Pathological Response in the Breast and Axillary Lymph Nodes after Neoadjuvant Systemic Treatment in Patients with Initially Node-Positive Breast Cancer Correlates with Disease Free Survival: An Exploratory Analysis of the GeparOcto Trial
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Objectives
2.3. Assessment of Endpoints
2.4. Statistical Analysis
3. Results
3.1. Patient and Tumor Baseline Characteristics
3.2. Management of Axillary Lymph Nodes after Neoadjuvant Systemic Treatment
3.3. Pathological Response after NAST
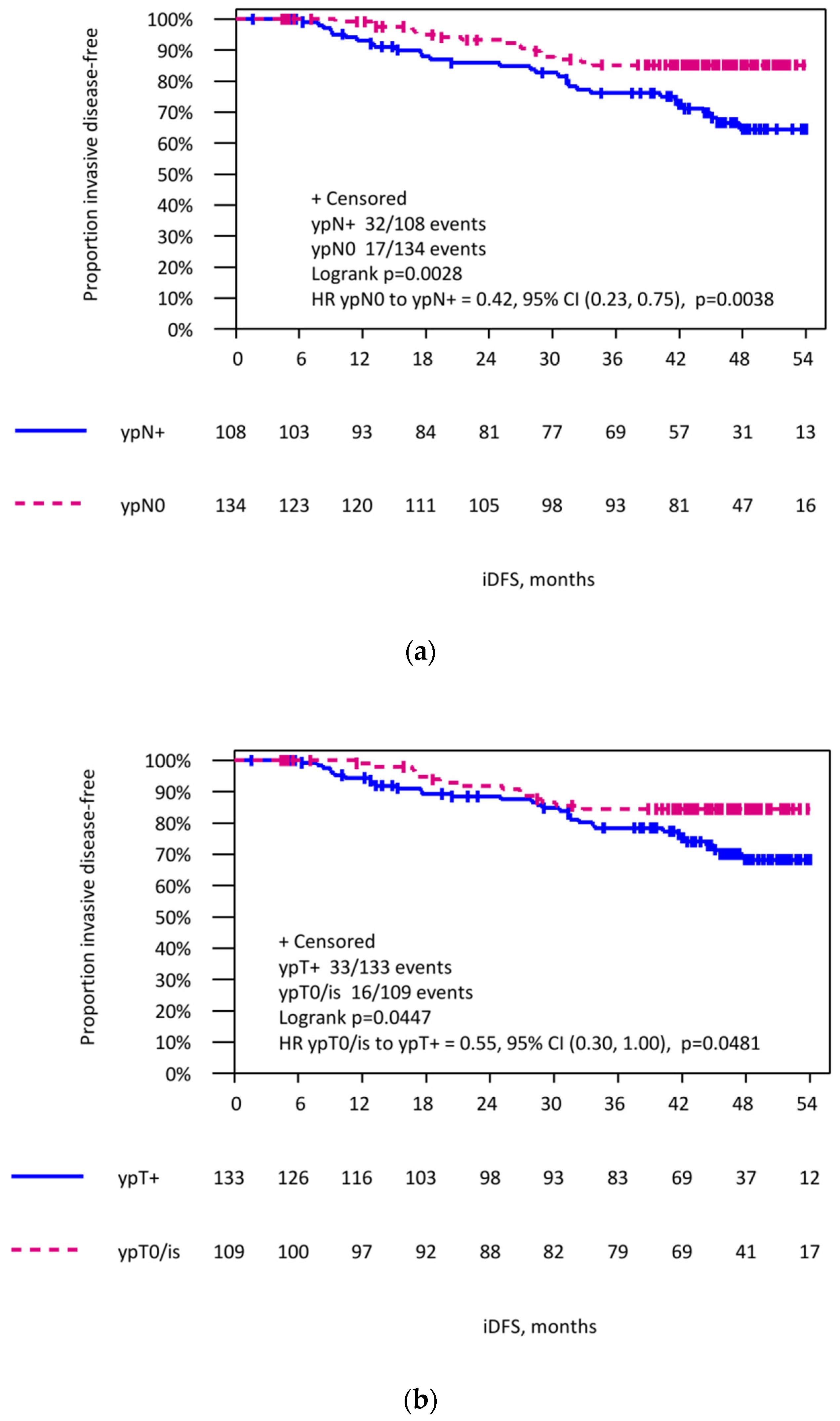
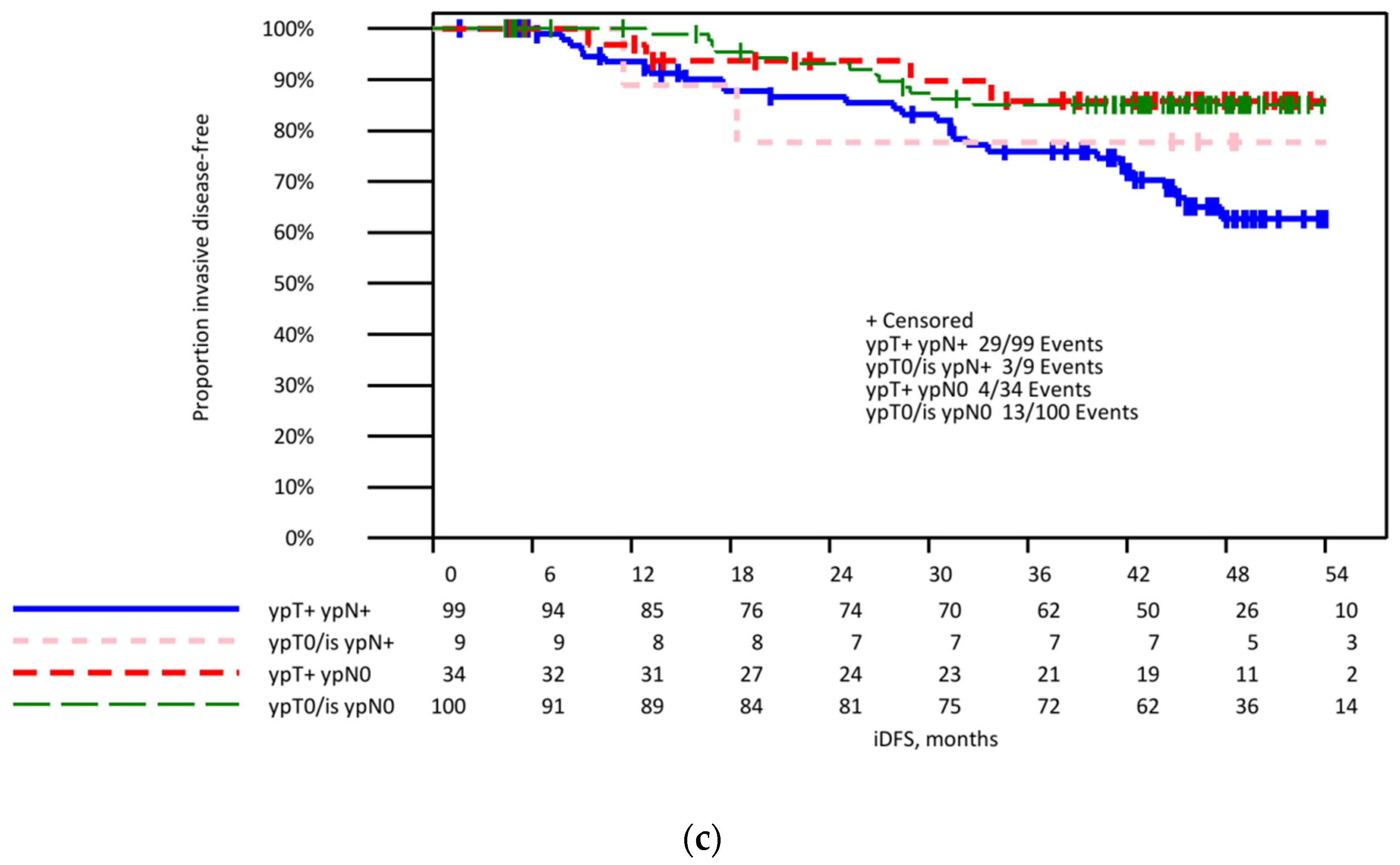
3.4. Invasive Disease-Free Survival in Patients with Initially Lymph Node-Positive Breast Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of axillary dissection vs. no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The acosog z0011 (alliance) randomized clinical trial. JAMA 2017, 318, 918–926. [Google Scholar] [CrossRef]
- Reimer, T.; Stachs, A.; Nekljudova, V.; Loibl, S.; Hartmann, S.; Wolter, K.; Hildenrandt, G.; Gerber, B. Restricted axillary staging in clinically and sonographically node-negative early invasive breast cancer (c/i t1-2) in the context of breast concerving therapy: First results following commencement of the intergroup-sentinel-mamma (insema) trial. Geburtsh Frauenheilk 2017, 77, 149–157. [Google Scholar] [PubMed] [Green Version]
- Gion, M.; Pérez-García, J.M.; Llombart-Cussac, A.; Sampayo-Cordero, M.; Cortés, J.; Malfettone, A. Surrogate endpoints for early-stage breast cancer: A review of the state of the art, controversies, and future prospects. Ther. Adv. Med. Oncol. 2021, 13, 17588359211059587. [Google Scholar] [CrossRef] [PubMed]
- Chiec, L.; Shah, A.N. Risk-based Approaches for Optimizing Treatment in HER2-Positive Early Stage Breast Cancer. Semin. Oncol. 2020, 47, 249–258. [Google Scholar] [CrossRef]
- Cortazar, P.; Geyer, C.E., Jr. Pathological complete response in neoadjuvant treatment of breast cancer. Ann. Surg. Oncol. 2015, 22, 1441–1446. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018, 19, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Cirier, J.; Body, G.; Jourdan, M.L.; Bedouet, L.; Fleurier, C.; Pilloy, J.; Arbion, F.; Ouldamer, L. Impact of pathological complete response to neoadjuvant chemotherapy in invasive breast cancer according to molecular subtype. Gynecol. Obstet. Fertil. Senol. 2017, 45, 535–544. [Google Scholar] [PubMed]
