High Dose Thoracic Re-Irradiation and Chemo-Immunotherapy for Centrally Recurrent NSCLC
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Literature Search
2.2. EQD2
2.3. Toxicity
2.4. Statistics
3. Results
3.1. Selected Studies
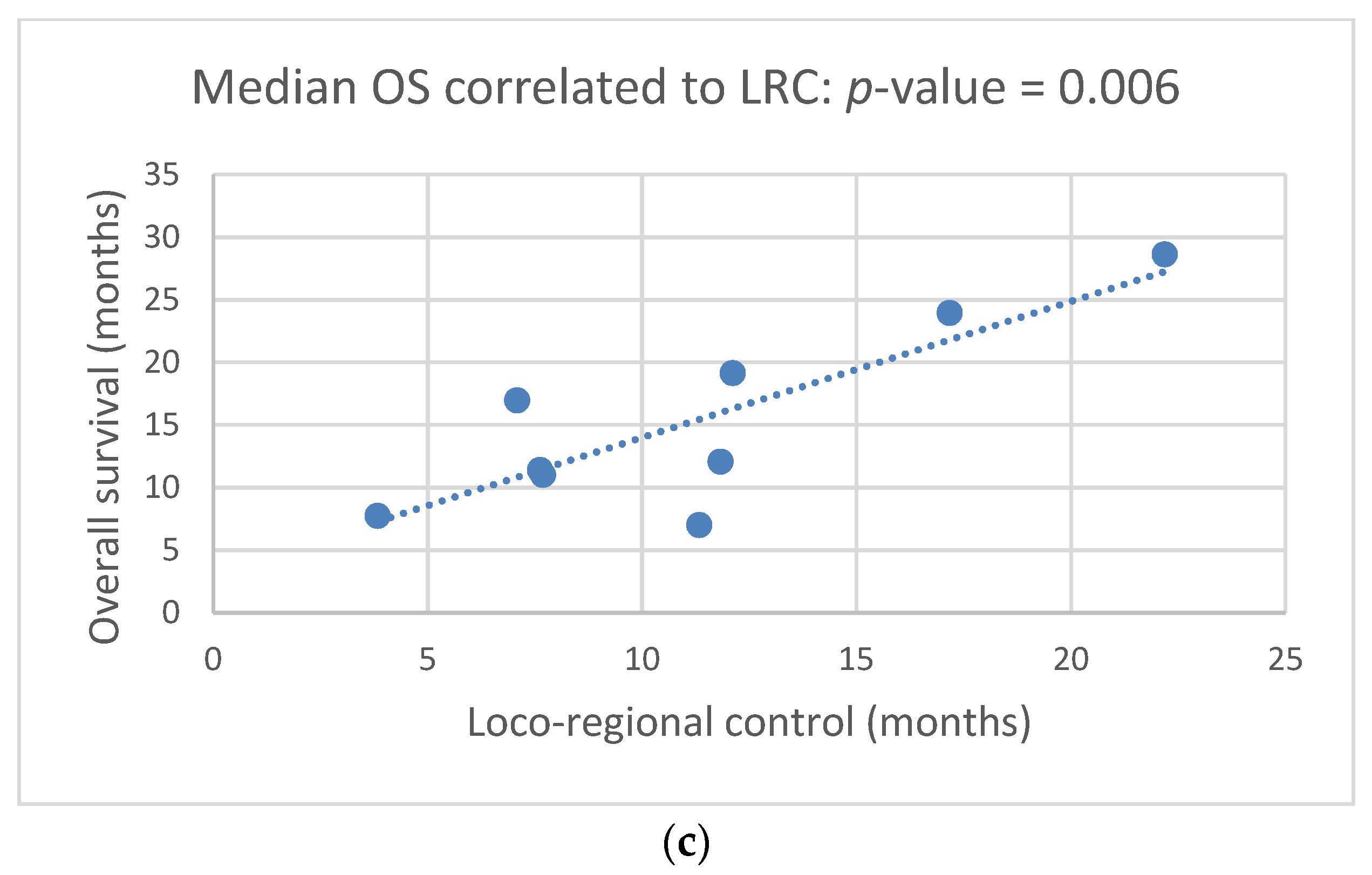
3.2. Clinical Outcome: mOS, mPFS, mLRC
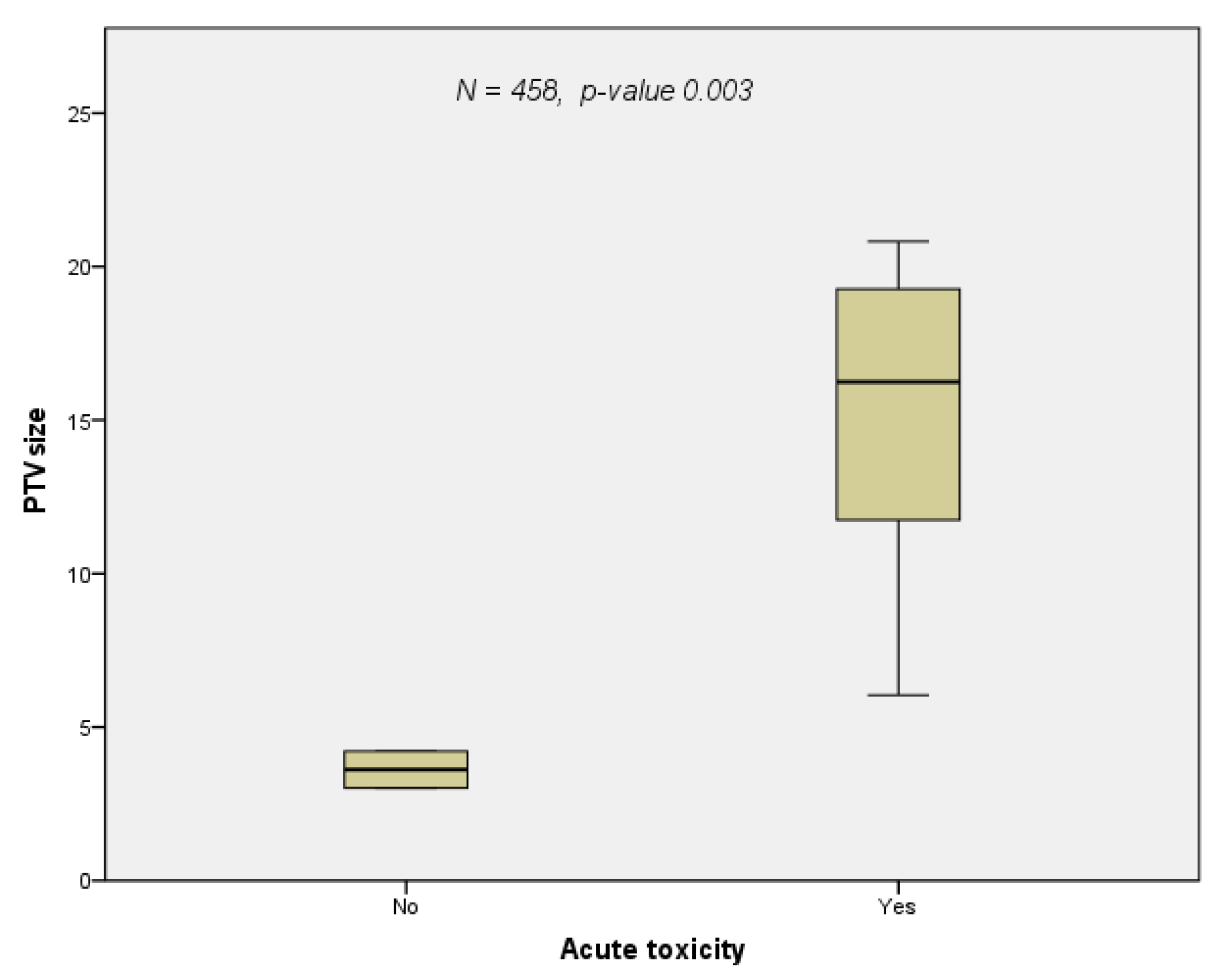
3.3. Toxicity
3.4. Prognostic Factors
3.5. Treatment
3.5.1. Re-Irradiation
3.5.2. Chemo-Immunotherapy at Re-Irradiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| cCRT | concomitant chemoradiotherapy |
| sCRT | sequential chemoradiotherapy |
| CT | chemotherapy |
| Dmax | Maximum dose |
| DSB | double strand break |
| EQD2 | biologically equivalent dose in 2 Gy fractions |
| FEV1 | forced expiratory volume in the first second |
| GI | genomic instability |
| GTV | gross tumor volume |
| HR | homologous recombination |
| ICI | immune checkpoint inhibitor |
| IGRT | image guided radiotherapy |
| IMRT | intensity modulated radiotherapy |
| IO | immune-oncological therapy |
| LA-NSCLC | locally advanced NSCLC |
| LC | local control |
| LRC | loco-regional control |
| MED | mean esophageal dose |
| MHD | mean heart dose |
| MLD | mean lung dose |
| NHEJ | non-homologous end-joining |
| NOS | not otherwise specified |
| NS | not stated |
| NSCLC | non-small cell lung cancer |
| NTCP | normal tissue complication probability |
| OAR | organs at risk |
| OS | overall survival |
| PD-1 | programmed cell death protein 1 |
| PFS | progression free survival |
| PRISMA | preferred reporting items for systematic reviews and meta-analyses |
| PTV | planning target volume |
| Re-RT | re-irradiation |
| RT | first course of radiotherapy |
| SABR | stereotactic ablative radiotherapy |
| SCLC | small cell lung cancer |
| TKI | tyrosine kinase inhibitor |
| VMAT | volumetric arc therapy |
References
- Green, N.; Melbye, R.W. Lung-Cancer-Retreatment of Local Recurrence after Definitive Irradiation. Cancer 1982, 49, 865–868. [Google Scholar] [CrossRef]
- Vansteenkiste, J.; De Ruysscher, D.; Eberhardt, W.E.; Lim, E.; Senan, S.; Felip, E.; Peters, S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. 6), vi89–vi98. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.H.; Berman, A.T.; Simone, C.B., 2nd; Ciunci, C.; Gabriel, P.; Lin, H.; Both, S.; Langer, C.; Lelionis, K.; Rengan, R.; et al. Multi-Institutional Prospective Study of Reirradiation with Proton Beam Radiotherapy for Locoregionally Recurrent Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, 281–292. [Google Scholar] [CrossRef] [Green Version]
- Curran, W.J.; Paulus, R.; Langer, C.J.; Komaki, R.; Lee, J.S.; Hauser, S.; Movsas, B.; Wasserman, T.; Rosenthal, S.A.; Gore, E.; et al. Sequential vs Concurrent Chemoradiation for Stage III Non-Small Cell Lung Cancer: Randomized Phase III Trial RTOG 9410. J. Natl. Cancer Inst. 2011, 103, 1452–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Ruysscher, D.; Faivre-Finn, C.; Le Pechoux, C.; Peeters, S.; Belderbos, J. High-dose re-irradiation following radical radiotherapy for non-small-cell lung cancer. Lancet Oncol. 2014, 15, e620–e624. [Google Scholar] [CrossRef]
