Comprehensive Evaluation of Vocal Outcomes and Quality of Life after Total Laryngectomy and Voice Restoration with J-Flap and Tracheoesophageal Puncture
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Technique
2.1.1. Tracheoesophageal Puncture
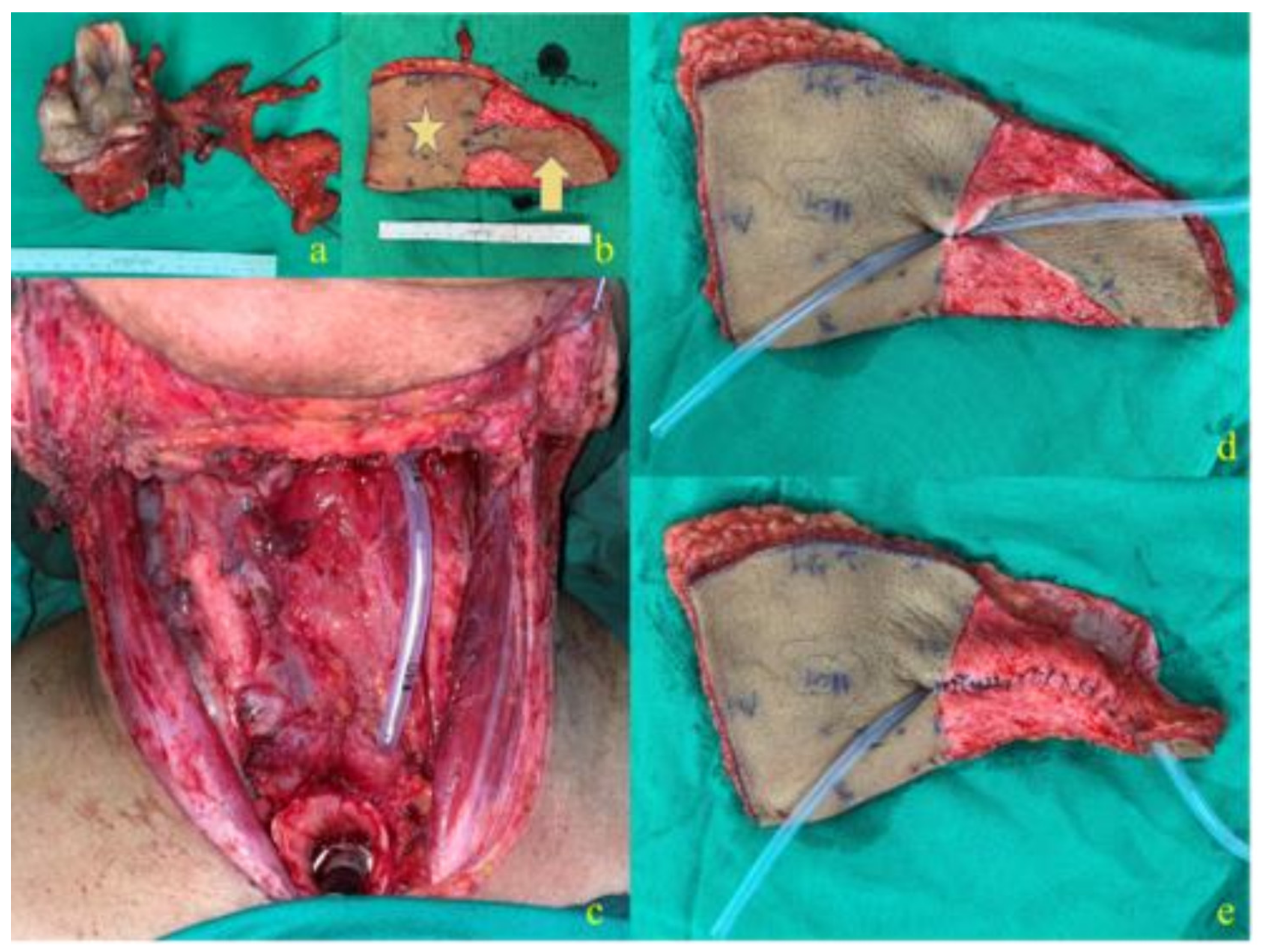
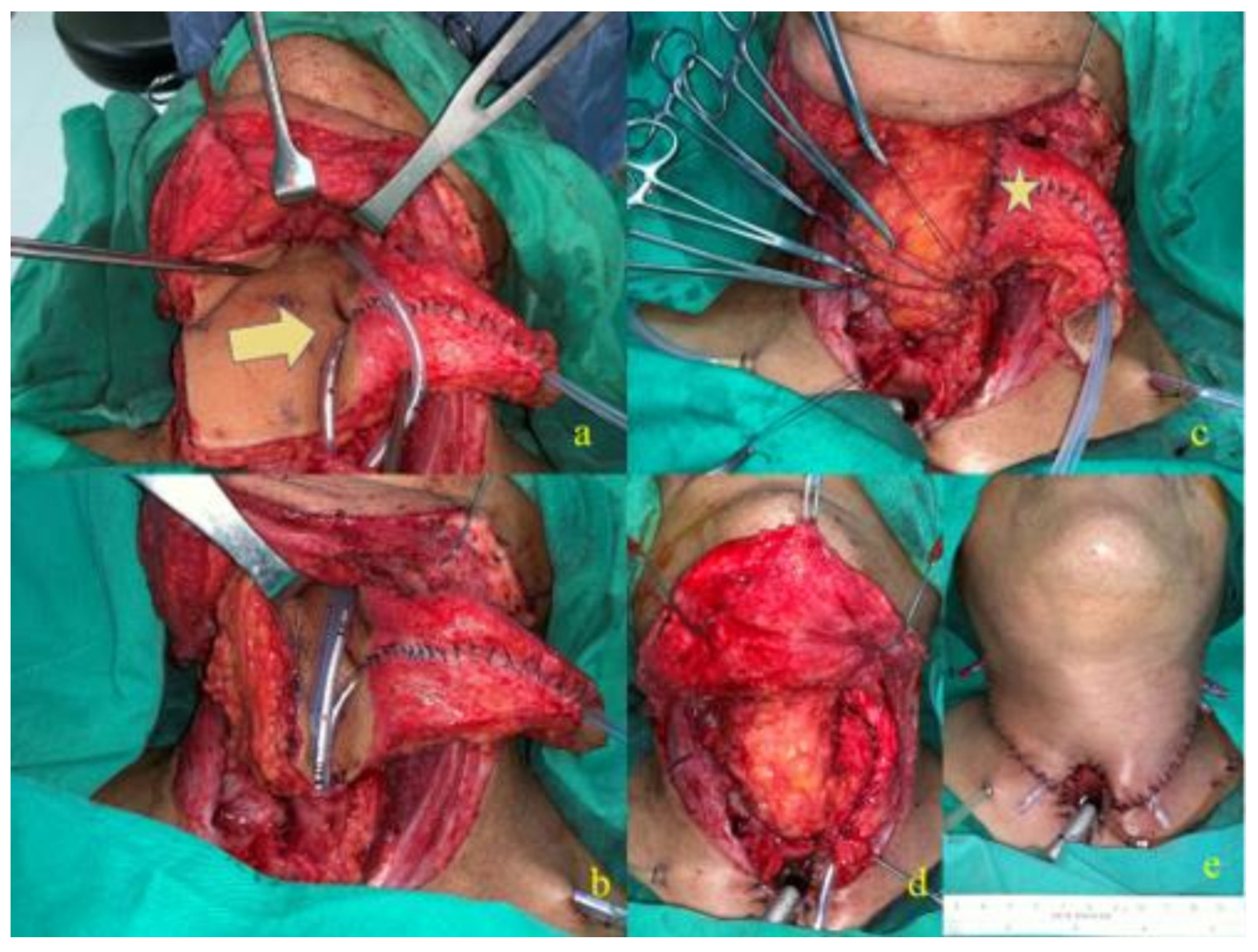
2.1.2. J-Flap Phonatory Tube
2.2. Speech and Voice Evaluation
2.2.1. Speech Intelligibility
2.2.2. Speech Accuracy
2.2.3. Voice Handicap Index
2.2.4. Subjective Assessment of Dysphonia (GIRBAS)
2.2.5. Acoustic Voice Analysis
2.2.6. Maximum Phonation Time
2.3. Quality of Life Evaluation: The University of Washington Quality of Life Questionnaire
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchi, F.; Missale, F.; Sampieri, C.; Filauro, M.; Iandelli, A.; Parrinello, G.; Incandela, F.; Smeele, L.E.; van den Brekel, M.W.M.; Del Bon, F.; et al. Laryngeal compartmentalization does not affect the prognosis of T3-T4 laryngeal cancer treated by upfront total laryngectomy. Cancers 2020, 12, 2241. [Google Scholar] [CrossRef]
- Sharpe, G.; Camoes Costa, V.; Doubé, W.; Sita, J.; McCarthy, C.; Carding, P. Communication changes with laryngectomy and impact on quality of life: A review. Qual. Life Res. 2019, 28, 863–877. [Google Scholar] [CrossRef]
- Tang, C.G.; Sinclair, C.F. Voice Restoration After Total Laryngectomy. Otolaryngol. Clin. N. Am. 2015, 48, 687–702. [Google Scholar] [CrossRef]
- Zenga, J.; Goldsmith, T.; Bunting, G.; Deschler, D.G. State of the art: Rehabilitation of speech and swallowing after total laryngectomy. Oral Oncol. 2018, 86, 38–47. [Google Scholar] [CrossRef]
