Molecular Modelling of Optical Biosensor Phosphorene-Thioguanine for Optimal Drug Delivery in Leukemia Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods and Materials
3. Results and Discussion
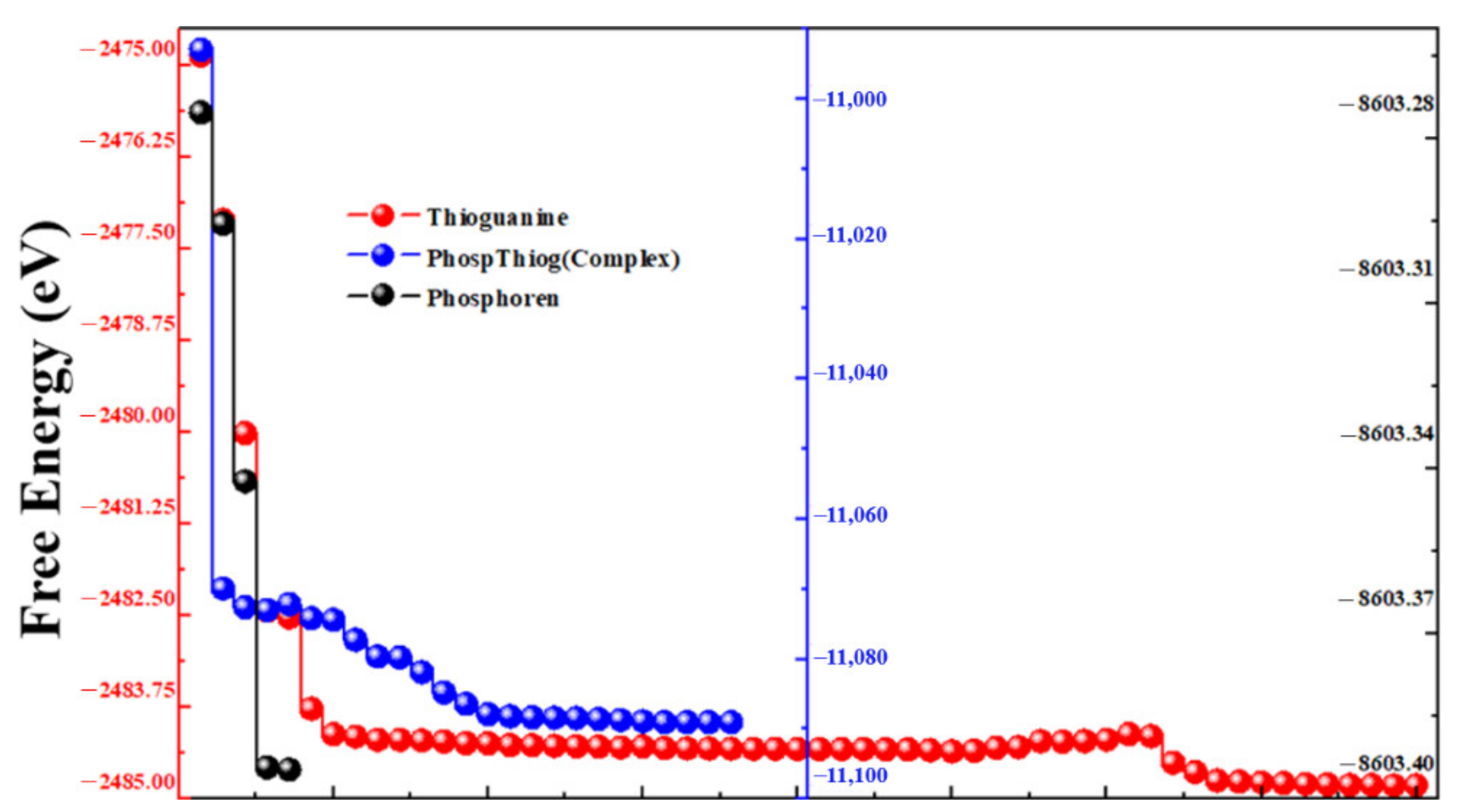
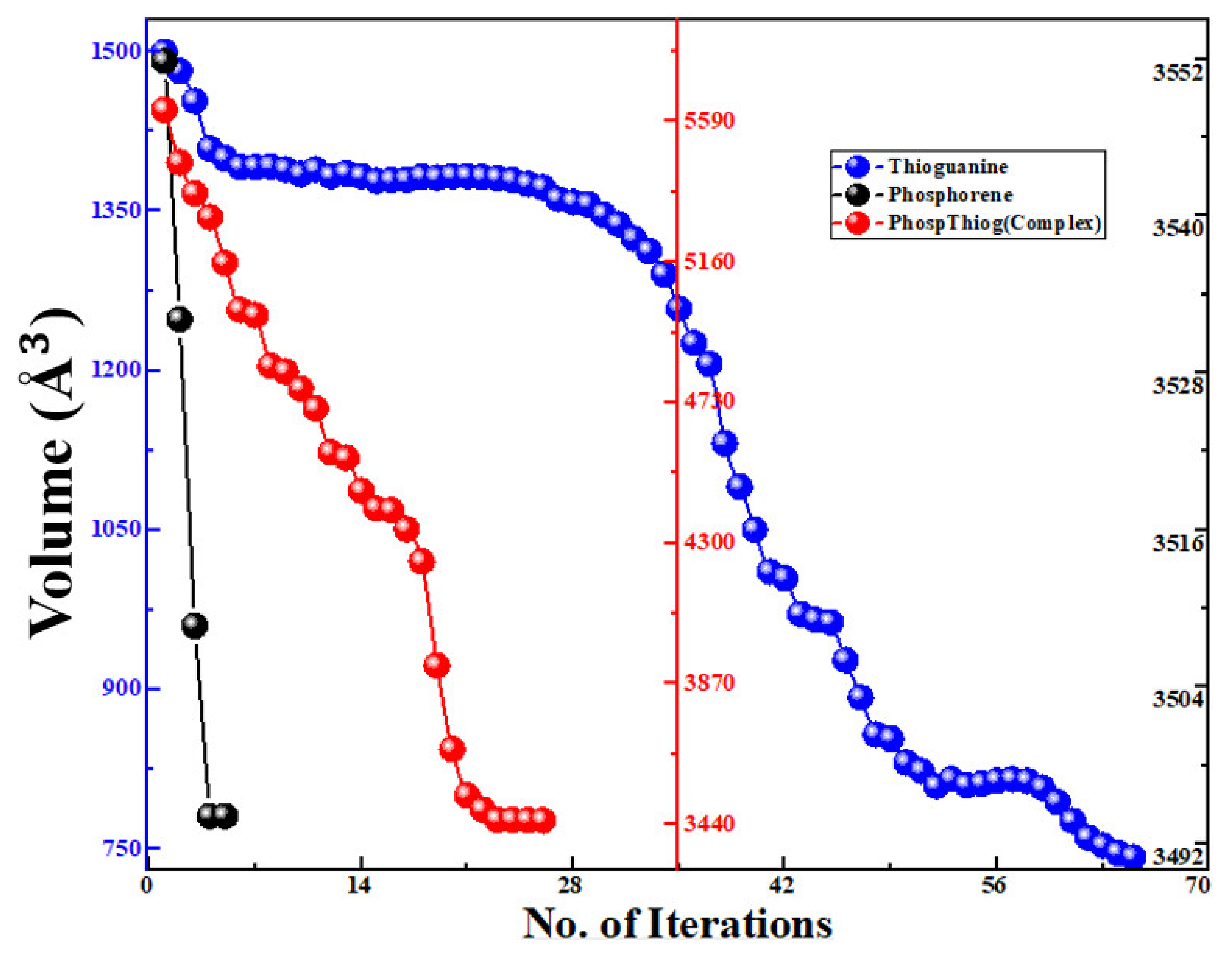
3.1. Geometries Analysis
3.2. Calculation of Adsorption Energy of the Complex
3.3. Frontier Molecular Orbital Analysis
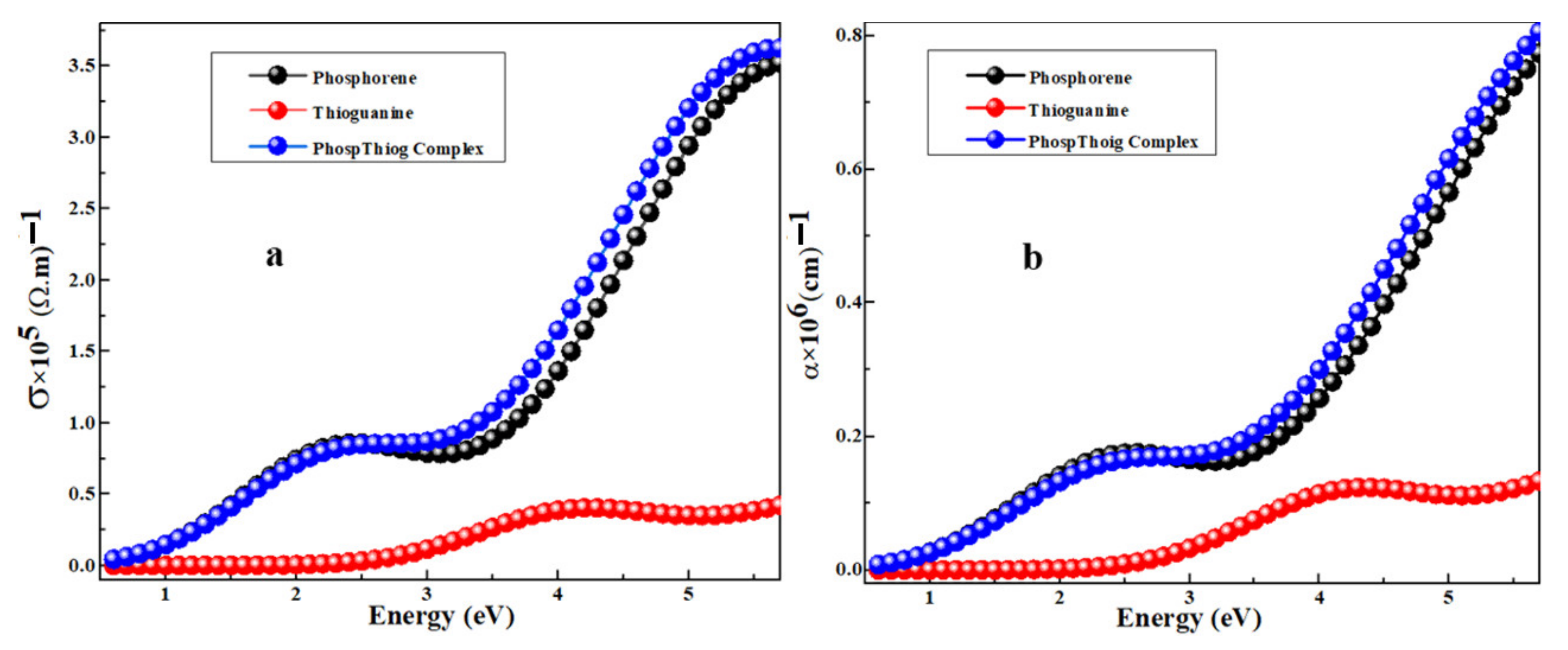
3.4. Optical Conductivity
3.5. Polarity of the Molecules
3.6. Quantum Molecular Descriptors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kebebe, D.; Liu, Y.; Wu, Y.; Vilakhamxay, M.; Liu, Z.; Li, J. Tumor-targeting delivery of herb-based drugs with cell-penetrating/tumor-targeting peptide-modified nanocarriers. Int. J. Nanomed. 2018, 13, 1425. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruthers, D.S.; Wickline, S.A.; Lanza, G.M. Nanotechnological applications in medicine. Curr. Opin. Biotechnol. 2007, 18, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Boisseau, P.; Loubaton, B. Nanomedicine, nanotechnology in medicine. Comptes. Rendus. Phys. 2011, 12, 620–636. [Google Scholar] [CrossRef] [Green Version]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, C.; Gu, X.; Gong, H.; Cheng, L.; Shi, X.; Feng, L.; Sun, B.; Liu, Z. Drug delivery with PEGylated MoS2 nano-sheets for combined photothermal and chemotherapy of cancer. Adv. Mater. 2014, 26, 3433–3440. [Google Scholar] [CrossRef]
- Cheng, L.; Yuan, C.; Shen, S.; Yi, X.; Gong, H.; Yang, K.; Liu, Z. Bottom-up synthesis of metal-ion-doped WS2 nanoflakes for cancer theranostics. ACS Nano 2015, 9, 11090–11101. [Google Scholar] [CrossRef] [PubMed]