- Faivre-Finn, C.; Vicente, D.; Kurata, T.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Spigel, D.R.; Garassino, M.C.; Reck, M.; Senan, S.; et al. Durvalumab after chemoradiotherapy in stage III NSCLC: 4-year survival update from the phase III PACIFIC trial. Ann. Oncol. 2020, 31, S1178–S1179. [Google Scholar] [CrossRef]
- de Wit, M.; Spigel, D.; Faivre-Finn, C.; Gray, J.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.; Garassino, M.; Hui, R.; et al. 5-Year Survival Data of Durvalumab after Chemoradiotherapy for unresectable Stage III NSCLC-an Update from the PACIFIC Study. Strahlenther. Und Onkol. 2021, 197 (Suppl. 1), S8–S9. [Google Scholar]
- Rulach, R.; Hanna, G.G.; Franks, K.; McAleese, J.; Harrow, S. Re-irradiation for Locally Recurrent Lung Cancer: Evidence, Risks and Benefits. Clin. Oncol. (R Coll. Radiol.) 2018, 30, 101–109. [Google Scholar] [CrossRef]
- Belderbos, J.; Walraven, I.; van Diessen, J.; Verheij, M.; de Ruysscher, D. Radiotherapy dose and fractionation for stage III NSCLC. Lancet Oncol. 2015, 16, e156–e157. [Google Scholar] [CrossRef] [Green Version]
- Timmerman, R.; McGarry, R.; Yiannoutsos, C.; Papiez, L.; Tudor, K.; DeLuca, J.; Ewing, M.; Abdulrahman, R.; DesRosiers, C.; Williams, M.; et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J. Clin. Oncol. 2006, 24, 4833–4839. [Google Scholar] [CrossRef]
- McAvoy, S.; Ciura, K.; Wei, C.; Rineer, J.; Liao, Z.; Chang, J.Y.; Palmer, M.B.; Cox, J.D.; Komaki, R.; Gomez, D.R. Definitive reirradiation for locoregionally recurrent non-small cell lung cancer with proton beam therapy or intensity modulated radiation therapy: Predictors of high-grade toxicity and survival outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Hunter, B.; Crockett, C.; Faivre-Finn, C.; Hiley, C.; Salem, A. Re-Irradiation of Recurrent Non-Small Cell Lung Cancer. Semin. Radiat. Oncol. 2021, 31, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, I.V.; Nikitaki, Z.; Souli, M.P.; Aziz, A.; Nowsheen, S.; Aziz, K.; Rogakou, E.; Georgakilas, A.G. Complex DNA Damage: A Route to Radiation-Induced Genomic Instability and Carcinogenesis. Cancers 2017, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Griffioen, G.H.M.J.; Dahele, M.; de Haan, P.F.; van de Ven, P.M.; Slotman, B.J.; Senan, S. High-dose, conventionally fractionated thoracic reirradiation for lung tumors. Lung Cancer 2014, 83, 356–362. [Google Scholar] [CrossRef]
- Tetar, S.; Dahele, M.; Griffioen, G.; Slotman, B.; Senan, S. High-dose conventional thoracic re-irradiation for lung cancer: Updated results. Lung Cancer 2015, 88, 235–236. [Google Scholar] [CrossRef]