- Gustavo Werutsky, G.; Untch, M.; Hanusch, C.; Fasching, P.A.; Blohmer, J.U.; Seiler, S.; Denkert, C.; Tesch, H.; Jackisch, C.; Gerber, B.; et al. Locoregional recurrence risk after neoadjuvant chemotherapy: A pooled analysis of nine prospective neoadjuvant breast cancer trials. Eur. J. Cancer 2020, 130, 92–101. [Google Scholar] [CrossRef]
- Kuehn, T.; Bauerfeind, I.; Fehm, T.; Fleige, B.; Hausschild, M.; Helms, G.; Lebeau, A.; Liedtke, C.; von Minckwitz, G.; Nekljudova, V.; et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (sentina): A prospective, multicentre cohort study. Lancet Oncol. 2013, 14, 609–618. [Google Scholar] [CrossRef]
- Boughey, J.C.; Suman, V.J.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Leitch, A.M.; Kuerer, H.M.; Bowling, M.; Flippo-Morton, T.S.; et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The acosog z1071 (alliance) clinical trial. JAMA 2013, 310, 1455–1461. [Google Scholar] [CrossRef] [Green Version]
- Carter, S.; Neuman, H.; Mamounas, E.P.; Bedrosian, I.; Moulder, S.; Montero, A.J.; Jagsi, R. Debating the optimal approach to nodal management after pathologic complete response to neoadjuvant chemotherapy in patients with breast cancer. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 42–48. [Google Scholar] [CrossRef]
- Simons, J.M.; van Nijnatten, T.J.A.; van der Pol, C.C.; Luiten, E.J.T.; Koppert, L.B.; Smidt, M.L. Diagnostic accuracy of different surgical procedures for axillary staging after neoadjuvant systemic therapy in node-positive breast cancer: A systematic review and meta-analysis. Ann. Surg. 2019, 269, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Tee, S.R.; Devane, L.A.; Evoy, D.; Rothwell, J.; Geraghty, J.; Prichard, R.S.; McDermott, E.W. Meta-analysis of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initial biopsy-proven node-positive breast cancer. Br. J. Surg. 2018, 105, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, A.; Mobus, V.; Tesch, H.; Hanusch, C.; Denkert, C.; Lubbe, K.; Huober, J.; Klare, P.; Kummel, S.; Untch, M.; et al. Intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer) for neoadjuvant treatment of high-risk early breast cancer (geparocto-gbg 84): A randomised phase iii trial. Eur. J. Cancer 2019, 106, 181–192. [Google Scholar] [PubMed]
