Bronchial Carcinoids: From Molecular Background to Treatment Approach
Abstract
Simple Summary
Abstract
1. Introduction
2. Molecular and Genetic Background of Bronchial Carcinoids
2.1. Differential Molecular Phenotype of Lung Neuroendocrine Carcinomas and Bronchial Carcinoids
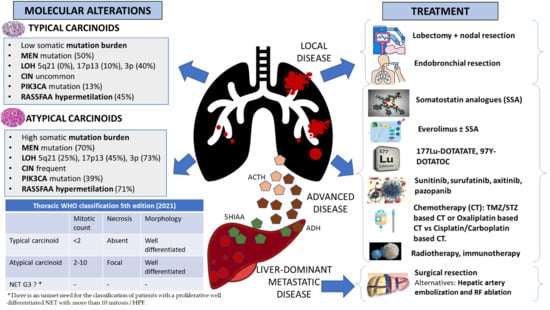
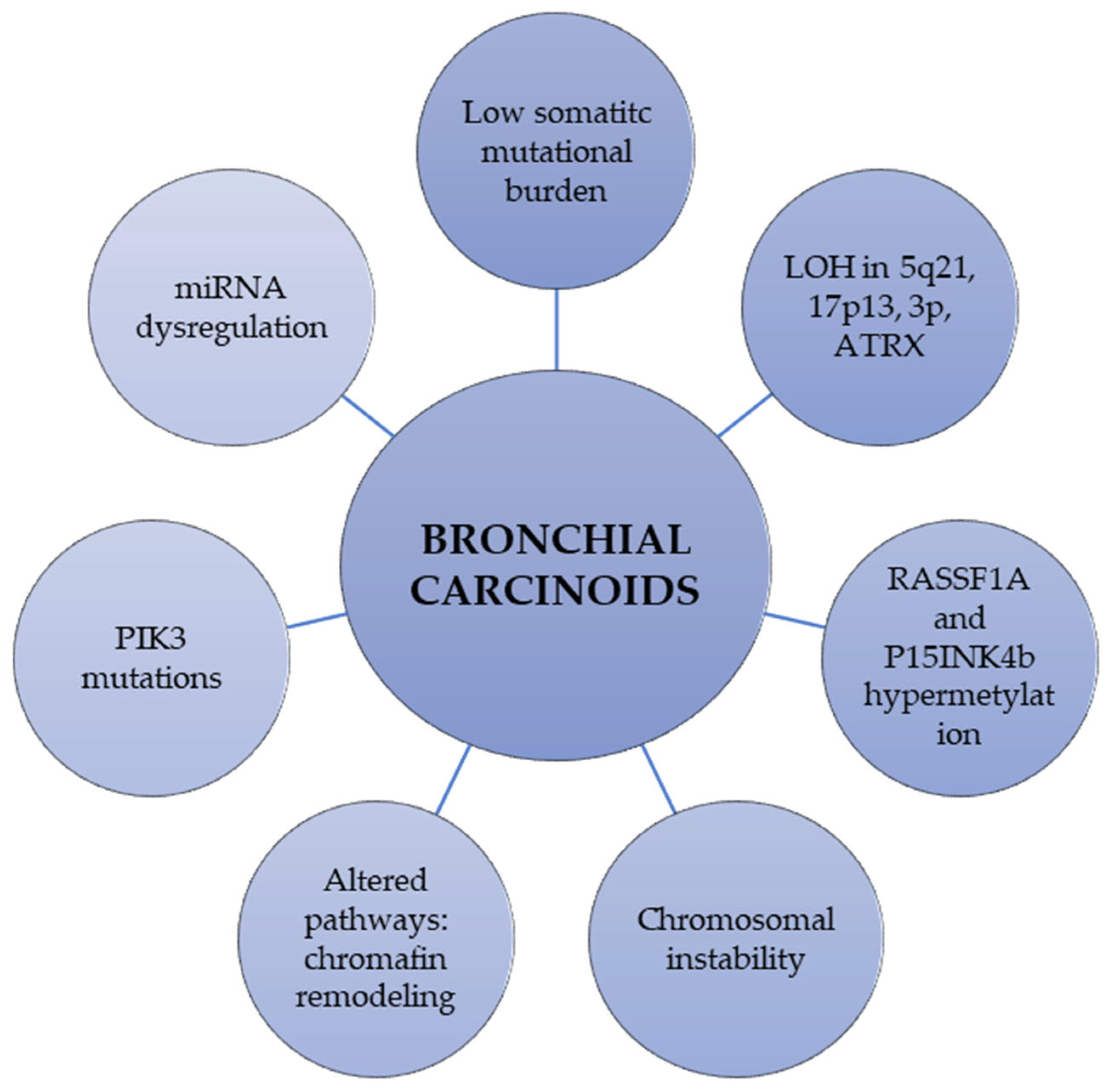
2.2. Molecular Alterations and Epigenetic Changes in Bronchial Carcinoids
| Molecular/Genetic Alteration | Description of the Findings in Bronchial Carcinoids |
|---|---|
| Mutations in chromatin-remodeling genes | Covalent histone modifiers and subunits of the SWI/SNF complex are mutated in 40% and 22.2% of BC, respectively [32]. Allelic losses of the MEN1 locus in 36% of sporadic BC |
| Somatic mutational burden | BC showed a lower mean number of mutations (TC, 0.7; AC, 1.8) than carcinomas (LCNEC, 4.6; SCLC, 5.8) [31] |
| Loss of heterozygosity (LOH) | LOH at 5q21 in 0% of TCs and 25% of ACs [37] LOH of 17p13 in 45% of ACs and 10% of TCs [38]. LOH of 3p in 40% of TCs, 73% of ACs [39]. LOH ATRX protein in 20% of BC [40], more common in AC than in TC. |
| Chromosomal instability (CIN) | CIN is increased in metastasized vs. non-metastasized carcinoids (gains 71% vs. 51%, losses 76% vs. 51%, cases with no chromosomal alterations 14% vs. 31%, respectively) [41] |
| Mutations in PIK3CA | Mutations in exon 9 and 20 in 13% of TCs and 39% of ACs [10] |
| Gene expression profiling | Upregulated genes: RET, ASPM, BIRC5, BUB1, CEP55, FANCA [42] Downregulated genes: OTP, PCK1, ASB4, FOLRI1, CD44 [42,43]. |
| Epigenetic changes | Promoter hypermethylation: RASSF1A [44] and P15INK4b [45] Histone modifications: Downregulation of H4KM20 and H4KA16 [46] miRNA upregulation: miR-129, -323-3p, -487b, -410, -369-3p, 376a [47] miRNA downregulation: miR-203, -224, -155, -302, -34b, -181b, -193a, -5p, -34b |
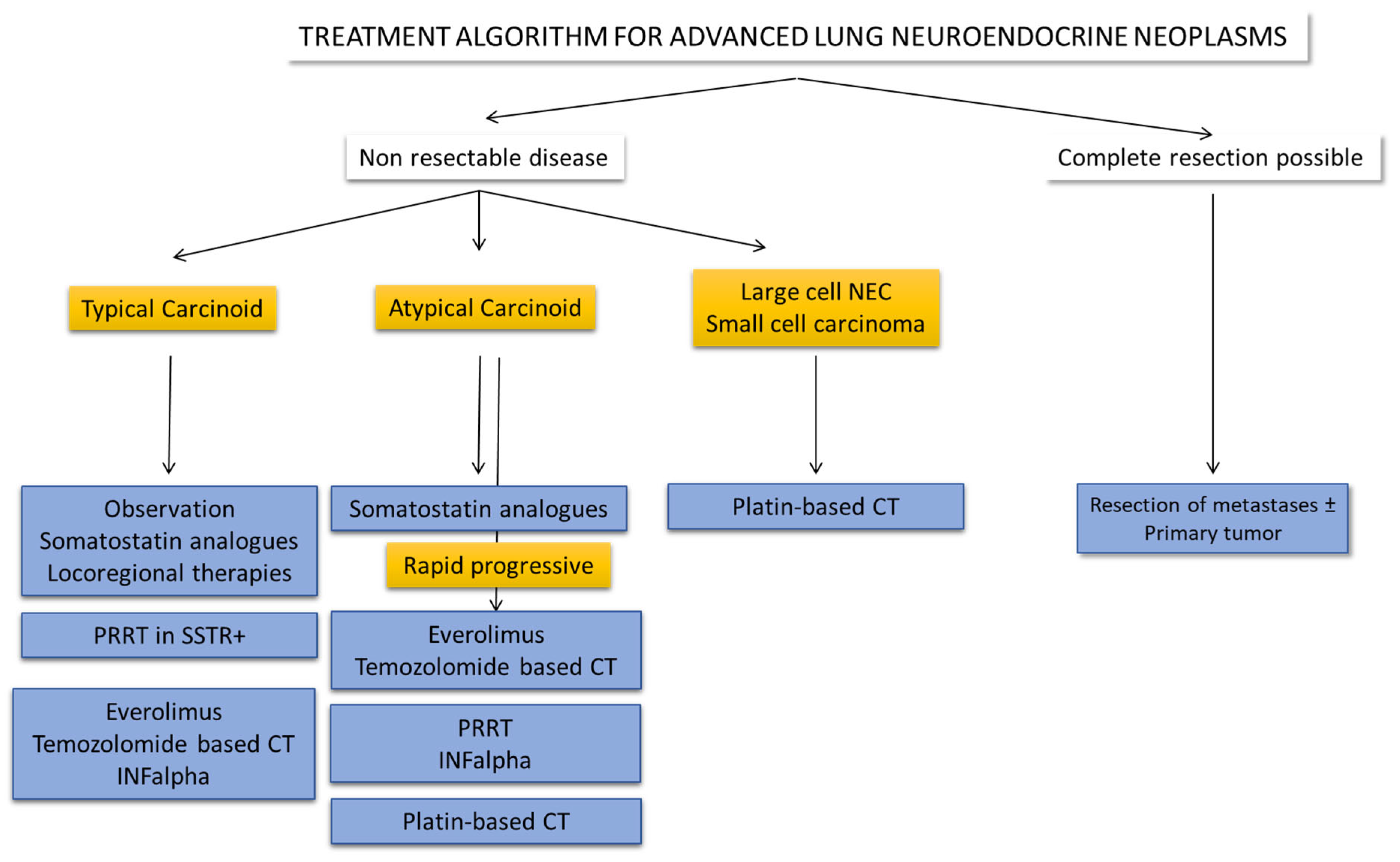
3. Treatment of Local Disease
3.1. Surgery
3.2. Endobronchial Resection
3.3. Other Treatments in Local Disease
4. Treatment of Advanced Disease
4.1. Somatostatin Analogues
4.2. Treatment of Liver-Dominant Metastatic Disease
4.3. Targeted Therapy
4.4. Peptide Receptor Radionuclide Therapy (PRRT)
4.5. Chemotherapy
4.6. Immunotherapy
4.7. Epigenetics
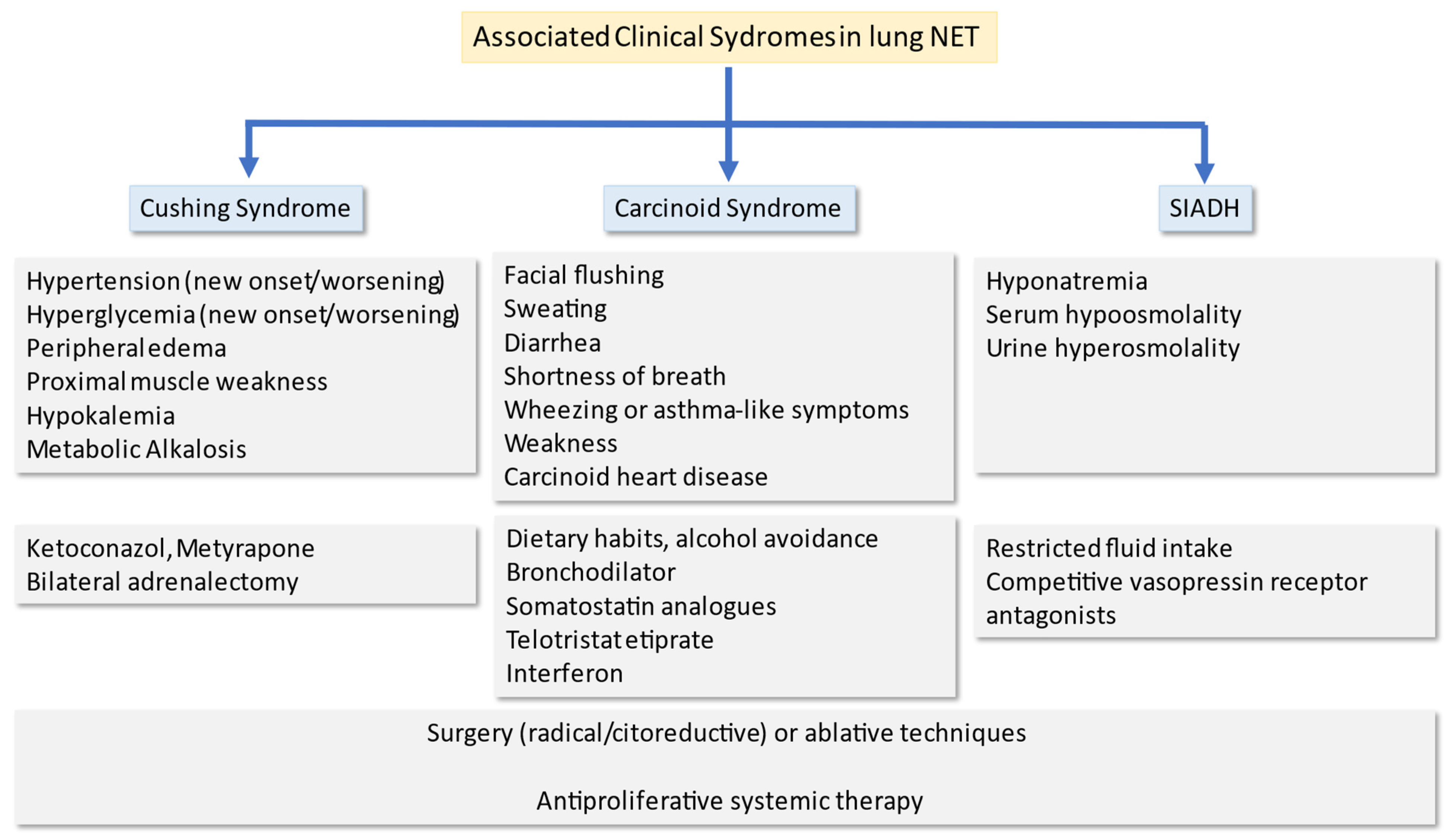
5. Treatment of Paraneoplastic Syndromes
5.1. Carcinoid Syndrome
5.2. Ectopic Cushing Syndrome
5.3. SIADH
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Travis, W.D. Lung tumours with neuroendocrine differentiation. Eur. J. Cancer 2009, 45, 251–266. [Google Scholar] [CrossRef]
- Modlin, I.M.; Lye, K.D.; Kidd, M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003, 97, 934–959. [Google Scholar] [CrossRef] [PubMed]
- Swarts, D.R.; Ramaekers, F.C.; Speel, E.-J.M. Molecular and cellular biology of neuroendocrine lung tumors: Evidence for separate biological entities. Biochim. Biophys. Acta Rev. Cancer 2012, 1826, 255–271. [Google Scholar] [CrossRef]
- Caplin, M.E.; Baudin, E.; Ferolla, P.; Filosso, P.; Garcia-Yuste, M.; Lim, E.; Oberg, K.; Pelosi, G.; Perren, A.; Rossi, R.E.; et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann. Oncol. 2015, 26, 1604–1620. [Google Scholar] [CrossRef] [PubMed]