- Yu, P.; Hanasono, M.M.; Skoracki, R.J.; Baumann, D.P.; Lewin, J.S.; Weber, R.S.; Robb, G.L. Pharyngoesophageal reconstruction with the anterolateral thigh flap after total laryngopharyngectomy. Cancer 2010, 116, 1718–1724. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, H.; Shiraishi, T.; Yasugawa, H.; Okamura, K.; Shirakusa, T. A new surgical technique for voice restoration after laryngopharyngoesophagectomy with a free ileocolic graft: Preliminary report. Surgery 1992, 111, 569–575. [Google Scholar] [PubMed]
- Hsiao, H.-T.; Leu, Y.-S.; Chang, Y.-C.; Yang, J.-C.; Tung, K.-Y. Voice and swallowing after laryngopharyngectomy and free ileocolic flap reconstruction for hypopharyngeal cancer. Ann. Plast. Surg. 2009, 62, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Gharb, B.B.; Rampazzo, A.; Perrone, F.; Chen, S.-H.; Trignano, E. Simultaneous restoration of voice function and digestive tract continuity in patients with synchronous primaries of hypopharynx and thoracic esophagus with pedicled ileocolon flap. Surgery 2011, 149, 662–671. [Google Scholar] [CrossRef]
- Karri, V.; Yang, M.-C.; Chung, K.-P.; Chen, S.-H.; Mardini, S.; Chen, H.-C. Total pharyngolaryngectomy and voice reconstruction with ileocolon free flap: Functional outcome and quality of life. J. Plast. Reconstr. Aesthetic Surg. 2011, 64, 911–920. [Google Scholar] [CrossRef]
- Lee, J.-C.; Hsu, W.-T.; Yang, C.-C.; Chang, S.-H. A fabricated forearm free flap with accompanying phonation tube for simultaneous reconstruction of a pharyngolaryngeal circumferential defect and voice loss: New surgical modification with functional phonation outcome. Laryngoscope 2013, 123, 344–349. [Google Scholar] [CrossRef]
