1 cervical carcinoma. Gynecol. Oncol. 2008, 111, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Boss, E.; van Golde, R.; Beerendonk, C.; Massuger, L. Pregnancy after radical trachelectomy: A real option? Gynecol. Oncol. 2005, 99, S152–S156. [Google Scholar] [CrossRef]
- Jolley, J.; Battista, L.; Wing, D. Management of Pregnancy after Radical Trachelectomy: Case Reports and Systematic Review of the Literature. Am. J. Perinatol. 2007, 24, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Salvo, G.; Ramirez, P.T.; Leitao, M.M.; Cibula, D.; Wu, X.; Falconer, H.; Persson, J.; Perrotta, M.; Mosgaard, B.J.; Kucukmetin, A.; et al. Open vs minimally invasive radical trachelectomy in early-stage cervical cancer: International Radical Trachelectomy Assessment Study. Am. J. Obstet. Gynecol. 2021. [Google Scholar] [CrossRef] [PubMed]
- de Vincenzo, R.; Ricci, C.; Fanfani, F.; Gui, B.; Gallotta, V.; Fagotti, A.; Ferrandina, G.; Scambia, G. Neoadjuvant chemotherapy followed by conization in stage IB2–IIA1 cervical cancer larger than 2 cm: A pilot study. Fertil. Steril. 2020, 115, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Kim, D.-Y.; Kim, J.-H.; Kim, Y.-M.; Nam, J.-H. Outcomes after radical hysterectomy according to tumor size divided by 2-cm interval in patients with early cervical cancer. Ann. Oncol. 2010, 22, 59–67. [Google Scholar] [CrossRef]
- Rotman, M.; Sedlis, A.; Piedmonte, M.R.; Bundy, B.; Lentz, S.S.; Muderspach, L.I.; Zaino, R.J. A phase III randomized trial of postoperative pelvic irradiation in stage IB cervical carcinoma with poor prognostic features: Follow-up of a gynecologic oncology group study. Int. J. Radiat. Oncol. 2006, 65, 169–176. [Google Scholar] [CrossRef]
- Monk, B.J.; Wang, J.; Im, S.; Stock, R.J.; Peters, W.A.; Liu, P.; Barrett, R.J.; Berek, J.S.; Souhami, L.; Grigsby, P.W.; et al. Rethinking the use of radiation and chemotherapy after radical hysterectomy: A clinical–pathologic analysis of a Gynecologic Oncology Group/Southwest Oncology Group/Radiation Therapy Oncology Group trial. Gynecol. Oncol. 2005, 96, 721–728. [Google Scholar] [CrossRef]
- Okazawa, M.; Mabuchi, S.; Isohashi, F.; Suzuki, O.; Yoshioka, Y.; Sasano, T.; Ohta, Y.; Kamiura, S.; Ogawa, K.; Kimura, T. Impact of the Addition of Concurrent Chemotherapy to Pelvic Radiotherapy in Surgically Treated Stage IB1-IIB Cervical Cancer Patients with Intermediate-Risk or High-Risk Factors: A 13-Year Experience. Int. J. Gynecol. Cancer 2013, 23, 567–575. [Google Scholar] [CrossRef]
- Rogers, L.; Siu, S.S.N.; Luesley, D.; Bryant, A.; Dickinson, H.O. Radiotherapy and chemoradiation after surgery for early cervical cancer. Cochrane Database Syst. Rev. 2012, 5, CD007583. [Google Scholar] [CrossRef] [Green Version]
- Gallotta, V.; Conte, C.; Federico, A.; Vizzielli, G.; Alletti, S.G.; Tortorella, L.; Anchora, L.P.; Cosentino, F.; Chiantera, V.; Fagotti, A.; et al. Robotic versus laparoscopic radical hysterectomy in early cervical cancer: A case matched control study. Eur. J. Surg. Oncol. (EJSO) 2018, 44, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, N.; Anchora, L.P.; Zannoni, G.F.; Carbone, V.; Bruno, M.; Fedele, C.; Gallotta, V.; Chiantera, V.; Avesani, G.; Gui, B.; et al. Validation of tumour-free distance as novel prognostic marker in early-stage cervical cancer: A retrospective, single-centre, cohort study. Br. J. Cancer 2021, 125, 561–568. [Google Scholar] [CrossRef]
- Nezhat, C.R.; Burrell, M.O.; Nezhat, F.R.; Benigno, B.B.; Welander, C.E. Laparoscopic radical hysterectomy with paraaortic and pelvic node dissection. Am. J. Obstet. Gynecol. 1992, 166, 864–865. [Google Scholar] [CrossRef]