- Sachithanandan, N.; Harle, R.A.; Burgess, J.R. Bronchopulmonary carcinoid in multiple endocrine neoplasia type 1. Cancer 2005, 103, 509–515. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Tazelaar, H.D.; Wentzlaff, K.A.; Kosugi, N.S.; Hai, N.; Benson, A.; Miller, D.L.; Yang, P. Familial pulmonary carcinoid tumors. Cancer 2001, 91, 2104–2109. [Google Scholar] [CrossRef]
- Swarts, D.R.A.; Scarpa, A.; Corbo, V.; Van Criekinge, W.; Van Engeland, M.; Gatti, G.; Henfling, M.E.R.; Papotti, M.; Perren, A.; Ramaekers, F.C.S.; et al. MEN1Gene Mutation and Reduced Expression Are Associated with Poor Prognosis in Pulmonary Carcinoids. J. Clin. Endocrinol. Metab. 2014, 99, E374–E378. [Google Scholar] [CrossRef]
- Terra, S.B.S.P.; Xie, H.; Boland, J.M.; Mansfield, A.S.; Molina, J.R.; Roden, A.C. Loss of ATRX expression predicts worse prognosis in pulmonary carcinoid tumors. Hum. Pathol. 2019, 94, 78–85. [Google Scholar] [CrossRef]
- Capodanno, A.; Boldrini, L.; Ali, G.; Pelliccioni, S.; Mussi, A.; Fontanini, G. Phosphatidylinositol-3-kinase α catalytic subunit gene somatic mutations in bronchopulmonary neuroendocrine tumours. Oncol. Rep. 2012, 28, 1559–1566. [Google Scholar] [CrossRef]
- Prinzi, N.; Rossi, R.E.; Proto, C.; Leuzzi, G.; Raimondi, A.; Torchio, M.; Milione, M.; Corti, F.; Colombo, E.; Prisciandaro, M.; et al. Recent Advances in the Management of Typical and Atypical Lung Carcinoids. Clin. Lung Cancer 2021, 22, 161–169. [Google Scholar] [CrossRef]
- Reuling, E.M.B.P.; Dickhoff, C.; Plaisier, P.W.; Coupe, V.; Mazairac, A.H.A.; Lely, R.J.; Bonjer, H.J.; Daniels, J.M.A. Endobronchial Treatment for Bronchial Carcinoid: Patient Selection and Predictors of Outcome. Respiration 2018, 95, 220–227. [Google Scholar] [CrossRef]
- Rinke, A.; Müller, H.-H.; Schade-Brittinger, C.; Klose, K.-J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.-F.; Bläker, M.C.; et al. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors: A Report from the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Ferolla, P.; Brizzi, M.P.; Meyer, T.; Mansoor, W.; Mazieres, J.; Cao, C.D.; Léna, H.; Berruti, A.; Damiano, V.; Buikhuisen, W.; et al. Efficacy and safety of long-acting pasireotide or everolimus alone or in combination in patients with advanced carcinoids of the lung and thymus (LUNA): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2017, 18, 1652–1664. [Google Scholar] [CrossRef]
- Shah, M.H.; Goldner, W.S.; Halfdanarson, T.R.; Bergsland, E.; Berlin, J.D.; Halperin, D.; Chan, J.; Kulke, M.H.; Benson, A.B.; Blaszkowsky, L.S.; et al. NCCN Guidelines Insights: Neuroendocrine and Adrenal Tumors, Version 2.2018. JNCCN J. Natl. Compr. Cancer Netw. 2018, 16, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Goldstraw, P.; Nicholson, A.G.; Travis, W.D.; Jett, J.R.; Ferolla, P.; Bomanji, J.; Rusch, V.W.; Asamura, H.; Skogseid, B.; et al. Proceedings of the IASLC International Workshop on Advances in Pulmonary Neuroendocrine Tumors 2007. J. Thorac. Oncol. 2008, 3, 1194–1201. [Google Scholar] [CrossRef]
- Fazio, N.; Buzzoni, R.; Fave, G.D.; Tesselaar, M.E.; Wolin, E.; Van Cutsem, E.; Tomassetti, P.; Strosberg, J.; Voi, M.; Bubuteishvili-Pacaud, L.; et al. Everolimus in advanced, progressive, well-differentiated, non-functional neuroendocrine tumors: RADIANT-4 lung subgroup analysis. Cancer Sci. 2018, 109, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Grande, E.; Capdevila, J.; Castellano, D.; Teulé, A.; Durán, I.; Fuster, J.; Sevilla, I.; Escudero, P.; Sastre, J.; Garcia-Donas, J.; et al. Pazopanib in pretreated advanced neuroendocrine tumors: A phase II, open-label trial of the Spanish Task Force Group for Neuroendocrine Tumors (GETNE). Ann. Oncol. 2015, 26, 1987–1993. [Google Scholar] [CrossRef]