- Lu, Y.A.; Pei, Y.C.; Chuang, H.F.; Lin, L.Y.; Hsin, L.J.; Kang, C.J.; Huang, S.F.; Chiang, H.C.; Tsao, C.K.; Fang, T.J. Speech Performance after Anterolateral Thigh Phonatory Tube Reconstruction for Total Laryngectomy. Laryngoscope 2021, 131, 1349–1357. [Google Scholar] [CrossRef]
- Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Anzai, Y.; Brizel, D.M.; Bruce, J.Y.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 873–898. [Google Scholar] [CrossRef]
- Doescher, J.; Veit, J.A.; Hoffmann, T.K. The 8th edition of the AJCC Cancer Staging Manual: Updates in otorhinolaryngology, head and neck surgery. HNO 2017, 65, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.H.; Fang, T.J.; Huang, S.F.; Kang, C.J.; Liao, C.T.; Hung, S.Y.; Cheong, C.F.; Tsao, C.K. Synchronous reconstruction of esophageal defect and voice with J-flap after laryngopharyngectomy: Indications and outcomes. Oral Oncol. 2020, 110, 104947. [Google Scholar] [CrossRef]
- Chuang, H.-F.; Yang, C.-C.; Chi, L.-Y.; Weismer, G.; Wang, Y.-T. Speech intelligibility, speaking rate, and vowel formant characteristics in Mandarin-speaking children with cochlear implant. Int. J. Speech-Lang. Pathol. 2012, 14, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Nikolopoulos, T.P.; Dyar, D.; O’Donoghue, G.M. Reliability of a rating scale for measuring speech intelligibility after pediatric cochlear implantation. Otol. Neurotol. 2001, 22, 631–633. [Google Scholar] [CrossRef]