- Pisibon, C.; Ouertani, A.; Bertolotto, C.; Ballotti, R.; Cheli, Y. Immune Checkpoints in Cancers: From Signaling to the Clinic. Cancers 2021, 13, 4573. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Q.; Xie, J.; Chen, M.; Liu, H.; Zhan, P.; Lv, T.; Song, Y. The Predictive Value of Clinical and Molecular Characteristics or Immunotherapy in Non-Small Cell Lung Cancer: A Meta-Analysis of Randomized Controlled Trials. Front. Oncol. 2021, 11, 732214. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef]
- Lin, P.Y.; Sun, L.; Thibodeaux, S.R.; Ludwig, S.M.; Vadlamudi, R.K.; Hurez, V.J.; Bahar, R.; Kious, M.J.; Livi, C.B.; Wall, S.R.; et al. B7-H1-dependent sex-related differences in tumor immunity and immunotherapy responses. J. Immunol. 2010, 185, 2747–2753. [Google Scholar] [CrossRef]
- Giordano, F.; Wenz, F. (Eds.) Strahlentherapie Kompakt; Elsevier: Munich, Germany, 2019; p. 35. [Google Scholar]
- Ward, M.; Tendulkar, R.D.; Videtic, G.M.M. (Eds.) Essentials of Clinical Radiation Oncology; Springer: New York, NY, USA, 2020; pp. 216–217. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Ohguri, T.; Imada, H.; Yahara, K.; Moon, S.D.; Yamaguchi, S.; Yatera, K.; Mukae, H.; Hanagiri, T.; Tanaka, F.; Korogi, Y. Re-irradiation plus regional hyperthermia for recurrent non-small cell lung cancer: A potential modality for inducing long-term survival in selected patients. Lung Cancer 2012, 77, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Grambozov, B.; Nussdorfer, E.; Kaiser, J.; Gerum, S.; Fastner, G.; Stana, M.; Gaisberger, C.; Wass, R.; Studnicka, M.; Sedlmayer, F.; et al. Re-Irradiation for Locally Recurrent Lung Cancer: A Single Center Retrospective Analysis. Curr. Oncol. 2021, 28, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, J.M.; Kuremsky, J.G.; Blackstock, A.W.; Munley, M.T.; Kearns, W.T.; Hinson, W.H.; Lovato, J.F.; Miller, A.A.; Petty, W.J.; Urbanic, J.J. Thoracic re-irradiation using stereotactic body radiotherapy (SBRT) techniques as first or second course of treatment. Radiother. Oncol. 2014, 110, 505–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.C.; Hsu, F.M.; Chen, Y.H.; Shih, J.Y.; Yu, C.J.; Lin, Z.Z.; Lu, S.H.; Yang, J.C.; Cheng, A.L.; Kuo, S.H. Clinical outcomes and toxicity predictors of thoracic re-irradiation for locoregionally recurrent lung cancer. Clin. Transl. Radiat. Oncol. 2020, 22, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Schlampp, I.; Rieber, J.; Adeberg, S.; Bozorgmehr, F.; Heussel, C.P.; Steins, M.; Kappes, J.; Hoffmann, H.; Welzel, T.; Debus, J.; et al. Re-irradiation in locally recurrent lung cancer patients. Strahlenther. Onkol. 2019, 195, 725–733. [Google Scholar] [CrossRef]
- Sumodhee, S.; Bondiau, P.Y.; Poudenx, M.; Cohen, C.; Naghavi, A.O.; Padovani, B.; Maneval, D.; Gal, J.; Leysalle, A.; Ghalloussi, H.; et al. Long term efficacy and toxicity after stereotactic ablative reirradiation in locally relapsed stage III non-small cell lung cancer. BMC Cancer 2019, 19, 305. [Google Scholar] [CrossRef]