- Frumovitz, M.; Dos Reis, R.; Sun, C.C.; Milam, M.R.; Bevers, M.W.; Brown, J.; Slomovitz, B.M.; Ramirez, P.T. Comparison of Total Laparoscopic and Abdominal Radical Hysterectomy for Patients with Early-Stage Cervical Cancer. Obstet. Gynecol. 2007, 110, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Gioiella, M.E.; Berkman, B.; Robinson, M. Spirituality and quality of life in gynecologic oncology patients. Cancer Pract. 1998, 6, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Kooby, D.A. Laparoscopic surgery for cancer: Historical, theoretical, and technical considerations. Oncology 2006, 20, 917–927. [Google Scholar]
- Zeng, Y.C.; Ching, S.S.; Loke, A.Y. Quality of life measurement in women with cervical cancer: Implications for Chinese cervical cancer survivors. Health Qual. Life Outcomes 2010, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Obermair, A.; Asher, R.; Pareja, R.; Frumovitz, M.; Lopez, A.; Marques, R.M.; Rendon, G.; Ribeiro, R.; Tsunoda, A.; Behan, V.; et al. Incidence of adverse events in minimally invasive vs open radical hysterectomy in early cervical cancer: Results of a randomized controlled trial. Am. J. Obstet. Gynecol. 2019, 222, 249.e1–249.e10. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Obermair, A.; Coleman, R.L.; Pareja, R.; Lopez, A.; Ribero, R.; Isla, D.; Rendon, G.; Bernardini, M.Q.; Buda, A.; et al. Quality of life in patients with cervical cancer after open versus minimally invasive radical hysterectomy (LACC): A secondary outcome of a multicentre, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2020, 21, 851–860. [Google Scholar] [CrossRef]
- Kim, S.I.; Cho, J.H.; Seol, A.; Kim, Y.I.; Lee, M.; Kim, H.S.; Chung, H.H.; Kim, J.-W.; Park, N.H.; Song, Y.-S. Comparison of survival outcomes between minimally invasive surgery and conventional open surgery for radical hysterectomy as primary treatment in patients with stage IB1–IIA2 cervical cancer. Gynecol. Oncol. 2019, 153, 3–12. [Google Scholar] [CrossRef]
- Paik, E.S.; Lim, M.C.; Kim, M.-H.; Kim, Y.H.; Song, E.S.; Seong, S.J.; Suh, D.H.; Lee, J.-M.; Lee, C.; Choi, C.H. Prognostic Model for Survival and Recurrence in Patients with Early-Stage Cervical Cancer: A Korean Gynecologic Oncology Group Study (KGOG 1028). Cancer Res. Treat. 2020, 52, 320–333. [Google Scholar] [CrossRef]
- Doo, D.W.; Kirkland, C.T.; Griswold, L.H.; McGwin, G.; Huh, W.K.; Leath, C.A.; Kim, K.H. Comparative outcomes between robotic and abdominal radical hysterectomy for IB1 cervical cancer: Results from a single high volume institution. Gynecol. Oncol. 2019, 153, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Odetto, D.; Puga, M.C.; Saadi, J.; Noll, F.; Perrotta, M. Minimally invasive radical hysterectomy: An analysis of oncologic outcomes from Hospital Italiano (Argentina). Int. J. Gynecol. Cancer 2019, 29, 863–868. [Google Scholar] [CrossRef]
- Cusimano, M.C.; Baxter, N.N.; Gien, L.T.; Moineddin, R.; Liu, N.; Dossa, F.; Willows, K.; Ferguson, S.E. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am. J. Obstet. Gynecol. 2019, 221, 619.e1–619.e24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melamed, A.; Margul, D.J.; Chen, L.; Keating, N.L.; Del Carmen, M.G.; Yang, J.; Seagle, B.-L.L.; Alexander, A.; Barber, E.L.; Rice, L.W.; et al. Survival after Minimally Invasive Radical Hysterectomy for Early-Stage Cervical Cancer. N. Engl. J. Med. 2018, 379, 1905–1914. [Google Scholar] [CrossRef]
- Köhler, C.; Hertel, H.; Herrmann, J.; Marnitz, S.; Mallmann, P.; Favero, G.; Plaikner, A.; Martus, P.; Gajda, M.; Schneider, A. Laparoscopic radical hysterectomy with transvaginal closure of vaginal cuff—A multicenter analysis. Int. J. Gynecol. Cancer 2019, 29, 845–850. [Google Scholar] [CrossRef]