- Jacobson, B.H.; Johnson, A.; Grywalski, C.; Silbergleit, A.; Jacobson, G.; Benninger, M.S.; Newman, C.W. The Voice Handicap Index (VHI): Development and Validation. Am. J. Speech-Lang. Pathol. 1997, 6, 66–69. [Google Scholar] [CrossRef]
- Kazi, R.; De Cordova, J.; Singh, A.; Venkitaraman, R.; Nutting, C.M.; Clarke, P.; Rhys-Evans, P.; Harrington, K.J. Voice-related Quality of Life in laryngectomees: Assessment using the VHI and V-RQOL symptom scales. J. Voice 2007, 21, 728–734. [Google Scholar] [CrossRef]
- Arffa, R.E.; Krishna, P.; Gartner-Schmidt, J.; Rosen, C.A. Normative values for the voice handicap index-10. J. Voice 2012, 26, 462–465. [Google Scholar] [CrossRef]
- Dejonckere, P.H.; Bradley, P.; Clemente, P.; Cornut, G.; Crevier-Buchman, L.; Friedrich, G.; Van De Heyning, P.; Remacle, M.; Woisard, V. A basic protocol for functional assessment of voice pathology, especially for investigating the efficacy of (phonosurgical) treatments and evaluating new assessment techniques: Guideline elaborated by the Committee on Phoniatrics of the European Laryngolo. Eur. Arch. Oto-Rhino-Laryngol. 2001, 258, 77–82. [Google Scholar] [CrossRef]
- Rogers, S.N.; Lowe, D.; Yueh, B.; Weymuller, E.A. The physical function and social-emotional function subscales of the University of Washington quality of life questionnaire. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 352–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.J.; Weymuller, E.A. Assessment of quality of life in head and neck cancer patients. Head Neck 1993, 15, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Bedre, R. Bioinfokit: Bioinformatics Data Analysis and Visualization Toolkit. Available online: https://github.com/reneshbedre/bioinfokit/tree/v0.6#readme (accessed on 10 April 2020).