- Barron, A.U.; Hoskin, T.L.; Day, C.N.; Hwang, E.S.; Kuerer, H.M.; Boughey, J.C. Association of low nodal positivity rate among patients with erbb2-positive or triple-negative breast cancer and breast pathologic complete response to neoadjuvant chemotherapy. JAMA Surg. 2018, 153, 1120–1126. [Google Scholar] [CrossRef] [Green Version]
- Samiei, S.; Simons, J.M.; Engelen, S.M.E.; Beets-Tan, R.G.H.; Classe, J.M.; Smidt, M.L. Axillary pathologic complete response after neoadjuvant systemic therapy by breast cancer subtype in patients with initially clinically node-positive disease: A systematic review and meta-analysis. JAMA Surg. 2021, 156, e210891. [Google Scholar] [CrossRef]
- Tadros, A.B.; Yang, W.T.; Krishnamurthy, S.; Rauch, G.M.; Smith, B.D.; Valero, V.; Black, D.M.; Lucci, A., Jr.; Caudle, A.S.; DeSnyder, S.M.; et al. Identification of patients with documented pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery. JAMA Surg. 2017, 152, 665–670. [Google Scholar] [CrossRef]
- Samiei, S.; van Nijnatten, T.J.A.; de Munck, L.; Keymeulen, K.; Simons, J.M.; Kooreman, L.F.S.; Siesling, S.; Lobbes, M.B.I.; Smidt, M.L. Correlation between pathologic complete response in the breast and absence of axillary lymph node metastases after neoadjuvant systemic therapy. Ann. Surg. 2018, 271, 574–580. [Google Scholar] [CrossRef]
- Kuemmel, S.; Heil, J.; Rueland, A.; Seiberling, C.; Harrach, H.; Schindowski, D.; Lubitz, J.; Hellerhoff, K.; Ankel, C.; Graßhoff, S.T.; et al. A prospective, multicenter registry study to evaluate the clinical feasibility of targeted axillary dissection (tad) in node-positive breast cancer patients. Ann. Surg. 2020. online ahead of print. [Google Scholar] [CrossRef]
- Banys-Paluchowski, M.; Gasparri, M.L.; de Boniface, J.; Gentilini, O.; Stickeler, E.; Hartmann, S.; Thill, M.; Rubio, I.T.; Di Micco, R.; Bonci, E.A.; et al. Surgical management of the axilla in clinically node-positive breast cancer patients converting to clinical node negativity through neoadjuvant chemotherapy: Current status, knowledge gaps, and rationale for the eubreast-03 axsana study. Cancers 2021, 13, 1565. [Google Scholar] [CrossRef]