- Chiva, L.; Zanagnolo, V.; Querleu, D.; Martin-Calvo, N.; Arévalo-Serrano, J.; Căpîlna, M.E.; Fagotti, A.; Kucukmetin, A.; Mom, C.; Chakalova, G.; et al. SUCCOR study: An international European cohort observational study comparing minimally invasive surgery versus open abdominal radical hysterectomy in patients with stage IB1 cervical cancer. Int. J. Gynecol. Cancer 2020, 30, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Casarin, J.; Bogani, G.; Papadia, A.; Ditto, A.; Pinelli, C.; Garzon, S.; Donadello, N.; Laganà, A.S.; Cromi, A.; Mueller, M.; et al. Preoperative Conization and Risk of Recurrence in Patients Undergoing Laparoscopic Radical Hysterectomy for Early Stage Cervical Cancer: A Multicenter Study. J. Minim. Invasive Gynecol. 2020, 28, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, N.; Anchora, L.P.; Kucukmetin, A.; Ratnavelu, N.; Korompelis, P.; Carbone, V.; Fedele, C.; Bruno, M.; Vizzielli, G.; Gallotta, V.; et al. Protective Role of Conization before Radical Hysterectomy in Early-Stage Cervical Cancer: A Propensity-Score Matching Study. Ann. Surg. Oncol. 2021, 28, 3585–3594. [Google Scholar] [CrossRef]
- Charo, L.M.; Vaida, F.; Eskander, R.N.; Binder, P.; Saenz, C.; McHale, M.; Plaxe, S. Rapid dissemination of practice-changing information: A longitudinal analysis of real-world rates of minimally invasive radical hysterectomy before and after presentation of the LACC trial. Gynecol. Oncol. 2020, 157, 494–499. [Google Scholar] [CrossRef]
- Anchora, L.P.; Turco, L.C.; Bizzarri, N.; Capozzi, V.A.; Lombisani, A.; Chiantera, V.; De Felice, F.; Gallotta, V.; Cosentino, F.; Fagotti, A.; et al. How to Select Early-Stage Cervical Cancer Patients Still Suitable for Laparoscopic Radical Hysterectomy: A Propensity-Matched Study. Ann. Surg. Oncol. 2020, 27, 1947–1955. [Google Scholar] [CrossRef]
- Nam, J.-H.; Park, J.-Y.; Kim, D.-Y.; Kim, J.-H.; Kim, Y.-M. Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: Long-term survival outcomes in a matched cohort study. Ann. Oncol. 2011, 23, 903–911. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, M.; Lee, S.; Suh, D.H.; Kim, H.S.; Kim, K.; Chung, H.H.; No, J.H.; Kim, J.-W.; Park, N.H.; et al. Impact of laparoscopic radical hysterectomy on survival outcome in patients with FIGO stage IB cervical cancer: A matching study of two institutional hospitals in Korea. Gynecol. Oncol. 2019, 155, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Mead-Harvey, C.; Polen-De, C.; Magtibay, P.; Butler, K.; Cliby, W.; Langstraat, C.; Dinh, T.; Chen, L.; Magrina, J. Survival outcomes in patients with cervical cancer treated with open versus robotic radical hysterectomy: Our surgical pathology interrogation. Gynecol. Oncol. 2020, 159, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Rauh-Hain, J.A.; Saenz, J.; Isla, D.O.; Pereira, G.J.R.; Odetto, D.; Martinelli, F.; Villoslada, V.; Zapardiel, I.; Trujillo, L.M.; et al. Oncological outcomes of laparoscopic radical hysterectomy versus radical abdominal hysterectomy in patients with early-stage cervical cancer: A multicenter analysis. Int. J. Gynecol. Cancer 2021, 31, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Uppal, S.; Gehrig, P.A.; Peng, K.; Bixel, K.L.; Matsuo, K.; Vetter, M.H.; Davidson, B.A.; Cisa, M.P.; Lees, B.F.; Brunette, L.L.; et al. Recurrence Rates in Patients With Cervical Cancer Treated With Abdominal Versus Minimally Invasive Radical Hysterectomy: A Multi-Institutional Retrospective Review Study. J. Clin. Oncol. 2020, 38, 1030–1040. [Google Scholar] [CrossRef]