- Kulke, M.H.; Lenz, H.-J.; Meropol, N.J.; Posey, J.; Ryan, D.P.; Picus, J.; Bergsland, E.; Stuart, K.; Tye, L.; Huang, X.; et al. Activity of Sunitinib in Patients with Advanced Neuroendocrine Tumors. J. Clin. Oncol. 2008, 26, 3403–3410. [Google Scholar] [CrossRef]
- Yao, J.C.; Phan, A.; Hoff, P.M.; Chen, H.X.; Charnsangavej, C.; Yeung, S.-C.J.; Hess, K.; Chaan, N.G.; Abbruzzese, J.L.; Ajani, J.A.; et al. Targeting Vascular Endothelial Growth Factor in Advanced Carcinoid Tumor: A Random Assignment Phase II Study of Depot Octreotide with Bevacizumab and Pegylated Interferon Alfa-2b. J. Clin. Oncol. 2008, 26, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, J.M.; Bergsland, E.; O’Neil, B.H.; Santoro, A.; Schellens, J.H.M.; Cohen, R.B.; Doi, T.; Ott, P.A.; Pishvaian, M.J.; Puzanov, I.; et al. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer 2020, 126, 3021–3030. [Google Scholar] [CrossRef]
- Lakhani, S.R.; Ellis, I.O.; Schnitt, S.J.; Tan, P.H.; van de Vijver, M.J. WHO Classification of Tumours; WHO Press: New York, NY, USA, 2012; p. 240. [Google Scholar]
- Rekhtman, N. Lung neuroendocrine neoplasms: Recent progress and persistent challenges. Mod. Pathol. 2022, 35, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Metovic, J.; Barella, M.; Bianchi, F.; Hofman, P.; Hofman, V.; Remmelink, M.; Kern, I.; Carvalho, L.; Pattini, L.; Sonzogni, A.; et al. Morphologic and molecular classification of lung neuroendocrine neoplasms. Virchows Arch. 2021, 478, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Shinmura, K.; Iwaizumi, M.; Igarashi, H.; Nagura, K.; Yamada, H.; Suzuki, M.; Fukasawa, K.; Sugimura, H. Induction of centrosome amplification and chromosome instability in p53-deficient lung cancer cells exposed to benzo[a]pyrene diol epoxide (B[a]PDE). J. Pathol. 2008, 216, 365–374. [Google Scholar] [CrossRef]
- Maitra, A.; Wistuba, I.I.; Washington, C.; Virmani, A.K.; Ashfaq, R.; Milchgrub, S.; Gazdar, A.F.; Minna, J.D. High-Resolution Chromosome 3p Allelotyping of Breast Carcinomas and Precursor Lesions Demonstrates Frequent Loss of Heterozygosity and a Discontinuous Pattern of Allele Loss. Am. J. Pathol. 2001, 159, 119–130. [Google Scholar] [CrossRef]
- Simbolo, M.; Mafficini, A.; Sikora, K.O.; Fassan, M.; Barbi, S.; Corbo, V.; Mastracci, L.; Rusev, B.; Grillo, F.; Vicentini, C.; et al. Lung neuroendocrine tumours: Deep sequencing of the four World Health Organization histotypes reveals chromatin-remodelling genes as major players and a prognostic role for TERT, RB1, MEN1 and KMT2D. J. Pathol. 2017, 241, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Battista, P.; Veschi, S.; Lattanzio, R.; Aceto, G.M.; Curia, M.C.; Magnasco, S.; Angelucci, D.; Cama, A.; Piantelli, M. Alterations of MEN1 and E-cadherin/β-catenin complex in sporadic pulmonary carcinoids. Int. J. Oncol. 2012, 41, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Kidd, M.; Filosso, P.-L.; Roffinella, M.; Lewczuk, A.; Cwikla, J.; Bodei, L.; Kolasinska-Cwikla, A.; Chung, K.-M.; Tesselaar, M.E.; et al. Molecular strategies in the management of bronchopulmonary and thymic neuroendocrine neoplasms. J. Thorac. Dis. 2017, 9, S1458–S1473. [Google Scholar] [CrossRef]