- Hartmann, S.; Kühn, T.; de Boniface, J.; Stachs, A.; Winckelmann, A.; Frisell, J.; Wiklander-Bråkenhielm, I.; Stubert, J.; Gerber, B.; Reimer, T. Carbon tattooing for targeted lymph node biopsy after primary systemic therapy in breast cancer: Prospective multicentre tattoo trial. Br. J. Surg. 2021, 108, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Barrio, A.V.; Montagna, G.; Mamtani, A.; Sevilimedu, V.; Edelweiss, M.; Capko, D.; Cody, H.S., 3rd; El-Tamer, M.; Gemignani, M.L.; Heerdt, A.; et al. Nodal recurrence in patients with node-positive breast cancer treated with sentinel node biopsy alone after neoadjuvant chemotherapy-a rare event. JAMA Oncol. 2021, 7, 1851–1855. [Google Scholar] [CrossRef]
- Wong, S.M.; Almana, N.; Choi, J.; Hu, J.; Gagnon, H.; Natsuhara, K.; Shen, A.H.; DeSantis, S.; Dominici, L.; Golshan, M.; et al. Prognostic Significance of Residual Axillary Nodal Micrometastases and Isolated Tumor Cells After Neoadjuvant Chemotherapy for Breast Cancer. Ann. Surg. Oncol. 2019, 26, 3502–3509. [Google Scholar] [CrossRef]
- Canavese, G.; Tinterri, C.; Carli, F.; Garrone, E.; Spinaci, S.; Della Valle, A.; Barbieri, E.; Marrazzo, E.; Bruzzi, P.; Dozin, B. Correlation between outcome and extent of residual disease in the sentinel node after neoadjuvant chemotherapy in clinically fine-needle proven node-positive breast cancer patients. Eur. J. Surg. Oncol. 2021, 47, 1920–1927. [Google Scholar] [CrossRef]
- Moo, T.A.; Jochelson, M.S.; Zabor, E.C.; Stempel, M.; Raiss, M.; Mamtani, A.; Tadros, A.B.; El-Tamer, M.; Morrow, M. Is clinical exam of the axilla sufficient to select node-positive patients who downstage after nac for slnb? A comparison of the accuracy of clinical exam versus mri. Ann. Surg. Oncol. 2019, 26, 4238–4243. [Google Scholar] [CrossRef] [PubMed]
- Boughey, J.C.; Ballman, K.V.; Hunt, K.K.; McCall, L.M.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Le-Petross, H.T. Axillary ultrasound after neoadjuvant chemotherapy and its impact on sentinel lymph node surgery: Results from the american college of surgeons oncology group z1071 trial (alliance). J. Clin. Oncol. 2015, 33, 3386–3393. [Google Scholar] [CrossRef] [Green Version]