- Varghese, B.T.; Mathew, A.; Sebastian, P.; Iype, E.M.; Vijay, A. Comparison of quality of life between voice rehabilitated and nonrehabilitated laryngectomies in a developing world community. Acta Otolaryngol. 2011, 131, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Finizia, C.; Bergman, B. Health-related quality of life in patients with laryngeal cancer: A post-treatment comparison of different modes of communication. Laryngoscope 2001, 111, 918–923. [Google Scholar] [CrossRef]
- Lewin, J.S.; Baumgart, L.M.; Barrow, M.P.; Hutcheson, K.A. Device Life of the Tracheoesophageal Voice Prosthesis Revisited. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 65–71. [Google Scholar] [CrossRef]
- Van As, C.J.; Hilgers, F.J.M.; Verdonck-de Leeuw, I.M.; Koopmans-van Beinum, F.J. Acoustical analysis and perceptual evaluation of tracheoesophageal prosthetic voice. J. Voice 1998, 12, 239–248. [Google Scholar] [CrossRef]
- Štajner-Katušić, S.; Horga, D.; Mušura, M.; Globlek, D. Voice and speech after laryngectomy. Clin. Linguist. Phon. 2006, 20, 195–203. [Google Scholar] [CrossRef]
- Silverman, D.A.; Puram, S.V.; Rocco, J.W.; Old, M.O.; Kang, S.Y. Salvage laryngectomy following organ-preservation therapy—An evidence-based review. Oral Oncol. 2019, 88, 137–144. [Google Scholar] [CrossRef]
- Yeh, D.H.; Sahovaler, A.; Fung, K. Reconstruction after salvage laryngectomy. Oral Oncol. 2017, 75, 22–27. [Google Scholar] [CrossRef]