- McAvoy, S.A.; Ciura, K.T.; Rineer, J.M.; Allen, P.K.; Liao, Z.; Chang, J.Y.; Palmer, M.B.; Cox, J.D.; Komaki, R.; Gomez, D.R. Feasibility of proton beam therapy for reirradiation of locoregionally recurrent non-small cell lung cancer. Radiother. Oncol. 2013, 109, 38–44. [Google Scholar] [CrossRef]
- Hong, J.H.; Kim, Y.S.; Lee, S.W.; Lee, S.J.; Kang, J.H.; Hong, S.H.; Hong, J.Y.; Cheon, G. High-Dose Thoracic Re-irradiation of Lung Cancer Using Highly Conformal Radiotherapy Is Effective with Acceptable Toxicity. Cancer Res. Treat. 2019, 51, 1156–1166. [Google Scholar] [CrossRef]
- Evans, J.D.; Gomez, D.R.; Amini, A.; Rebueno, N.; Allen, P.K.; Martel, M.K.; Rineer, J.M.; Ang, K.K.; McAvoy, S.; Cox, J.D.; et al. Aortic dose constraints when reirradiating thoracic tumors. Radiother. Oncol. 2013, 106, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Grambozov, B.; Wass, R.; Stana, M.; Gerum, S.; Karner, J.; Fastner, G.; Studnicka, M.; Sedlmayer, F.; Zehentmayr, F. Impact of reirradiation, chemotherapy, and immunotherapy on survival of patients with recurrent lung cancer: A single-center retrospective analysis. Thorac. Cancer 2021, 12, 1162–1170. [Google Scholar] [CrossRef]
- Mac Manus, M.P.; Everitt, S.; Bayne, M.; Ball, D.; Plumridge, N.; Binns, D.; Herschtal, A.; Cruickshank, D.; Bressel, M.; Hicks, R.J. The use of fused PET/CT images for patient selection and radical radiotherapy target volume definition in patients with non-small cell lung cancer: Results of a prospective study with mature survival data. Radiother. Oncol. 2013, 106, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Murakami, M.; Yoden, E.; Sasaki, R.; Okuno, Y.; Nakajima, T.; Kuroda, Y. Reirradiation for locally recurrent lung cancer previously treated with radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 390–396. [Google Scholar] [CrossRef]
- Maddalo, M.; D’Angelo, E.; Fiorica, F.; Argenone, A.; Scricciolo, M.; Cozzi, S.; Nardangeli, A.; Dionisi, F.; Costantino, G.; Vagge, S.; et al. Thoracic re-irradiation with 3D-conformal or more advanced techniques: A systematic review of treatment safety by the Re-irradiation Study Group of the Italian Association of Radiation and Oncology AIRO. Crit. Rev. Oncol. Hematol. 2021, 167, 103500. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, L.; Di Muzio, J.; Agolli, L.; Alongi, F.; Mazzola, R.; Valeriani, M.; Badellino, S.; Osti, M.F.; Ricardi, U. What is the role of reirradiation in the management of locoregionally relapsed non small-cell lung cancer? Lung Cancer 2020, 146, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Meijneke, T.R.; Petit, S.F.; Wentzler, D.; Hoogeman, M.; Nuyttens, J.J. Reirradiation and stereotactic radiotherapy for tumors in the lung: Dose summation and toxicity. Radiother. Oncol. 