- Volante, M.; Mete, O.; Pelosi, G.; Roden, A.C.; Speel, E.J.M.; Uccella, S. Molecular Pathology of Well-Differentiated Pulmonary and Thymic Neuroendocrine Tumors: What Do Pathologists Need to Know? Endocr. Pathol. 2021, 32, 154–168. [Google Scholar] [CrossRef]
- Centonze, G.; Biganzoli, D.; Prinzi, N.; Pusceddu, S.; Mangogna, A.; Tamborini, E.; Perrone, F.; Busico, A.; Lagano, V.; Cattaneo, L.; et al. Beyond Traditional Morphological Characterization of Lung Neuroendocrine Neoplasms: In Silico Study of Next-Generation Sequencing Mutations Analysis across the Four World Health Organization Defined Groups. Cancers 2020, 12, 2753. [Google Scholar] [CrossRef]
- Fernandez-Cuesta, L.; Peifer, M.; Lu, X.; Sun, R.; Ozretić, L.; Seidel, D.; Zander, T.; Leenders, F.; George, J.; Müller, C.; et al. Frequent mutations in chromatin-remodelling genes in pulmonary carcinoids. Nat. Commun. 2014, 5, 3518. [Google Scholar] [CrossRef]
- Laddha, S.V.; Da Silva, E.M.; Robzyk, K.; Untch, B.R.; Ke, H.; Rekhtman, N.; Poirier, J.T.; Travis, W.D.; Tang, L.H.; Chan, C.S. Integrative Genomic Characterization Identifies Molecular Subtypes of Lung Carcinoids. Cancer Res. 2019, 79, 4339–4347. [Google Scholar] [CrossRef] [PubMed]
- Görtz, B.; Roth, J.; Krähenmann, A.; de Krijger, R.R.; Muletta-Feurer, S.; Rütimann, K.; Saremaslani, P.; Speel, E.J.; Heitz, P.U.; Komminoth, P. Mutations and Allelic Deletions of the MEN1 Gene Are Associated with a Subset of Sporadic Endocrine Pancreatic and Neuroendocrine Tumors and Not Restricted to Foregut Neoplasms. Am. J. Pathol. 1999, 154, 429–436. [Google Scholar] [CrossRef]
- Gustafsson, B.I.; Kidd, M.; Chan, A.K.; Malfertheiner, M.V.; Modlin, I.M. Bronchopulmonary neuroendocrine tumors. Cancer 2008, 113, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, G.; Scarpa, A.; Puppa, G.; Veronesi, G.; Spaggiari, L.; Pasini, F.; Maisonneuve, P.; Iannucci, A.; Arrigoni, G.; Viale, G. Alteration of the E-cadherin/β-catenin cell adhesion system is common in pulmonary neuroendocrine tumors and is an independent predictor of lymph node metastasis in atypical carcinoids. Cancer 2005, 103, 1154–1164. [Google Scholar] [CrossRef]
- Walch, A.K.; Zitzelsberger, H.F.; Aubele, M.M.; Mattis, A.E.; Bauchinger, M.; Candidus, S.; Präuer, H.W.; Werner, M.; Höfler, H. Typical and Atypical Carcinoid Tumors of the Lung Are Characterized by 11q Deletions as Detected by Comparative Genomic Hybridization. Am. J. Pathol. 1998, 153, 1089–1098. [Google Scholar] [CrossRef]
- Leotlela, P.D.; Jauch, A.; Holtgreve-Grez, H.; Thakker, R.V. Genetics of neuroendocrine and carcinoid tumours. Endocr.-Relat. Cancer 2003, 10, 437–450. [Google Scholar] [CrossRef]
- Onuki, N.; Wistuba, I.I.; Travis, W.D.; Virmani, A.K.; Yashima, K.; Brambilla, E.; Hasleton, P.; Gazdar, A.F. Genetic changes in the spectrum of neuroendocrine lung tumors. Cancer 1999, 85, 600–607. [Google Scholar] [CrossRef]
- Marinoni, I.; Kurrer, A.S.; Vassella, E.; Dettmer, M.; Rudolph, T.; Banz, V.; Hunger, F.; Pasquinelli, S.; Speel, E.; Perren, A. Loss of DAXX and ATRX Are Associated with Chromosome Instability and Reduced Survival of Patients with Pancreatic Neuroendocrine Tumors. Gastroenterology 2014, 146, 453–460.e5. [Google Scholar] [CrossRef] [PubMed]