- Schwentner, L.; Helms, G.; Nekljudova, V.; Ataseven, B.; Bauerfeind, I.; Ditsch, N.; Fehm, T.; Fleige, B.; Hauschild, M.; Heil, J.; et al. Using ultrasound and palpation for predicting axillary lymph node status following neoadjuvant chemotherapy—Results from the multi-center sentina trial. Breast 2017, 31, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Le-Petross, H.T.; McCall, L.M.; Hunt, K.K.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Ballman, K.V.; Boughey, J.C. Axillary ultrasound identifies residual nodal disease after chemotherapy: Results from the american college of surgeons oncology group z1071 trial (alliance). AJR Am. J. Roentgenol. 2018, 210, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Kim, H.J.; Park, H.Y.; Park, J.Y.; Chae, Y.S.; Lee, S.M.; Cho, S.H.; Shin, K.M.; Lee, S.Y. Axillary pathologic complete response to neoadjuvant chemotherapy in clinically node-positive breast cancer patients: A predictive model integrating the imaging characteristics of ultrasound restaging with known clinicopathologic characteristics. Ultrasound Med. Biol. 2019, 45, 702–709. [Google Scholar] [CrossRef]
- Liedtke, C.; Gorlich, D.; Bauerfeind, I.; Fehm, T.; Fleige, B.; Helms, G.; Lebeau, A.; Staebler, A.; Ataseven, B.; Denkert, C.; et al. Validation of a nomogram predicting non-sentinel lymph node metastases among patients with breast cancer after primary systemic therapy—A transsentina substudy. Breast Care 2018, 13, 440–446. [Google Scholar] [CrossRef]
- Kantor, O.; Sipsy, L.M.; Yao, K.; James, T.A. A predictive model for axillary node pathologic complete response after neoadjuvant chemotherapy for breast cancer. Ann. Surg. Oncol. 2018, 25, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
| Biological Subtype | ypN0 N (%) | ypN+ N (%) | p-Value a | |
|---|---|---|---|---|
| ycN0 | luminal HER2- (N = 58) | 15 (25.9) | 43 (74.1) | <0.001 |
| TNBC (N = 36) | 27 (75.0) | 9 (25.0) | ||
| HER2+ (N = 38) | 31 (81.6) | 7 (18.4) | ||
| Overall (N = 132) | 73 (55.3) | 59 (44.7) | ||
| ycN+ | luminal HER2- (N = 35) | 14 (40.0) | 21 (60.0) | 0.010 |
| TNBC (N = 21) | 13 (61.9) | 8 (38.1) | ||