- Galli, A.; Giordano, L.; Biafora, M.; Tulli, M.; Di Santo, D.; Bussi, M. Voice prosthesis rehabilitation after total laryngectomy: Are satisfaction and quality of life maintained over time? Acta Otorhinolaryngol. Ital. 2019, 39, 162–168. [Google Scholar] [CrossRef]
| Variables | Categories | Total | J-Flap | TEP | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Age | 60.1 | 8.6 | 58.33 | 9.39 | 61.7 | 7.71 | 0.2 | |
| Height | 1.69 | 0.06 | 1.65 | 0.05 | 1.72 | 0.04 | <0.001 | |
| Weight | 67.65 | 9.72 | 62.82 | 8.63 | 72 | 8.68 | 0.002 | |
| BMI | 23.53 | 2.69 | 22.96 | 2.82 | 24.05 | 2.53 | 0.22 | |
| No. | % | No. | % | No. | % | |||
| Sex | Male | 38 | 100 | 18 | 100 | 20 | 100 | |
| Female | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Tumor Site | Larynx | 22 | 57.9 | 4 | 22.2 | 18 | 90 | <0.001 |
| Pharynx | 16 | 42.1 | 14 | 77.8 | 2 | 10 | ||
| Tumor | Primary | 16 | 42.1 | 4 | 22.2 | 12 | 60 | 0.019 |
| Recurrence | 22 | 57.9 | 14 | 77.8 | 8 | 40 | ||
| Radiotherapy | Preoperative | 19 | 50 | 15 | 83.3 | 4 | 20 | <0.001 |
| Postoperative | 18 | 47.4 | 3 | 16.7 | 15 | 75 | ||
| Speech Pathologist Evaluation | J-Flap Group | TEP Group | p-Value |
|---|---|---|---|
| Speech intelligibility | |||
| NTID scale | 3.50 ± 0.63 | 4.25 ± 1.11 | 0.005 |
| GIRBAS | |||
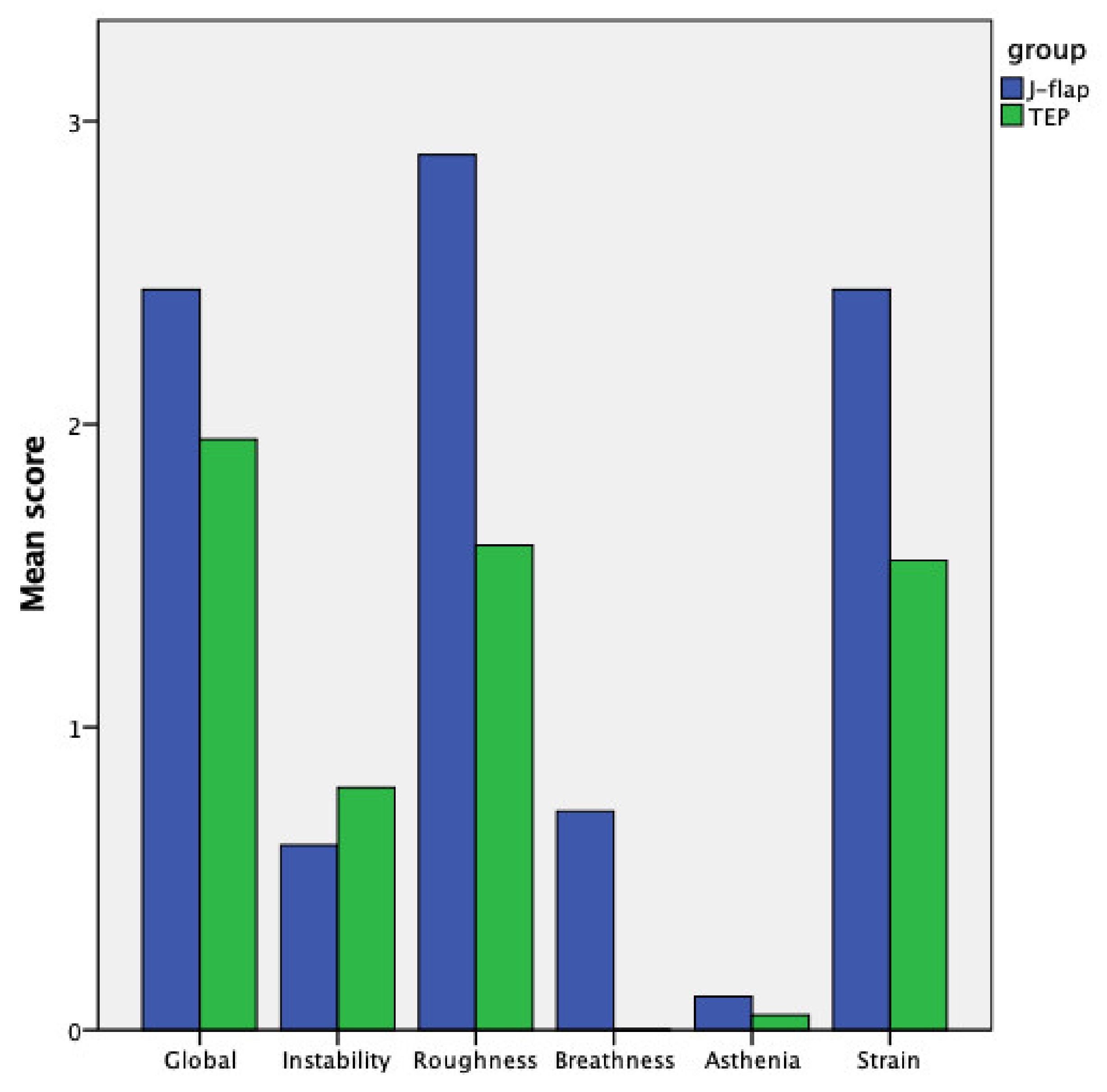
| Voice quality Globally | 2.446 ± 0.85 | 1.95 ± 0.82 | 0.04 |
| Voice quality Instability | 0.61 ± 0.97 | 0.80 ± 1.00 | 0.43 |
| Voice quality Roughness | 2.89 ± 0.47 | 1.60 ± 0.68 | <0.001 |