- Warth, A.; Herpel, E.; Krysa, S.; Hoffmann, H.; Schnabel, P.A.; Schirmacher, P.; Mechtersheimer, G.; Bläker, H. Chromosomal instability is more frequent in metastasized than in non-metastasized pulmonary carcinoids but is not a reliable predictor of metastatic potential. Exp. Mol. Med. 2009, 41, 349–353. [Google Scholar] [CrossRef]
- Swarts, D.R.A.; Van Neste, L.; Henfling, M.E.R.; Eijkenboom, I.; Eijk, P.P.; van Velthuysen, M.-L.; Vink, A.; Volante, M.; Ylstra, B.; Van Criekinge, W.; et al. An exploration of pathways involved in lung carcinoid progression using gene expression profiling. Carcinogenesis 2013, 34, 2726–2737. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Swarts, D.R.A.; Henfling, M.E.R.; Van Neste, L.; Van Suylen, R.-J.; Dingemans, A.-M.C.; Dinjens, W.N.M.; Haesevoets, A.; Rudelius, M.; Thunnissen, E.; Volante, M.; et al. CD44 and OTP Are Strong Prognostic Markers for Pulmonary Carcinoids. Clin. Cancer Res. 2013, 19, 2197–2207. [Google Scholar] [CrossRef]
- Shilo, K.; Wu, X.; Sharma, S.; Welliver, M.; Duan, W.; Villalona-Calero, M.; Fukuoka, J.; Sif, S.; Baiocchi, R.; Hitchcock, C.L.; et al. Cellular localization of protein arginine methyltransferase-5 correlates with grade of lung tumors. Diagn. Pathol. 2013, 8, 201. [Google Scholar] [CrossRef]
- Findeis-Hosey, J.J.; Huang, J.; Li, F.; Yang, Q.; McMahon, L.A.; Xu, H. High-grade neuroendocrine carcinomas of the lung highly express enhancer of zeste homolog 2, but carcinoids do not. Hum. Pathol. 2011, 42, 867–872. [Google Scholar] [CrossRef]
- Mairinger, F.D.; Walter, R.F.H.; Theegarten, D.; Hager, T.; Vollbrecht, C.; Christoph, D.C.; Worm, K.; Ting, S.; Werner, R.; Stamatis, G.; et al. Gene Expression Analysis of the 26S Proteasome Subunit PSMB4 Reveals Significant Upregulation, Different Expression and Association with Proliferation in Human Pulmonary Neuroendocrine Tumours. J. Cancer 2014, 5, 646–654. [Google Scholar] [CrossRef]
- Rapa, I.; Votta, A.; Felice, B.; Righi, L.; Giorcelli, J.; Scarpa, A.; Speel, E.-J.M.; Scagliotti, G.V.; Papotti, M.; Volante, M. Identification of MicroRNAs Differentially Expressed in Lung Carcinoid Subtypes and Progression. Neuroendocrinology 2015, 101, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, R.; Petzmann, S.; Klemen, H.; Fraire, A.E.; Hasleton, P.; Popper, H.H. The position of pulmonary carcinoids within the spectrum of neuroendocrine tumors of the lung and other tissues. Genes Chromosom. Cancer 2002, 34, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, T.; Bellio, M.; Gentilin, E.; Molè, D.; Tagliati, F.; Schiavon, M.; Cavallesco, N.G.; Andriolo, L.G.; Ambrosio, M.R.; Rea, F.; et al. mTOR, p70S6K, AKT, and ERK1/2 levels predict sensitivity to mTOR and PI3K/mTOR inhibitors in human bronchial carcinoids. Endocr.-Relat. Cancer 2013, 20, 463–475. [Google Scholar] [CrossRef][Green Version]
- Zatelli, M.C.; Minoia, M.; Martini, C.; Tagliati, F.; Ambrosio, M.R.; Schiavon, M.; Buratto, M.; Calabrese, F.; Gentilin, E.; Cavallesco, G.; et al. Everolimus as a new potential antiproliferative agent in aggressive human bronchial carcinoids. Endocr.-Relat. Cancer 2010, 17, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, G.; Fumagalli, C.; Trubia, M.; Sonzogni, A.; Rekhtman, N.; Maisonneuve, P.; Galetta, D.; Spaggiari, L.; Veronesi, G.; Scarpa, A.; et al. Dual role of RASSF1 as a Tumor Suppressor and an Oncogene in Neuroendocrine Tumors of the Lung. Anticancer Res. 2010, 30, 4269–4281. Available online: https://air.unimi.it/handle/2434/148128#.YRkjmYgzbIU (accessed on 25 May 2021).