- Weng, Q.; Wang, B.; Wang, X.; Hanagata, N.; Li, X.; Liu, D.; Wang, X.; Jiang, X.; Bando, Y.; Golberg, D. Highly water-soluble, porous, and biocompatible boron nitrides for anticancer drug delivery. ACS Nano 2014, 8, 6123–6130. [Google Scholar] [CrossRef]
- Chen, L.; Zhong, X.; Yi, X.; Huang, M.; Ning, P.; Liu, T.; Ge, C.; Chai, Z.; Liu, Z.; Yang, K. Radionuclide 131I labeled reduced graphene oxide for nuclear imaging guided combined radio-and photothermal therapy of cancer. Biomaterials 2015, 66, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Genovese, F.; Aiello, E.; Amantea, M.; Makeeva, I.; Zavan, B.; Rengo, S.; Fortunato, L. Phosphorene is the new graphene in biomedical applications. Materials 2019, 12, 2301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tariq, A.; Nazir, S.; Arshad, A.W.; Nawaz, F.; Ayub, K.; Iqbal, J. DFT study of the therapeutic potential of phosphorene as a new drug-delivery system to treat cancer. RSC Adv. 2019, 9, 24325–24332. [Google Scholar] [CrossRef] [Green Version]
- Chimene, D.; Alge, D.L.; Gaharwar, A.K. Two-dimensional nanomaterials for biomedical applications: Emerging trends and future prospects. Adv. Mater. 2015, 27, 7261–7284. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Nilghaz, A.; Lin, Y.; Xu, J.; Lu, X. Black phosphorus and its biomedical applications. Theranostics 2018, 8, 1005. [Google Scholar] [CrossRef]
- Luo, M.; Fan, T.; Zhou, Y.; Zhang, H.; Mei, L. 2D black phosphorus–based biomedical applications. Adv. Funct. Mater. 2019, 29, 1808306. [Google Scholar] [CrossRef]
- Yang, A.; Wang, D.; Wang, X.; Zhang, D.; Koratkar, N.; Rong, M. Recent advances in phosphorene as a sensing material. Nano Today 2018, 20, 13–32. [Google Scholar] [CrossRef]
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.; Tománek, D.; Ye, P.D. Phosphorene: An unexplored 2D semiconductor with a high hole mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef] [Green Version]