2013, 107, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Wurstbauer, K.; Zehentmayr, F.; Deutschmann, H.; Dagn, K.; Exeli, A.K.; Kopp, P.; Porsch, P.; Maurer, B.; Studnicka, M.; Sedlmayer, F. DART-bid for loco-regionally advanced NSCLC: Summary of acute and late toxicity with long-term follow-up; experiences with pulmonary dose constraints. Strahlenther. Onkol. 2017, 193, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abusaris, H.; Storchi, P.R.M.; Brandwijk, R.P.; Nuyttens, J.J. Second re-irradiation: Efficacy, dose and toxicity in patients who received three courses of radiotherapy with overlapping fields. Radiother. Oncol. 2011, 99, 235–239. [Google Scholar] [CrossRef]
- Gagliardi, G.; Constine, L.S.; Moiseenko, V.; Correa, C.; Pierce, L.J.; Allen, A.M.; Marks, L.B. Radiation dose-volume effects in the heart. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76 (Suppl. 3), S77–S85. [Google Scholar] [CrossRef]
- Viani, G.A.; Arruda, C.V.; De Fendi, L.I. Effectiveness and Safety of Reirradiation with Stereotactic Ablative Radiotherapy of Lung Cancer after a First Course of Thoracic Radiation: A Meta-analysis. Am. J. Clin. Oncol. 2020, 43, 575–581. [Google Scholar] [CrossRef]
- Muller, D.A.; Dutta, S.W.; Aliotta, E.; Sanders, J.C.; Wijesooriya, K.; Watkins, W.T.; Larner, J.M. Clinical Outcomes and Predictors of Lung Toxicity after Multiple Courses of Lung Stereotactic Body Radiotherapy for Early-Stage Non-Small Cell Lung Cancer. Clin. Lung Cancer 2021, 22, 234–241. [Google Scholar] [CrossRef]
- Ricco, A.; Barlow, S.; Feng, J.; Jacob, J.; Lozano, A.; Hanlon, A.; Arrigo, S.; Obayomi-Davies, O.; Lamond, J.; Yang, J.; et al. Repeat Thoracic Stereotactic Body Radiation Therapy (SBRT) for Nonsmall Cell Lung Cancer: Long-Term Outcomes, Toxicity, and Dosimetric Considerations. Adv. Radiat. Oncol. 2020, 5, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Andruska, N.; Stowe, H.B.; Crockett, C.; Liu, W.; Palma, D.; Faivre-Finn, C.; Badiyan, S.N.; Canc, I.A.S.L. Stereotactic Radiation for Lung Cancer: A Practical Approach to Challenging Scenarios. J. Thorac. Oncol. 2021, 16, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Schroder, C.; Stiefel, I.; Tanadini-Lang, S.; Pytko, I.; Vu, E.; Guckenberger, M.; Andratschke, N. Re-irradiation in the thorax-An analysis of efficacy and safety based on accumulated EQD2 doses. Radiother. Oncol. 2020, 152, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Troost, E.G.C.; Wink, K.C.J.; Roelofs, E.; Simone, C.B.; Makocki, S.; Lock, S.; van Kollenburg, P.; Dechambre, D.; Minken, A.W.H.; van der Stoep, J.; et al. Photons or protons for reirradiation in (non-)small cell lung cancer: Results of the multicentric ROCOCO in silico study. Brit. J. Radiol. 2020, 93, 20190879. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [Green Version]
- Wallis, C.J.D.; Butaney, M.; Satkunasivam, R.; Freedland, S.J.; Patel, S.P.; Hamid, O.; Pal, S.K.; Klaassen, Z. Association of Patient Sex With Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Advanced Cancers: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 529–536. [Google Scholar] [CrossRef]