- Toyooka, S.; Gazdar, A.F. Methylation Profiling of Lung Cancer: A Decade of Progress. Mol. Cancer Ther. 2011, 10, 61–67. [Google Scholar] [CrossRef][Green Version]
- Toyooka, S.; Toyooka, K.O.; Maruyama, R.; Virmani, A.K.; Girard, L.; Miyajima, K.; Harada, K.; Ariyoshi, Y.; Takahashi, T.; Sugio, K.; et al. DNA methylation profiles of lung tumors. Mol. Cancer Ther. 2001, 1, 61–67. [Google Scholar]
- Warneboldt, J.; Haller, F.; Horstmann, O.; Danner, B.C.; Füzesi, L.; Doenecke, D.; Happel, N. Histone H1x is highly expressed in human neuroendocrine cells and tumours. BMC Cancer 2008, 8, 388. [Google Scholar] [CrossRef]
- La Torre, A.; Muscarella, L.A.; Parrella, P.; Balsamo, T.; Bisceglia, M.; Valori, V.M.; La Torre, A.; Barbano, R.; Perrella, E.; Poeta, M.L.; et al. Aberrant Genes Promoter Methylation in Neural Crest-Derived Tumors. Int. J. Biol. Mark. 2012, 27, 389–394. [Google Scholar] [CrossRef]
- Mairinger, F.; Ting, S.; Werner, R.; Walter, R.; Hager, T.; Vollbrecht, C.; Christoph, D.C.; Worm, K.; Mairinger, T.; Sheu-Grabellus, S.-Y.; et al. Different micro-RNA expression profiles distinguish subtypes of neuroendocrine tumors of the lung: Results of a profiling study. Mod. Pathol. 2014, 27, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Detassis, S.; Del Vescovo, V.; Grasso, M.; Masella, S.; Cantaloni, C.; Cima, L.; Cavazza, A.; Graziano, P.; Rossi, G.; Barbareschi, M.; et al. miR375-3p Distinguishes Low-Grade Neuroendocrine from Non-neuroendocrine Lung Tumors in FFPE Samples. Front. Mol. Biosci. 2020, 7, 86. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Motoi, N.; Yamamoto, N.; Nagano, H.; Ushijima, M.; Matsuura, M.; Okumura, S.; Yamaguchi, T.; Fukayama, M.; Ishikawa, Y. Pulmonary Carcinoids and Low-Grade Gastrointestinal Neuroendocrine Tumors Show Common MicroRNA Expression Profiles, Different from Adenocarcinomas and Small Cell Carcinomas. Neuroendocrinology 2017, 106, 47–57. [Google Scholar] [CrossRef]
- Singh, S.; Bergsland, E.K.; Card, C.M.; Hope, T.A.; Kunz, P.L.; Laidley, D.T.; Lawrence, B.; Leyden, S.; Metz, D.C.; Michael, M.; et al. Commonwealth Neuroendocrine Tumour Research Collaboration and the North American Neuroendocrine Tumor Society Guidelines for the Diagnosis and Management of Patients with Lung Neuroendocrine Tumors: An International Collaborative Endorsement and Update of the 2015 European Neuroendocrine Tumor Society Expert Consensus Guidelines. J. Thorac. Oncol. 2020, 15, 1577–1598. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Moua, T.; Midthun, D.E.; Mullon, J.J.; Decker, P.A.; Ryu, J.H. Diagnostic Yield and Bleeding Complications Associated with Bronchoscopic Biopsy of Endobronchial Carcinoid Tumors. J. Bronchol.-Interv. Pulmonol. 2020, 27, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Christopher, D.J.; Balamugesh, T.; Shah, A. Clinico-pathologic study of pulmonary carcinoid tumours—A retrospective analysis and review of literature. Respir. Med. 2008, 102, 1611–1614. [Google Scholar] [CrossRef] [PubMed]
- Raz, D.J.; Nelson, R.A.; Grannis, F.W.; Kim, J.Y. Natural History of Typical Pulmonary Carcinoid Tumors: A comparison of non-surgical and surgical treatment. Chest 2015, 147, 1111–1117. [Google Scholar] [CrossRef]
- Gosain, R.; Mukherjee, S.; Yendamuri, S.S.; Iyer, R. Management of Typical and Atypical Pulmonary Carcinoids Based on Different Established Guidelines. Cancers 2018, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Cooke, D.T.; Jett, J.R.; David, E.A. Extent of Resection and Lymph Node Assessment for Clinical Stage T1aN0M0 Typical Carcinoid Tumors. Ann. Thorac. Surg. 2018, 105, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Gosain, R.; Groman, A.; Gosain, R.; Dasari, A.; Halfdanarson, T.; Mukherjee, S. Incidence and Survival Outcomes in Patients with Lung Neuroendocrine Neoplasms in the United States. Cancers 2021, 13, 1753. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Sigel, K.; Martin, J.; Jordan, R.; Beasley, M.B.; Smith, C.; Kaufman, A.; Wisnivesky, J.; Kim, M.K. Evaluation of the Prognostic Significance of TNM Staging Guidelines in Lung Carcinoid Tumors. J. Thorac. Oncol. 2019, 14, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pang, Z.; Wang, Y.; Bie, F.; Zeng, Y.; Wang, G.; Du, J. The role of surgery for atypical bronchopulmonary carcinoid tumor: Development and validation of a model based on Surveillance, Epidemiology, and End Results (SEER) database. Lung Cancer 2020, 139, 94–102. [Google Scholar] [CrossRef]
- Kneuertz, P.J.; Kamel, M.K.; Stiles, B.M.; Lee, B.E.; Rahouma, M.; Harrison, S.W.; Altorki, N.K.; Port, J.L. Incidence and Prognostic Significance of Carcinoid Lymph Node Metastases. Ann. Thorac. Surg. 2018, 106, 981–988. [Google Scholar] [CrossRef]
- Dalar, L.; Ozdemir, C.; Sokucu, S.N.; Karasulu, L.; Urer, H.N.; Altin, S.; Abul, Y. Endobronchial Treatment of Carcinoid Tumors of the Lung. Thorac. Cardiovasc. Surg. 2015, 64, 166–171. [Google Scholar] [CrossRef]
- Guarino, C.; Mazzarella, G.; De Rosa, N.; Cesaro, C.; La Cerra, G.; Grella, E.; Perrotta, F.; Curcio, C.; Guerra, G.; Bianco, A. Pre-surgical bronchoscopic treatment for typical endobronchial carcinoids. Int. J. Surg. 2016, 33, S30–S35. [Google Scholar] [CrossRef]
- Brokx, H.A.P.; Paul, M.; Postmus, P.E.; Sutedja, T.G. Long-term follow-up after first-line bronchoscopic therapy in patients with bronchial carcinoids. Thorax 2015, 70, 468–472. [Google Scholar] [CrossRef]
- Singh, D.; Chen, Y.; Cummings, M.A.; Milano, M.T. Inoperable Pulmonary Carcinoid Tumors: Local Control Rates with Stereotactic Body Radiotherapy/Hypofractionated RT with Image-Guided Radiotherapy. Clin. Lung Cancer 2019, 20, e284–e290. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.T.; Öberg, K.; Choi, J.; Harrison, L.H.; Hassan, M.M.; Strosberg, J.R.; Krenning, E.P.; Kocha, W.; Woltering, E.A.; Maples, W.J. NANETS Consensus Guideline for the Diagnosis and Management of Neuroendocrine Tumors: Well-Differentiated Neuroendocrine Tumors of the Thorax (Includes Lung and Thymus). Pancreas 2010, 39, 784–798. [Google Scholar] [CrossRef]