- Pica, M.; D’Amato, R. Chemistry of Phosphorene: Synthesis, Functionalization and Biomedical Applications in an Update Review. Inorganics 2020, 8, 29. [Google Scholar] [CrossRef]
- Akhtar, M.; Anderson, G.; Zhao, R.; Alruqi, A.; Mroczkowska, J.E.; Sumanasekera, G.; Jasinski, J.B. Recent advances in synthesis, properties, and applications of phosphorene. NPJ 2D Mater. Appl. 2017, 1, 1–13. [Google Scholar] [CrossRef]
- Liu, B.; Bai, L.; Korznikova, E.A.; Dmitriev, S.V.; Law, A.W.K.; Zhou, K. Thermal conductivity and tensile response of phosphorene nanosheets with vacancy defects. J. Phys. Chem. C 2017, 121, 13876–13887. [Google Scholar] [CrossRef]
- Fan, T.; Zhou, Y.; Qiu, M.; Zhang, H. Black phosphorus: A novel nanoplatform with potential in the field of bio-photonic nanomedicine. J. Innov. Opt. Health Sci. 2018, 11, 1830003. [Google Scholar] [CrossRef] [Green Version]
- Murugan, C.; Sharma, V.; Murugan, R.K.; Malaimegu, G.; Sundaramurthy, A. Two-dimensional cancer theranostic nanomaterials: Synthesis, surface functionalization and applications in photothermal therapy. J. Control. Release 2019, 299, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Herrlinger, K.R.; Kreisel, W.; Schwab, M.; Schoelmerich, J.; Fleig, W.E.; Ruhl, A.; Reinshagen, M.; Deibert, P.; Fellermann, K.; Greinwald, R.; et al. 6-Thioguanine—Efficacy and safety in chronic active Crohn’s disease. Aliment. Pharmacol. Ther. 2003, 17, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.Y.; Yang, H.W.; Liu, H.L.; Tsai, R.Y.; Pang, S.T.; Chuang, K.L.; Chang, Y.S.; Hwang, T.L.; Chang, Y.H.; Chuang, H.C.; et al. Superhigh-magnetization nanocarrier as a doxorubicin delivery platform for magnetic targeting therapy. Biomaterials 2011, 32, 8999–9010. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, L.; Zhang, J.; Ma, R.; Gao, J.; Liu, Y.; Zhang, C.; Zhang, Z. A tumor-targeting near-infrared laser-triggered drug delivery system based on GO@ Ag nanoparticles for chemo-photothermal therapy and X-ray imaging. Biomaterials 2014, 35, 5847–5861. [Google Scholar] [CrossRef] [PubMed]
- Soler, J.M.; Artacho, E.; Gale, J.D.; García, A.; Junquera, J.; Ordejón, P.; Sánchez-Portal, D. The SIESTA method for ab initio order-N materials simulation. J. Phys. Condens. Matter. 2002, 14, 2745. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Cohen, R.E. More accurate generalized gradient approximation for solids. Phys. Rev. B 2006, 73, 235116. [Google Scholar] [CrossRef] [Green Version]
- Hammer, B.; Hansen, L.B.; Nørskov, J.K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; di Valentin, C.; Pacchioni, G. Electronic and structural properties of WO3: A systematic hybrid DFT study. J. Phys. Chem. C 2011, 115, 8345–8353. [Google Scholar] [CrossRef]
- Caravaca, M.; Casali, R. Ab initio localized basis set study of structural parameters and elastic properties of HfO2 polymorphs. J. Phys. Condens. Matter. 2005, 17, 5795. [Google Scholar] [CrossRef]
- Liang, W.; Luo, X. Theoretical Studies of MoS2 and Phosphorene Drug Delivery for Antituberculosis Drugs. J. Phys. Chem. C 2020, 124, 8279–8287. [Google Scholar] [CrossRef]
- Rahimi, R.; Solimannejad, M. BC3 graphene-like monolayer as a drug delivery system for nitrosourea anticancer drug: A first-principles perception. Appl. Surf. Sci. 2020, 525, 146577. [Google Scholar] [CrossRef]
- Jafari, Z.; Baharfar, R.; Rad, A.S.; Asghari, S. Potential of graphene oxide as a drug delivery system for Sumatriptan: A detailed density functional theory study. J. Biomol. Struct. Dyn. 2020, 39, 1611–1622. [Google Scholar] [CrossRef]