| Voice quality Breathiness | 0.72 ± 1.22 | 0.00 ± 0.00 | 0.01 |
| Voice quality Asthenia | 0.11 ± 0.32 | 0.05 ± 0.22 | 0.49 |
| Voice quality Strain | 2.44 ± 0.92 | 1.55 ± 1.05 | 0.009 |
| Speech accuracy | |||
| Vowel correct rate (%) | 96.66 ± 6.51 | 93.54 ± 17.19 | 0.29 |
| Consonant correct rate (%) | 65.77 ± 11.02 | 86.02 ± 20.79 | <0.001 |
| Word correct rate (%) | 69.27 ± 11.52 | 77.56 ± 22.23 | 0.185 |
| Acoustic and Phonatory Parameters | J-Flap Group | TEP Group | p-Value |
|---|---|---|---|
| Phonatory performances | |||
| Maximum phonation time (s) | 12.66 ± 7.36 | 11.26 ± 5.90 | 0.53 |
| S/Z ratio | 1.06 ± 0.78 | 1.24 ± 0.35 | 0.43 |
| Acoustic voice analysis | |||
| Fundamental frequency (Hz) | 183.15 ± 114.21 | 270.20 ± 183.80 | 0.12 |
| Jitter (%) | 9.66 ± 6.99 | 9.78 ± 7.38 | 0.96 |
| Shimmer (dB) | 1.90 ± 0.96 | 1.66 ± 0.72 | 0.39 |
| Harmonic-to-noise ratio | 2.13 ± 1.11 | 1.88 ± 0.91 | 0.50 |
| Voice Handicap Index | J-Flap Group | TEP Group | p-Value |
|---|---|---|---|
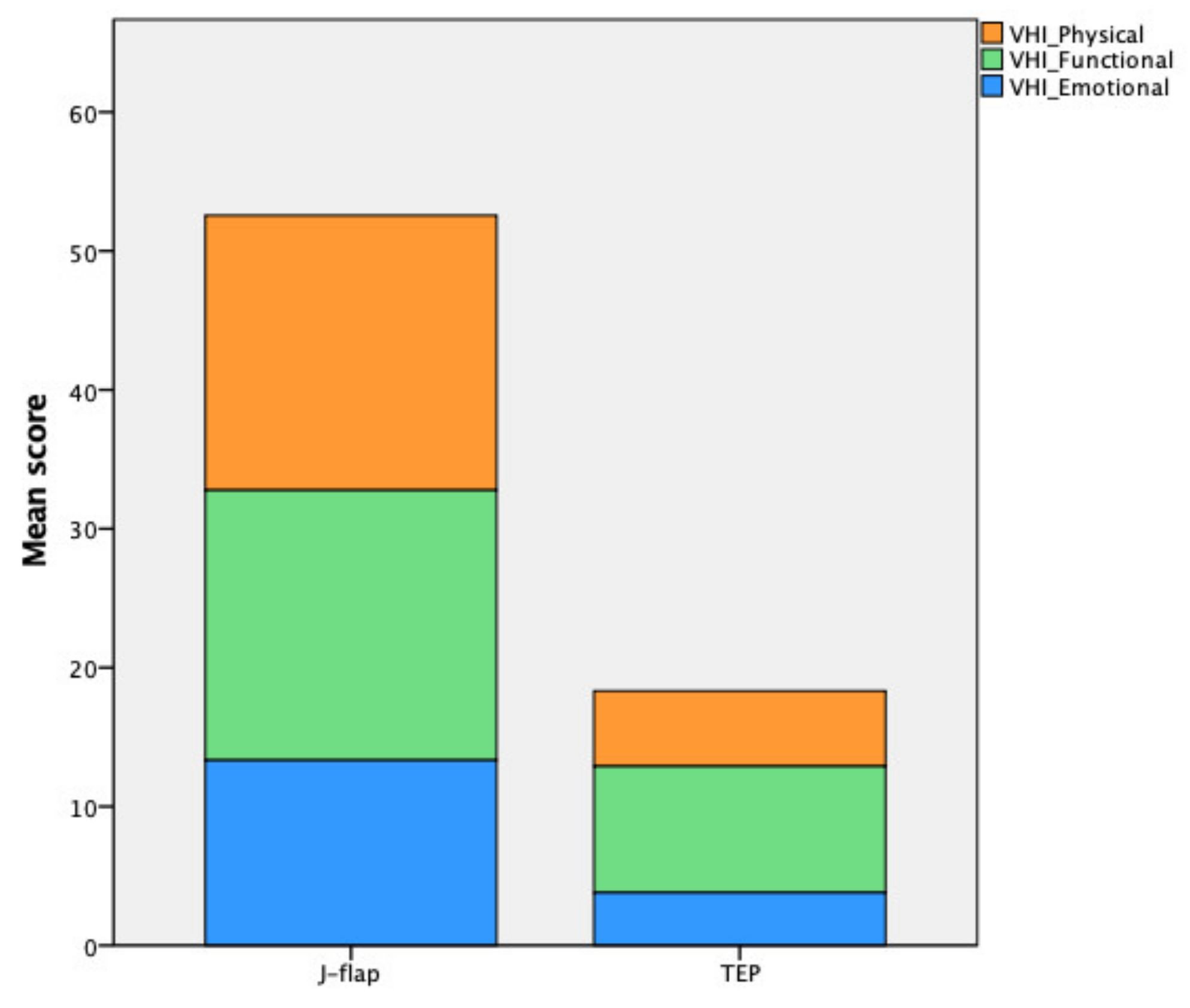
| Physical score | 19.78 ± 8.57 | 5.42 ± 3.87 | <0.001 |
| Functional score | 19.44 ± 10.25 | 9.11 ± 6.07 | 0.0001 |
| Emotional score | 13.33 ± 9.74 | 3.79 ± 4.46 | <0.001 |
| VHI total score | 52.56 ± 26.78 | 18.32 ± 11.62 | <0.001 |
| University of Washington Quality of Life (QoL) Questionnaire | J-Flap Group | TEP Group | p-Value |
|---|---|---|---|
| Domain | |||
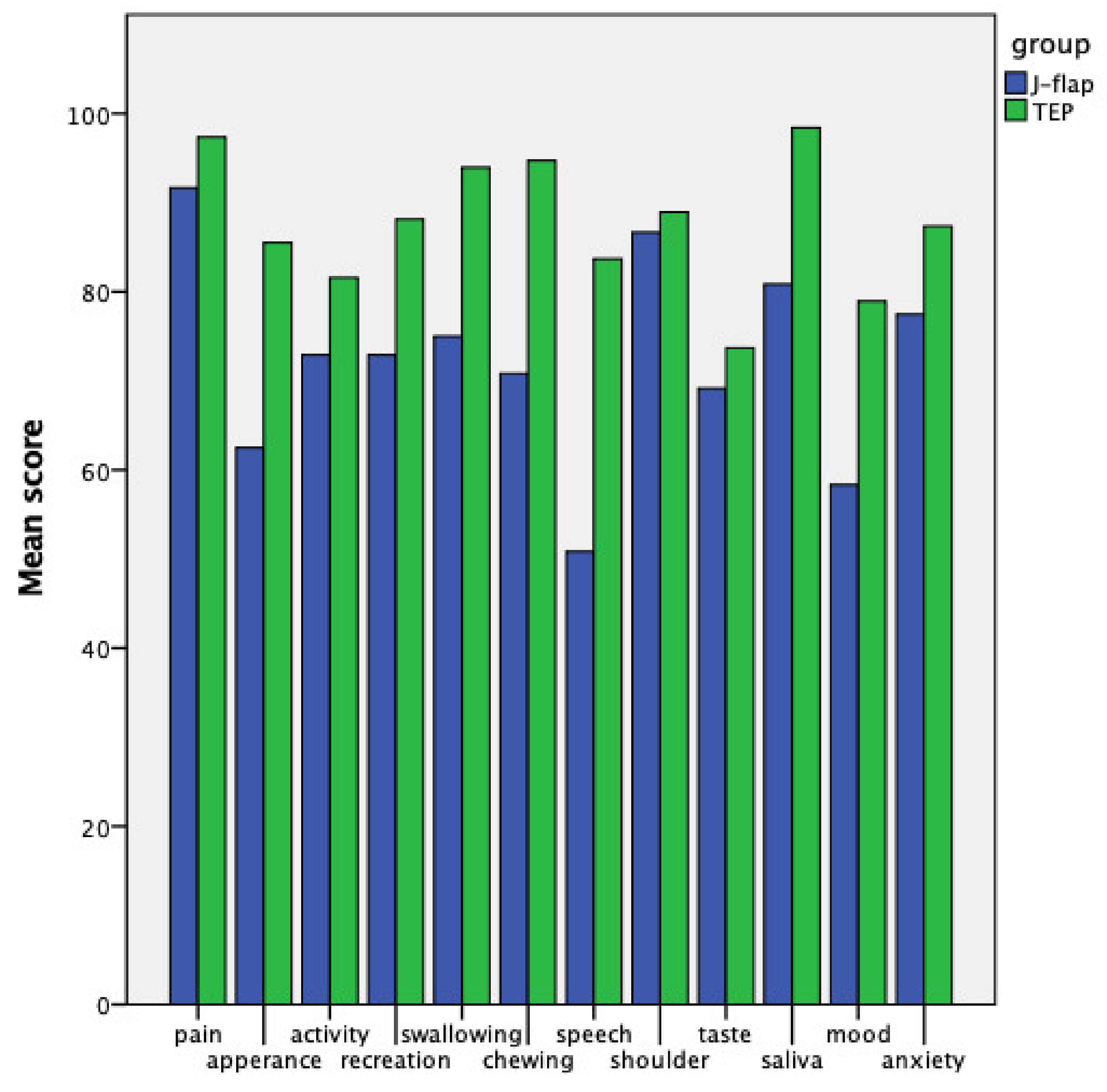
| Physical function subdomain | 68.19 ± 21.65 | 88.33 ± 9.46 | 0.009 |
| Social-emotional function subdomain | 76.67 ± 19.14 | 87.06 ± 7.65 | 0.09 |