- Deshpande, R.P.; Sharma, S.; Watabe, K. The Confounders of Cancer Immunotherapy: Roles of Lifestyle, Metabolic Disorders and Sociological Factors. Cancers 2020, 12, 2983. [Google Scholar] [CrossRef]


| Study | Patient and Tumor Characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients Included (N) | Median Age at Re-RT (years) | Re-RT Completed (N) | Histology | ECOG | Interval (Months) | Central Tumors (%) | UICC Stage at RT | UICC Stage at Re-RT | |||
| Retrospective | Grambozov | 2021 | 47 | 66 | 47 | NSCLC: 74% SCLC: 21% NOS: 5% | 0–1: 85% 2: 15% | 20 | 53 | I: 15% II: 17% III: 53% IV: 15% | ns |
| Griffioen (update Tetar) | 2014/2015 | 30 | 63 | 30 | NSCLC: 88% SCLC: 8% None: 4% | 0–1: 83% 2: 17% | 51 | 83 | I–II: 12% III: 63% IV: 8% SCLC: 17% | I–II: 21% III: 63% IV: 8% SCLC: 8% | |
| Hong | 2019 | 31 | 64 | 31 | NSCLC: 74% SCLC: 22% NOS: 3% | 0–1: 94%, 2: 6% | 15 | 74 | I: 10% II: 13% III: 45% IV: 6% NOS: 3% SCLC 22% | ns | |
| Kilburn | 2014 | 33 | 64 | 33 | NSCLC: 72% SCLC: 12% Mixed: 3% Other: 12% | 0–1: 79% 2: 18% (1) | 18 | 52 | I: 31% II: 14% III: 45% IV: 10% | ns | |
| McAvoy | 2013 | 33 | 69 | 31 | NSCLC: 100% | 0–1: 67% >1: 33% | 36 | 85 | I: 21% II: 15% III: 61% IV: 3% | ns | |
| McAvoy | 2014 | 102 | 68 | 99 | NSCLC: 100% | 0–1: 81% >1: 19% | 17 | 87 | I: 29% II: 16% III: 45% IV: 9% | ns | |
| Ohguri | 2012 | 33 | 68 | 33 | NSCLC: 100% | 0–1: 73% >1: 27% | 8 | 58 | I: 6% II: 12% III: 52% IV: 12% (2) | ns | |
| Schlampp | 2019 | 62 | 63 | 62 | NSCLC: 78% SCLC 16% NOS 6% | 0–1: 100% | 14 | 100 | III: 100% | ns | |
| Sumodhee | 2019 | 46 | 66 | 46 | NSCLC: 100% | 0–1: 100% | 23 | 52 | III: 100% | ns | |
| Yang | 2020 | 50 | 65 | 50 | NSCLC: 78% SCLC 8% NOS 14% | ns | 13 | 86 | ns | IIb–IIIc: 68% IV: 32% | |
| Prospective | Chao | 2016 | 57 | 65 | 52 (3) | NSCLC: 100% | 0–2: 100% | 19 | 61 | ns | I: 21% II: 7% III: 62% IV: 11% |
| Study | Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Re-RT-Technique | Median PTV at RT (mL) | Median PTV at Re-RT (mL) | Median Total Dose at RT (Gy) | Median Total Dose at Re-RT (Gy) | Cumulative EQD2 (Gy) | Systemic Treatment at Re-RT | |||
| Retrospective | Grambozov | 2021 | IMRT | ns | 47 | 74 | 51 | 131 | sCRT (1): 57% none: 43% |
| Griffioen (update Tetar) | 2014/2015 | IMRT | 539 | 248 | 60 | 60 | 120 | cCRT: 8% sCRT: 54% | |
| Hong | 2019 | IMRT: 68% SABR: 32% | 353 | 51 | 64 (2) | 55 (2) | 119 | cCRT:10% sCRT: 61% none: 29% | |
| Kilburn | 2014 | SABR | ns | 2.5 cm | 60 | 50 | 209 (3) | cCRT: 61% sCRT: 9% none: 30% (4) | |
| McAvoy | 2013 | Protons | ns | 96 (5) | 62 | 66 | 128 | cCRT: 24% sCRT: 51% none: 25% | |
| McAvoy | 2014 | Protons SABR | ns | 94 | 70 | 61 | 131 | cCRT: 33% sCRT: 48% none: 19% | |
| Ohguri | 2012 | 3D (6) | 112 cm2 | 38 cm2 | 70 | 50 | 115 | cCRT: 46% sCRT: ns | |