- Hashemzadeh, H.; Raissi, H. Loading and release of anticancer drug from phosphorene as a template material with high efficient carrier: From vacuum to cell membrane. J. Mol. Liq. 2019, 291, 111346. [Google Scholar] [CrossRef]
- Seminario, J.M. Recent Developments and Applications of Modern Density Functional Theory; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Fukui, K. The role of frontier orbitals in chemical reactions (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1982, 21, 801–809. [Google Scholar] [CrossRef]
- Das, S.; Gulotty, R.; Sumant, A.V.; Roelofs, A. Erratum: Tunable Transport Gap in Phosphorene (Nano Letters (2014) 14: 10 (5733–5739) 10 1021/nl5025535). Nano Lett. 2016, 16, 2861–2866. [Google Scholar]
- Sa, B.; Li, Y.L.; Qi, J.; Ahuja, R.; Sun, Z. Strain engineering for phosphorene: The potential application as a photocatalyst. J. Phys. Chem. C 2014, 118, 26560–26568. [Google Scholar] [CrossRef] [Green Version]
- Gunasekaran, S.; Kumaresan, S.; Arunbalaji, R.; Anand, G.; Seshadri, S.; Muthu, S. Vibrational assignments and electronic structure calculations for 6-thioguanine. J. Raman Spectrosc. Int. J. Orig. Work. All Asp. RamanSpectrosc. Incl. High. Order Processes Also Brillouin Rayleigh Scatt. 2009, 40, 1675–1681. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C.R. Natural bond orbitals and extensions of localized bonding concepts. Chem. Educ. Res. Pract. 2001, 2, 91–104. [Google Scholar] [CrossRef]
- Sheikhi, M.; Shahab, S.; Khaleghian, M.; Kumar, R. Interaction between new anti-cancer drug syndros and CNT (6, 6-6) nanotube for medical applications: Geometry optimization, molecular structure, spectroscopic (NMR, UV/Vis, excited state), FMO, MEP and HOMO-LUMO investigation. Appl. Surf. Sci. 2018, 434, 504–513. [Google Scholar] [CrossRef]