- Chen, C.; Liu, P.; Ni, Y.; Tang, L.; Xu, Y.; Bin, X.; Lang, J. Laparoscopic versus abdominal radical hysterectomy for stage IB1 cervical cancer patients with tumor size ≤ 2 cm: A case-matched control study. Int. J. Clin. Oncol. 2020, 25, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Paik, E.S.; Lim, M.C.; Kim, M.-H.; Kim, Y.H.; Song, E.S.; Seong, S.J.; Suh, D.H.; Lee, J.-M.; Lee, C.; Choi, C.H. Comparison of laparoscopic and abdominal radical hysterectomy in early stage cervical cancer patients without adjuvant treatment: Ancillary analysis of a Korean Gynecologic Oncology Group Study (KGOG 1028). Gynecol. Oncol. 2019, 154, 547–553. [Google Scholar] [CrossRef]
- Nasioudis, D.; Albright, B.B.; Haggerty, A.F.; Ko, E.M.; Kim, S.H.; Morgan, M.A.; Latif, N.A. Survival following minimally invasive radical hysterectomy for patients with cervical carcinoma and tumor size ≤ 2 cm. Am. J. Obstet. Gynecol. 2021, 224, 317–318. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, N.; Ye, P.; Chen, J.; Nan, X.; Zhao, H.; Zhou, K.; Zhang, Y.; Xue, J.; Zhou, H.; et al. Comparison of laparoscopic and open radical hysterectomy in cervical cancer patients with tumor size ≤ 2 cm. Int. J. Gynecol. Cancer 2020, 30, 564–571. [Google Scholar] [CrossRef] [Green Version]
- Nasioudis, D.; Albright, B.B.; Ko, E.M.; Haggerty, A.F.; Ii, R.L.G.; Kim, S.H.; Morgan, M.A.; Latif, N.A. Oncologic outcomes of minimally invasive versus open radical hysterectomy for early stage cervical carcinoma and tumor size <2 cm: A systematic review and meta-analysis. Int. J. Gynecol. Cancer 2021, 31, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Sevin, B.U.; Nadji, M.; Lampe, B.; Lu, Y.; Hilsenbeck, S.; Koechli, O.R.; Averette, H.E. Prognostic factors of early stage cervi-cal cancer treated by radical hysterectomy. Cancer 1995, 76, 1978–1986. [Google Scholar] [CrossRef]
- Wright, J.D.; Matsuo, K.; Huang, Y.; Tergas, A.I.; Hou, J.Y.; Khoury-Collado, F.; Clair, C.M.S.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L. Prognostic Performance of the 2018 International Federation of Gynecology and Obstetrics Cervical Cancer Staging Guidelines. Obstet. Gynecol. 2019, 134, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.E.; Pappas, L.; Ghia, A.J.; Gaffney, D.K. Impact of tumor size on survival in cancer of the cervix and validation of stage IIA1 and IIA2 subdivisions. Gynecol. Oncol. 2013, 129, 517–521. [Google Scholar] [CrossRef]
- Rutledge, T.L.; Kamelle, S.A.; Tillmanns, T.D.; Gould, N.S.; Wright, J.D.; Cohn, D.; Herzog, T.J.; Rader, J.S.; Gold, M.A.; Johnson, G.A.; et al. A comparison of stages IB1 and IB2 cervical cancers treated with radical hysterectomy. Is size the real difference? Gynecol. Oncol. 2004, 95, 70–76. [Google Scholar] [CrossRef]
- Creasman, W.T.; Kohler, M.F. Is lymph vascular space involvement an independent prognostic factor in early cervical cancer? Gynecol. Oncol. 2004, 92, 525–529. [Google Scholar] [CrossRef] [PubMed]

| Stage | Description |
|---|---|
| I | The carcinoma is strictly confined to the cervix (extension to the uterine corpus should be disregarded) |
| IA | Invasive carcinoma that can be diagnosed only by microscopy, with maximum depth of invasion ≤ 5 mm a |
| IA1 | Measured stromal invasion ≤ 3 mm in depth |
| IA2 | Measured stromal invasion > 3 mm and ≤5 mm in depth |
| IB | Invasive carcinoma with measured deepest invasion > 5 mm (greater than Stage IA); lesion limited to the cervix uteri with size measure by maximum tumor diameter b |
| IB1 | Invasive carcinoma > 5 mm depth of stromal invasion, and ≤2 cm in greatest dimension |
| IB2 | Invasive carcinoma > 2 cm and ≤4 cm in greatest dimension |
| IB3 | Invasive carcinoma > 4 cm in greatest dimension |
| II | The cervical carcinoma has invaded beyond the uterus, but has not extended onto the lower third of the vagina or to the pelvic wall |