- Bertino, E.M.; Confer, P.D.; Colonna, J.E.; Ross, P.; Otterson, G.A. Pulmonary neuroendocrine/carcinoid tumors: A review article. Cancer 2009, 115, 4434–4441. [Google Scholar] [CrossRef]
- Ramirez, R.A.; Thomas, K.; Jacob, A.; Lin, K.; Mattison, Y.B.; Chauhan, A. Adjuvant therapy for lung neuroendocrine neoplasms. World J. Clin. Oncol. 2021, 12, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef]
- Welin, S.; Sorbye, H.; Sebjornsen, S.; Knappskog, S.; Busch, C.; Öberg, K. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer 2011, 117, 4617–4622. [Google Scholar] [CrossRef]
- Naraev, B.G.; Ramirez, R.A.; Kendi, A.T.; Halfdanarson, T.R. Peptide Receptor Radionuclide Therapy for Patients with Advanced Lung Carcinoids. Clin. Lung Cancer 2019, 20, e376–e392. [Google Scholar] [CrossRef]
- Sundin, A.; Arnold, R.; Baudin, E.; Cwikla, J.B.; Eriksson, B.; Fanti, S.; Fazio, N.; Giammarile, F.; Hicks, R.J.; Kjaer, A.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine and Hybrid Imaging. Neuroendocrinology 2017, 105, 212–244. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, T.; Ducreux, M.; Baudin, E.; Sabourin, J.-C.; De Baere, T.; Mitry, E.; Schlumberger, M.; Rougier, P. Antitumour activity of somatostatin analogues in progressive metastatic neuroendocrine tumours. Eur. J. Cancer 2001, 37, 1014–1019. [Google Scholar] [CrossRef]
- Ducreux, M.; Ruszniewski, P.; Chayvialle, J.-A.; Blumberg, J.; Cloarec, D.; Michel, H.; Raymond, J.M.; Dupas, J.-L.; Gouerou, H.; Jian, R.; et al. The antitumoral effect of the long-acting somatostatin analog lanreotide in neuroendocrine tumors. Am. J. Gastroenterol. 2000, 95, 3276–3281. [Google Scholar] [CrossRef]
- Faiss, S.; Pape, U.-F.; Böhmig, M.; Dörffel, Y.; Mansmann, U.; Golder, W.; Riecken, E.O.; Wiedenmann, B. Prospective, Randomized, Multicenter Trial on the Antiproliferative Effect of Lanreotide, Interferon Alfa, and Their Combination for Therapy of Metastatic Neuroendocrine Gastroenteropancreatic Tumors—The International Lanreotide and Interferon Alfa Study Group. J. Clin. Oncol. 2003, 21, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Ruszniewski, P. Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2014, 371, 1555–1557. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Phan, A.T.; Ćwikła, J.B.; Sedláčková, E.; Thanh, X.-M.T.; Wolin, E.M.; Ruszniewski, P.; on behalf of the CLARINET Investigators. Lanreotide autogel/depot in advanced enteropancreatic neuroendocrine tumours: Final results of the CLARINET open-label extension study. Endocrine 2021, 71, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, I.; Le Teuff, G.; Guigay, J.; Caramella, C.; Berdelou, A.; Leboulleux, S.; Déandréis, D.; Hadoux, J.; Ducreux, M.; Duvillard, P.; et al. Antitumour activity of somatostatin analogues in sporadic, progressive, metastatic pulmonary carcinoids. Eur. J. Cancer 2017, 75, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Lenotti, E.; Alberti, A.; Spada, F.; Amoroso, V.; Maisonneuve, P.; Grisanti, S.; Baggi, A.; Bianchi, S.; Fazio, N.; Berruti, A. Outcome of Patients with Metastatic Lung Neuroendocrine Tumors Submitted to First Line Monotherapy with Somatostatin Analogs. Front. Endocrinol. 2021, 12, 669484. [Google Scholar] [CrossRef]
- Horsch, D.; Baudin, E.; Singh, S.; Caplin, M.E.; Ferone, D.; Wolin, E.M.; Capdevila, J.; Buikhuisen, W.A.; Raderer, M.; Dansin, E.; et al. Lanreotide autogel/depot (LAN) in patients with advanced bronchopulmonary (BP) neuroendocrine tumors (NETs): Results from the phase III SPINET study. Ann. Oncol. 2021, 32, S906–S920. [Google Scholar] [CrossRef]
- Ferolla, P.; Berruti, A.; Spada, F.; Brizzi, M.P.; Ibrahim, T.; Cola, A.; Faggiano, A.; Marconcini, R.; Vaccaro, V.; Giuffrida, D.; et al. Lanreotide autogel (LAN) and temozolomide (TMZ) combination therapy in progressive thoracic neuroendocrine tumours (TNETs): ATLANT study results. Ann. Oncol. 2020, 31, S711–S724. [Google Scholar] [CrossRef]
- Reidy-Lagunes, D.; Kulke, M.; Wolin, E.; Singh, S.; Ferone, D.; Hoersch, D.; Mirakhur, B.; Hoffmanns, P.; Houchard, A.; Caplin, M.; et al. PUB119 Lanreotide in Patients with Lung Neuroendocrine Tumors: The Randomized Double-Blind Placebo-Controlled International Phase 3 SPINET Study. J. Thorac. Oncol. 2017, 12, S1516–S1517. [Google Scholar] [CrossRef][Green Version]
- Pavel, M.E.; Baudin, E.; Öberg, K.E.; Hainsworth, J.D.; Voi, M.; Rouyrre, N.; Peeters, M.; Gross, D.J.; Yao, J.C. Efficacy of everolimus plus octreotide LAR in patients with advanced neuroendocrine tumor and carcinoid syndrome: Final overall survival from the randomized, placebo-controlled phase 3 RADIANT-2 study. Ann. Oncol. 2017, 28, 1569–1575. [Google Scholar] [CrossRef]
- Xu, J.; Shen, L.; Zhou, Z.; Li, J.; Bai, C.; Chi, Y.; Li, Z.; Xu, N.; Li, E.; Liu, T.; et al. Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 1500–1512. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Benavent, M.; Fonseca, P.J.; Castellano, D.; Alonso, T.; Teule, A.; Custodio, A.; Tafuto, S.; Munoa, A.L.C.; Spada, F.; et al. A phase II/III randomized double-blind study of octreotide acetate LAR with axitinib versus octreotide acetate LAR with placebo in patients with advanced G1-G2 NETs of non-pancreatic origin (AXINET trial-GETNE-1107). J. Clin. Oncol. 2021, 39, 360. [Google Scholar] [CrossRef]
- Pavel, M.; Baudin, E.; Couvelard, A.; Krenning, E.; Öberg, K.; Steinmüller, T.; Anlauf, M.; Wiedenmann, B.; Salazar, R. ENETS Consensus Guidelines for the Management of Patients with Liver and Other Distant Metastases from Neuroendocrine Neoplasms of Foregut, Midgut, Hindgut, and Unknown Primary. Neuroendocrinology 2012, 95, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Steinmüller, T.; Kianmanesh, R.; Falconi, M.; Scarpa, A.; Taal, B.; Kwekkeboom, D.J.; Lopes, J.M.; Perren, A.; Nikou, G.; Yao, J.; et al. Consensus Guidelines for the Management of Patients with Liver Metastases from Digestive (Neuro)endocrine Tumors: Foregut, Midgut, Hindgut, and Unknown Primary. Neuroendocrinology 2008, 87, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Jermendy, G.; Kónya, A.; Kárpáti, P. Hepatic artery embolization—New approach for treatment of malignant carcinoid syndrome. Dtsch. Z. Verdau. Stoffwechs. 1986, 46, 130–136. [Google Scholar]