- Nikfar, Z.; Shariatinia, Z. DFT computational study on the phosphate functionalized SWCNTs as efficient drug delivery systems for anti-osteoporosis zolendronate and risedronate drugs. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 91, 41–59. [Google Scholar] [CrossRef]
- Chernyak, Y. Dielectric constant, dipole moment, and solubility parameters of some cyclic acid esters. J. Chem. Eng. Data 2006, 51, 416–418. [Google Scholar] [CrossRef]
- Politzer, P.; Peralta-Inga Shields, Z.; Bulat, F.A.; Murray, J.S. Average local ionization energies as a route to intrinsic atomic electronegativities. J. Chem. Theory Comput. 2011, 7, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Shweta; Khan, E.; Poonam, T.; Rakesh, M.; Padam, K. A theoretical study on molecular structure, chemical reactivity, and molecular docking studies on dalbergin and methyldalbergin. J. Mol. Struct. 2019, 1183, 100–106. [Google Scholar] [CrossRef]
- Ahmadi, R.; Pirahan-Foroush, M. Ab initio studies of fullerene effect on chemical properties of naphazoline drop. Ann. Mil. Health Sci. Res. 2014, 12, 86–90. [Google Scholar]



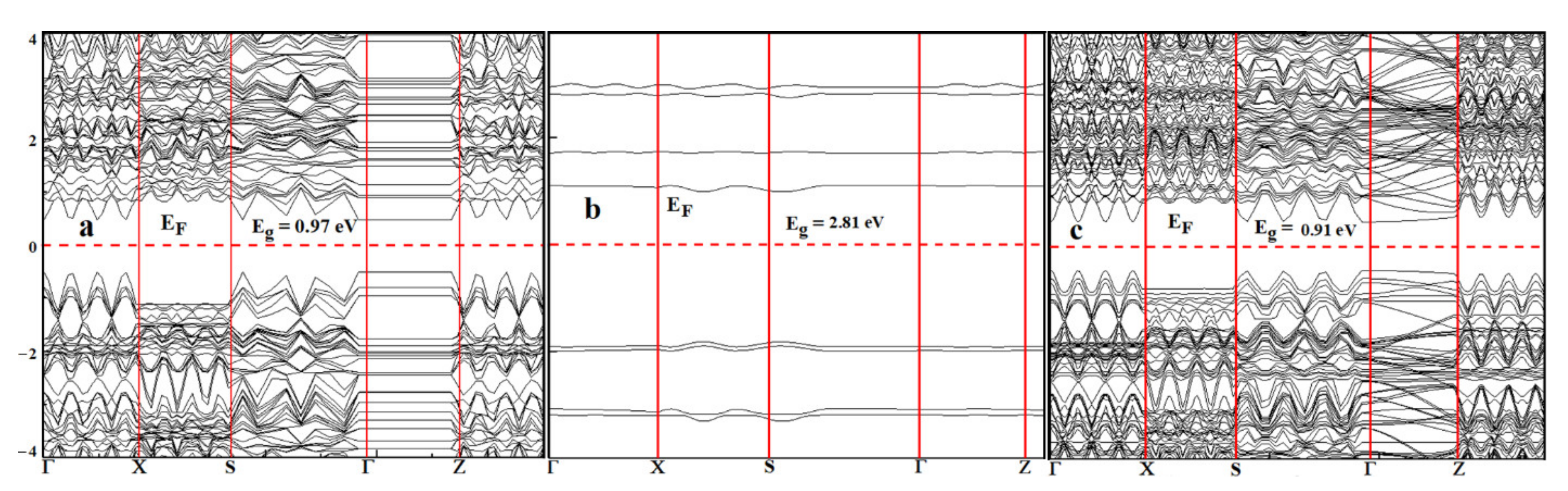

| Material | Method | ELUMO (eV) | EHOMO (eV) | Eg (eV) | Experiment | Other DFT |
|---|---|---|---|---|---|---|
| Phosphorene | RPBE | −2.90 | −3.88 | 0.97 | 1–1.45 [16,36] | 0.76–0.92 [37] |
| PBE | −3.01 | −3.95 | 0.94 | |||
| revPBE | −2.91 | −3.88 | 0.97 | |||
| Hybrid | −3.08 | −4.05 | 0.97 | |||
| Thioguanine | RPBE | −1.23 | −4.06 | 2.83 | 3.58 [38] | 7.20–0.14 [38] |
| PBE | −1.36 | −4.18 | 2.82 | |||
| revPBE | −1.24 | −4.06 | 2.82 | |||
| Hybrid | −1.44 | −4.34 | 2.81 | |||