| QoL composite | 72.43 ± 18.30 | 87.69 ± 6.42 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsao, C.-K.; Marchi, F.; Kang, C.-J.; Sampieri, C.; Lu, Y.-A.; Huang, S.-F.; Chen, Y.-T.; Giordano, G.; Peretti, G.; Parrinello, G.; et al. Comprehensive Evaluation of Vocal Outcomes and Quality of Life after Total Laryngectomy and Voice Restoration with J-Flap and Tracheoesophageal Puncture. Cancers 2022, 14, 544. https://doi.org/10.3390/cancers14030544
Tsao C-K, Marchi F, Kang C-J, Sampieri C, Lu Y-A, Huang S-F, Chen Y-T, Giordano G, Peretti G, Parrinello G, et al. Comprehensive Evaluation of Vocal Outcomes and Quality of Life after Total Laryngectomy and Voice Restoration with J-Flap and Tracheoesophageal Puncture. Cancers. 2022; 14(3):544. https://doi.org/10.3390/cancers14030544
Chicago/Turabian StyleTsao, Chung-Kan, Filippo Marchi, Chung-Jan Kang, Claudio Sampieri, Yi-An Lu, Shiang-Fu Huang, Yu-Ting Chen, Giorgio Giordano, Giorgio Peretti, Giampiero Parrinello, and et al. 2022. "Comprehensive Evaluation of Vocal Outcomes and Quality of Life after Total Laryngectomy and Voice Restoration with J-Flap and Tracheoesophageal Puncture" Cancers 14, no. 3: 544. https://doi.org/10.3390/cancers14030544
APA StyleTsao, C.-K., Marchi, F., Kang, C.-J., Sampieri, C., Lu, Y.-A., Huang, S.-F., Chen, Y.-T., Giordano, G., Peretti, G., Parrinello, G., Iandelli, A., & Fang, T.-J. (2022). Comprehensive Evaluation of Vocal Outcomes and Quality of Life after Total Laryngectomy and Voice Restoration with J-Flap and Tracheoesophageal Puncture. Cancers, 14(3), 544. https://doi.org/10.3390/cancers14030544