| IIA | Involvement limited to the upper two-thirds of the vagina without parametrial invasion |
| IIA1 | Invasive carcinoma ≤ 4 cm in greatest dimension |
| IIA2 | Invasive carcinoma > 4 cm in greatest dimension |
| IIB | With parametrial invasion but not up to the pelvic wall |
| III | The carcinoma involves the lower third of the vagina and/or extends to the pelvic wall and/or causes hydronephrosis or non-functioning kidney and/or involves pelvic and/or paraaortic lymph nodes c |
| IIIA | Carcinoma involves lower third of the vagina, with no extension to the pelvic wall |
| IIIB | Extension to the pelvic wall and/or hydronephrosis or non-functioning kidney (unless known to be due to another cause) |
| IIIC | Involvement of pelvic and/or paraaortic lymph nodes (including micrometastasis) c, irrespective of tumor size and extent (with r and p notations) d |
| IIIC1 | Pelvic lymph node metastasis only |
| IIIC2 | Paraaortic lymph node metastasis |
| IV | The carcinoma has extended beyond the true pelvis or has involved (biopsy proven) the mucosa of the bladder or rectum. A bullous edema, as such, does not permit a case to be allotted to Stage IV |
| IVA | Spread of the growth to adjacent organs |
| IVB | Spread to distant organs |
| Author | Year | N | Outcomes |
|---|---|---|---|
| Nam, et al. [95] | 2012 | 526 (335 < 2 cm) | No difference between open surgery (OP) and minimally invasive surgery (MIS) for oncologic outcomes |
| Paik, et al. [101] | 2019 | 476 (248 < 2 cm) | Difference observed: MIS was associated with a lower rate of disease-free survival (DFS) |
| Kim, et al. [96] | 2019 | 565 (283 < 2 cm) | No difference between open surgery (OP) and minimally invasive surgery (MIS) for oncologic outcomes |
| Pedone Anchora, et al. [94] | 2020 | 423 (251 < 2 cm) | No difference between open surgery (OP) and minimally invasive surgery (MIS) for oncologic outcomes |
| Chen, et al. [103] | 2020 | 325 | Difference observed: MIS was associated with worse 5-year disease-free survival |
| Yang, et al. [97] | 2020 | 333 (111 < 2 cm) | No difference between open surgery (OP) and minimally invasive surgery (MIS) for oncologic outcomes |
| Chiva, et al. [90] | 2020 | 693 (303 < 2 cm) | No difference between open surgery (OP) and minimally invasive surgery (MIS) for oncologic outcomes |
| Uppal, et al. [99] | 2020 | 815 (264 < 2 cm) | Difference observed: MIS was associated with increased risk of recurrence and inferior disease-free survival |
| Rodriguez, et al. [98] | 2021 | 1379 (979 < 2 cm) | No difference between open surgery (OP) and minimally invasive surgery (MIS) for oncologic outcomes |
| Nasioudis, et al. [102] | 2021 | 2046 | Difference observed: MIS was associated with worse overall survival (OS) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, Y.M.; Godoy, L.R.; Longatto-Filho, A.; Reis, R.d. Management of Early-Stage Cervical Cancer: A Literature Review. Cancers 2022, 14, 575. https://doi.org/10.3390/cancers14030575
Guimarães YM, Godoy LR, Longatto-Filho A, Reis Rd. Management of Early-Stage Cervical Cancer: A Literature Review. Cancers. 2022; 14(3):575. https://doi.org/10.3390/cancers14030575
Chicago/Turabian StyleGuimarães, Yasmin Medeiros, Luani Rezende Godoy, Adhemar Longatto-Filho, and Ricardo dos Reis. 2022. "Management of Early-Stage Cervical Cancer: A Literature Review" Cancers 14, no. 3: 575. https://doi.org/10.3390/cancers14030575
APA StyleGuimarães, Y. M., Godoy, L. R., Longatto-Filho, A., & Reis, R. d. (2022). Management of Early-Stage Cervical Cancer: A Literature Review. Cancers, 14(3), 575. https://doi.org/10.3390/cancers14030575