| Phosp/Thiog | RPBE | −2.80 | −3.71 | 0.91 | - | - |
| PBE | −2.91 | −3.79 | 0.88 | |||
| revPBE | −2.81 | −3.72 | 0.91 | |||
| Hybrid | −3.00 | −3.91 | 0.91 |
| Chemical Properties | Phosphorene | Thioguanine | PT Complex |
|---|---|---|---|
| Dipole moment (D) | 0.0005 | 0.28 | 1.74 |
| Electron affinity | 3.08 | 1.44 | 3.00 |
| Ionization potential (eV) | 4.05 | 4.24 | 3.91 |
| Electronegativity (eV) | 3.56 | 2.84 | 3.45 |
| Chemical potential (eV) | −3.56 | −2.84 | −3.45 |
| Chemical hardness | 0.48 | 1.40 | 0.45 |
| Chemical softness (eV)−1 | 1.04 | 0.35 | 1.11 |
| Electrophilicity index (eV) | 13.20 | 2.88 | 13.22 |
| Molecular charge transfer (ΔN) | 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mian, S.A.; Khan, S.U.; Hussain, A.; Rauf, A.; Ahmed, E.; Jang, J. Molecular Modelling of Optical Biosensor Phosphorene-Thioguanine for Optimal Drug Delivery in Leukemia Treatment. Cancers 2022, 14, 545. https://doi.org/10.3390/cancers14030545
Mian SA, Khan SU, Hussain A, Rauf A, Ahmed E, Jang J. Molecular Modelling of Optical Biosensor Phosphorene-Thioguanine for Optimal Drug Delivery in Leukemia Treatment. Cancers. 2022; 14(3):545. https://doi.org/10.3390/cancers14030545
Chicago/Turabian StyleMian, Shabeer Ahmad, Shafqat Ullah Khan, Akbar Hussain, Abdur Rauf, Ejaz Ahmed, and Joonkyung Jang. 2022. "Molecular Modelling of Optical Biosensor Phosphorene-Thioguanine for Optimal Drug Delivery in Leukemia Treatment" Cancers 14, no. 3: 545. https://doi.org/10.3390/cancers14030545
APA StyleMian, S. A., Khan, S. U., Hussain, A., Rauf, A., Ahmed, E., & Jang, J. (2022). Molecular Modelling of Optical Biosensor Phosphorene-Thioguanine for Optimal Drug Delivery in Leukemia Treatment. Cancers, 14(3), 545. https://doi.org/10.3390/cancers14030545