| HER2+ (N = 21) | 17 (81.0) | 4 (19.0) | ||
| Overall (N = 77) | 44 (57.1) | 33 (42.9) | ||
| ycNx * | luminal HER2- (N = 17) | 6 (35.3) | 11 (64.7) | 0.080 |
| TNBC (N = 6) | 3 (50.0) | 3 (50.0) | ||
| HER2+ (N = 10) | 8 (80.0) | 2 (20.0) | ||
| Overall (N = 33) | 17 (51.5) | 16 (48.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerber, B.; Schneeweiss, A.; Möbus, V.; Golatta, M.; Tesch, H.; Krug, D.; Hanusch, C.; Denkert, C.; Lübbe, K.; Heil, J.; et al. Pathological Response in the Breast and Axillary Lymph Nodes after Neoadjuvant Systemic Treatment in Patients with Initially Node-Positive Breast Cancer Correlates with Disease Free Survival: An Exploratory Analysis of the GeparOcto Trial. Cancers 2022, 14, 521. https://doi.org/10.3390/cancers14030521
Gerber B, Schneeweiss A, Möbus V, Golatta M, Tesch H, Krug D, Hanusch C, Denkert C, Lübbe K, Heil J, et al. Pathological Response in the Breast and Axillary Lymph Nodes after Neoadjuvant Systemic Treatment in Patients with Initially Node-Positive Breast Cancer Correlates with Disease Free Survival: An Exploratory Analysis of the GeparOcto Trial. Cancers. 2022; 14(3):521. https://doi.org/10.3390/cancers14030521
Chicago/Turabian StyleGerber, Bernd, Andreas Schneeweiss, Volker Möbus, Michael Golatta, Hans Tesch, David Krug, Claus Hanusch, Carsten Denkert, Kristina Lübbe, Jörg Heil, and et al. 2022. "Pathological Response in the Breast and Axillary Lymph Nodes after Neoadjuvant Systemic Treatment in Patients with Initially Node-Positive Breast Cancer Correlates with Disease Free Survival: An Exploratory Analysis of the GeparOcto Trial" Cancers 14, no. 3: 521. https://doi.org/10.3390/cancers14030521
APA StyleGerber, B., Schneeweiss, A., Möbus, V., Golatta, M., Tesch, H., Krug, D., Hanusch, C., Denkert, C., Lübbe, K., Heil, J., Huober, J., Ataseven, B., Klare, P., Hahn, M., Untch, M., Kast, K., Jackisch, C., Thomalla, J., Seither, F., ... Kühn, T. (2022). Pathological Response in the Breast and Axillary Lymph Nodes after Neoadjuvant Systemic Treatment in Patients with Initially Node-Positive Breast Cancer Correlates with Disease Free Survival: An Exploratory Analysis of the GeparOcto Trial. Cancers, 14(3), 521. https://doi.org/10.3390/cancers14030521