| Schlampp | 2019 | IMRT | 459 | 176 | 60 | 40 | 100 | cCRT: 0% sCRT: 92% | |
| Sumodhee | 2019 | SABR | ns | 13 | 66 | 60 | 196 | cCRT: 0% sCRT: 22% none: 78% | |
| Yang | 2020 | 3D: 14% IMRT: 86% | 529 | 202 | 60 | 51 | 106 | cCRT: 18% sCRT (7): 42% | |
| Prospective | Chao | 2016 | Protons | ns | 108 (8) | ns | 67 | ns | cCRT: 68% sCRT: ns |
| Study | Outcome after Re-RT | |||||
|---|---|---|---|---|---|---|
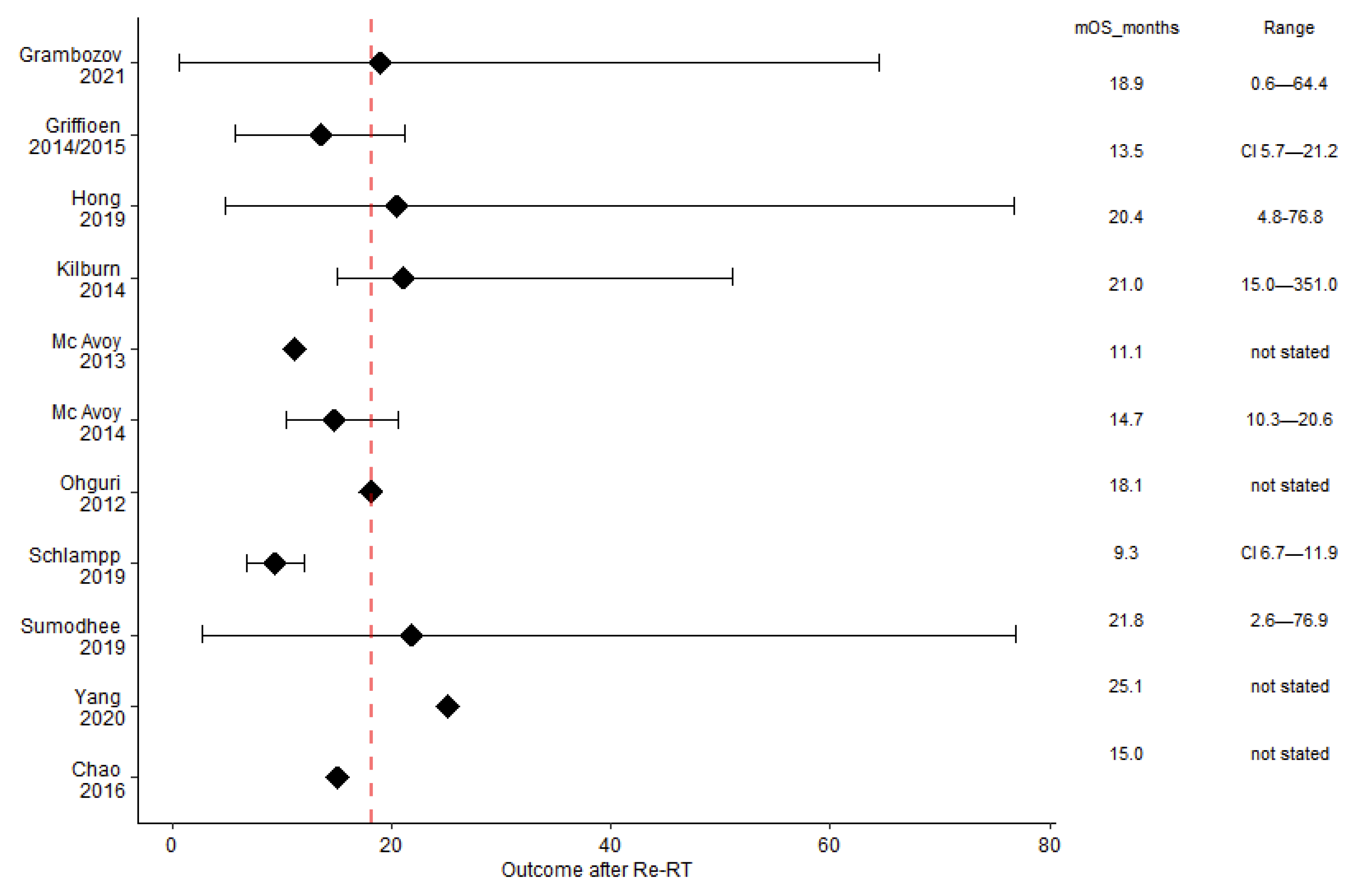
| mOS (Months) | mPFS (Months) | mLRC (Months) | Patients Alive at 24 Months after Re-RT (%) | |||
| Retrospective | Grambozov | 2021 | 18.9 (16.5–21.3) | ns | 7.9 (6.7–9) | 30 |
| Griffioen (update Tetar) | 2014/2015 | 13.5 (5.7–21.2) | 8.4 (5.5–11.3) | 6.7 (2.5–11.0) | 23 | |
| Hong | 2019 | 20.4 (4.8–76.8) | 15.4 (3.4–76.8) | 20 (1) | 39 | |
| Kilburn | 2014 | 21 (2) (15–51) | 16 (2) (6.6–nr) | not reached | 45 | |
| McAvoy | 2013 | 11.1 | 4.5 | 18 | 33 | |
| McAvoy | 2014 | 14.7 (10.3–20.6) | 11.4 (6.8–23.8) | 11.4 (8.6–22.7) | 33 | |
| Ohguri | 2012 | 18.1 | 6.7 | 12.1 | 45 (1) | |
| Schlampp | 2019 | 9.3 (6.7–11.9) | ns | 6.5 (6.0–7.0) | 15 (1) | |
| Sumodhee | 2019 | 21.8 (2.6–76.9) | 9.6 (1–62.5) | 13.8 (1–76.9) | 45 | |
| Yang | 2020 | 25.1 | 5.9 | 18 | 50 | |
| Prospective | Chao | 2016 | 15 | 14 | ns | 43 |
| Study | Toxicity | ||||
|---|---|---|---|---|---|
| Acute | Late | Lethal | |||
| Retrospective | Grambozov | 2021 | G1-2: 9% (esophageal), 2% (pulmonary) G3-4: 4% (esophageal) | G1-2: 0%, G3-4: 2% (hemorrhage) | 2% (cardiac) |
| Griffioen (update Tetar) | 2014/2015 | G1-2: 88%, G3-4: 10% | G2: 21% (vertebral collapse, pulmonary) | 13% (hemorrhage) | |
| Hong | 2019 | G1-2: 90% (pulmonary), 19%(esophageal) G3-4: 0% | G1-2: 49% (pulmonary) 6% (esophageal) G3-4: 3% (pericarditis) | 0% | |
| Kilburn | 2014 | G1-2: 36% (pulmonary, chestwall pain) G3-4: 3% (pulmonary) | G1-4: 0% | 3% (aorto-esophageal fistula) | |
| McAvoy | 2013 | G1-2: 36% (pulmonary), 36% (chestwall), 15% (esophageal), 15% (cardiac) G3-4: 21% (pulmonary), 9% (esophageal) 3% (cardiac) | G1-4: 0% | 0% | |
| McAvoy | 2014 | G1-2: 18% (esophageal), 27% (pneumonitis) G3-4: 7% (esophageal), 10% (pneumonitis) | G1-2: ns G3-4: 1% (pulmonary) | 0% | |
| Ohguri | 2012 | G1-2: 9% (pneumonitis), 15% (dermatitis), 6% (hematological) G3-4: 9% (thermal burns), 3% (pleuritis) | G1-2: 0% G3-4: 3% (brachial plexus neuritis) | 0% | |
| Schlampp | 2019 | G1-2: 24% (including 19% pneumonitis) G3-4: 8% (pneumonitis) | G1-2: 21% (pulmonary) G3-4: 5% (esophageal, pulmonary) | 2% (pulmonary) | |
| Sumodhee | 2019 | ns | ns | 4% (pulmonary) (1) | |
| Yang | 2020 | G1-2: 22% (pulmonary) G3-4: 26% (pulmonary) | G1-4: 0% | 14% (pulmonary) | |
| Prospective | Chao | 2016 | G1-2: ns G3-4: 39% | G1-2: ns G3-4: 12% | 11% (2) |
| Study | Esophageal Toxicity | Pulmonary Toxicity | OS | PFS | LRC | Statistics | ||
|---|---|---|---|---|---|---|---|---|
| Retrospective | Grambozov | 2021 | ns | ns | PTV (p-value 0.000, HR 1.007) | none | none | multivariate |
| Griffioen (update Tetar) | 2014/2015 | ns | ns | PTV | PTV | ns | univariate | |
| Hong | 2018 | ns | MLD | EQD2 (Re-RT), cumulative EQD2 (both courses) | gender, CT after Re-RT, GTV, PTV, fraction size | EQD2 (Re-RT), cumulative EQD2 (both courses) | univariate | |
| Kilburn | 2014 | ns | ns | ns | ns | ns | ns | |
| McAvoy | 2013 | none | none | ns | ns | ns | univariate | |
| McAvoy | 2014 | none | none | histology (p-value 0.004, HR 0.4), cCRT (p-value 0.0045, HR 2.6), EQD2 at Re-RT (p-value 0.021, HR 0.2), ECOG (p-value 0.028; HR 2.5) | T4 (p-value 0.013, HR 3.5), cCRT (p-value 0.027, HR 0.5) | cCRT (p-value 0.004, HR 6.5), interval (p-value 0.012, HR 0.4) | multivariate | |
| Ohguri | 2012 | ns | ns | histology (p-value 0.04, HR 3.5), recurrent tumor size (p-value 0.001, HR 17.3) | none | histology (p-value 0.03, HR 3.7) | multivariate | |
| Schlampp | 2019 | ns | ns | nodal involvement, total Re-RT dose, dose to aorta, interval, FEV1 | ns | none | univariate | |
| Sumodhee | 2019 | in-field relapse combined with central tumor (p-value 0.03, HR 6.4) | in-field relapse combined with central tumor (p-value 0.03, HR 6.4) | ns | ns | recurrent tumor size (p-value 0.04, HR 0.2) | multivariate | |
| Yang | 2020 | ns | ns | none | extrathoracic disease (p-value 0.02, HR 2.9) | none | multivariate | |
| Prospective | Chao | 2016 | cCRT, MED, MHD, central volume overlap | cCRT, MED, MHD, central volume overlap | MED | none | none | univariate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grambozov, B.; Stana, M.; Kaiser, B.; Karner, J.; Gerum, S.; Ruznic, E.; Zellinger, B.; Moosbrugger, R.; Studnicka, M.; Fastner, G.; et al. High Dose Thoracic Re-Irradiation and Chemo-Immunotherapy for Centrally Recurrent NSCLC. Cancers 2022, 14, 573. https://doi.org/10.3390/cancers14030573
Grambozov B, Stana M, Kaiser B, Karner J, Gerum S, Ruznic E, Zellinger B, Moosbrugger R, Studnicka M, Fastner G, et al. High Dose Thoracic Re-Irradiation and Chemo-Immunotherapy for Centrally Recurrent NSCLC. Cancers. 2022; 14(3):573. https://doi.org/10.3390/cancers14030573
Chicago/Turabian StyleGrambozov, Brane, Markus Stana, Bernhard Kaiser, Josef Karner, Sabine Gerum, Elvis Ruznic, Barbara Zellinger, Raphaela Moosbrugger, Michael Studnicka, Gerd Fastner, and et al. 2022. "High Dose Thoracic Re-Irradiation and Chemo-Immunotherapy for Centrally Recurrent NSCLC" Cancers 14, no. 3: 573. https://doi.org/10.3390/cancers14030573
APA StyleGrambozov, B., Stana, M., Kaiser, B., Karner, J., Gerum, S., Ruznic, E., Zellinger, B., Moosbrugger, R., Studnicka, M., Fastner, G., Sedlmayer, F., & Zehentmayr, F. (2022). High Dose Thoracic Re-Irradiation and Chemo-Immunotherapy for Centrally Recurrent NSCLC. Cancers, 14(3), 573. https://doi.org/10.3390/cancers14030573