- Milkiewicz, P.; Olliff, S.; Johnson, A.P.; Elias, E. Obstructive sleep apnoea syndrome (OSAS) as a complication of carcinoid syndrome treated successfully by hepatic artery embolization. Eur. J. Gastroenterol. Hepatol. 1997, 9, 217–220. [Google Scholar] [CrossRef]
- Hay, N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell 2005, 8, 179–183. [Google Scholar] [CrossRef]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef]
- Baudin, E.; Caplin, M.; Garcia-Carbonero, R.; Fazio, N.; Ferolla, P.; Filosso, P.; Frilling, A.; de Herder, W.; Hörsch, D.; Knigge, U.; et al. Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 439–451. [Google Scholar] [CrossRef]
- Fazio, N.; Granberg, D.; Grossman, A.; Saletan, S.; Klimovsky, J.; Panneerselvam, A.; Wolin, E.M. Everolimus Plus Octreotide Long-Acting Repeatable in Patients with Advanced Lung Neuroendocrine Tumors: Analysis of the phase 3, randomized, placebo-controlled RADIANT-2 study. Chest 2013, 143, 955–962. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.-L.; Bang, Y.-J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib Malate for the Treatment of Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef]
- Yao, J.C.; Guthrie, K.A.; Moran, C.; Strosberg, J.R.; Kulke, M.H.; Chan, J.A.; LoConte, N.; McWilliams, R.R.; Wolin, E.M.; Mattar, B.; et al. Phase III Prospective Randomized Comparison Trial of Depot Octreotide Plus Interferon Alfa-2b Versus Depot Octreotide Plus Bevacizumab in Patients with Advanced Carcinoid Tumors: SWOG S0518. J. Clin. Oncol. 2017, 35, 1695–1703. [Google Scholar] [CrossRef]
- Castellano, D.; Capdevila, J.; Sastre, J.; Alonso, V.; Llanos, M.; Garcia-Carbonero, R.; Mozo, J.L.M.; Sevilla, I.; Durán, I.; Salazar, R. Sorafenib and bevacizumab combination targeted therapy in advanced neuroendocrine tumour: A phase II study of Spanish Neuroendocrine Tumour Group (GETNE0801). Eur. J. Cancer 2013, 49, 3780–3787. [Google Scholar] [CrossRef]
- Dasari, A.; Li, D.; Sung, M.W.; Tucci, C.; Kauh, J.S.; Kania, M.K.; Paulson, A.S. Efficacy and safety of surufatinib in United States (US) patients (pts) with neuroendocrine tumors (NETs). J. Clin. Oncol. 2020, 38, 4610. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Benavent, M.; Fonseca, P.J.; Castellano, D.; Alonso-Gordoa, T.; Teulé, A.; Custodio, A.; Tafuto, S.; La Casta, A.; Spada, F.; et al. 1097O The AXINET trial (GETNE1107): Axitinib plus octreotide LAR improves PFS by blinded central radiological assessment vs placebo plus octreotide LAR in G1-2 extrapancreatic NETs. Ann. Oncol. 2021, 32, S907–S908. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; De Herder, W.W.; Kam, B.L.; Van Eijck, C.H.; Van Essen, M.; Kooij, P.P.; Feelders, R.A.; Van Aken, M.O.; Krenning, E.P. Treatment With the Radiolabeled Somatostatin Analog [177Lu-DOTA0,Tyr3] Octreotate: Toxicity, Efficacy, and Survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Russo, G.L.; Pusceddu, S.; Prinzi, N.; Imbimbo, M.; Proto, C.; Signorelli, D.; Vitali, M.; Ganzinelli, M.; Maccauro, M.; Buzzoni, R.; et al. Peptide receptor radionuclide therapy: Focus on bronchial neuroendocrine tumors. Tumor Biol. 2016, 37, 12991–13003. [Google Scholar] [CrossRef]
- Ianniello, A.; Sansovini, M.; Severi, S.; Nicolini, S.; Grana, C.M.; Massri, K.; Bongiovanni, A.; Antonuzzo, L.; Di Iorio, V.; Sarnelli, A.; et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE in advanced bronchial carcinoids: Prognostic role of thyroid transcription factor 1 and 18F-FDG PET. Eur. J. Nucl. Med. Mol. Imaging 2015, 43, 1040–1046. [Google Scholar] [CrossRef]
- Mariniello, A.; Bodei, L.; Tinelli, C.; Baio, S.M.; Gilardi, L.; Colandrea, M.; Papi, S.; Valmadre, G.; Fazio, N.; Galetta, D.; et al. Long-term results of PRRT in advanced bronchopulmonary carcinoid. Eur. J. Nucl. Med. Mol. Imaging 2015, 43, 441–452. [Google Scholar] [CrossRef]
- Van Vliet, E.I.; Krenning, E.P.; Teunissen, J.J.; Bergsma, H.; Kam, B.L.; Kwekkeboom, D.J. Comparison of Response Evaluation in Patients with Gastroenteropancreatic and Thoracic Neuroendocrine Tumors After Treatment with [177Lu-DOTA0,Tyr3] Octreotate. J. Nucl. Med. 2013, 54, 1689–1696. [Google Scholar] [CrossRef]
- Imhof, A.; Brunner, P.; Marincek, N.; Briel, M.; Schindler, C.; Rasch, H.; Mäcke, H.R.; Rochlitz, C.; Müller-Brand, J.; Walter, M.A. Response, Survival, and Long-Term Toxicity After Therapy with the Radiolabeled Somatostatin Analogue [90Y-DOTA]-TOC in Metastasized Neuroendocrine Cancers. J. Clin. Oncol. 2011, 29, 2416–2423. [Google Scholar] [CrossRef]
- Van Essen, M.; Krenning, E.P.; Bakker, W.H.; de Herder, W.W.; van Aken, M.O.; Kwekkeboom, D.J. Peptide receptor radionuclide therapy with 177Lu-octreotate in patients with foregut carcinoid tumours of bronchial, gastric and thymic origin. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1219–1227. [Google Scholar] [CrossRef]
- Brabander, T.; Van Der Zwan, W.A.; Teunissen, J.J.; Kam, B.L.; Feelders, R.A.; De Herder, W.W.; Van Eijck, C.H.; Franssen, G.J.; Krenning, E.P.; Kwekkeboom, D.J. Long-Term Efficacy, Survival, and Safety of [177Lu-DOTA0,Tyr3] Octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin. Cancer Res. 2017, 23, 4617–4624. [Google Scholar] [CrossRef]
- Zidan, L.; Iravani, A.; Oleinikov, K.; Ben-Haim, S.; Gross, D.J.; Meirovitz, A.; Maimon, O.; Akhurst, T.; Michael, M.; Hicks, R.J.; et al. Efficacy and safety of 177Lu-DOTATATE in lung neuroendocrine tumors: A bi-center study. J. Nucl. Med. 2021, 63, 260760. [Google Scholar] [CrossRef]
- Waldherr, C.; Pless, M.; Maecke, H.R.; Haldemann, A.; Mueller-Brand, J. The clinical value of [90Y-DOTA]-D-Phe1-Tyr3-octreotide (90Y-DOTATOC) in the treatment of neuroendocrine tumours: A clinical phase II study. Ann. Oncol. 2001, 12, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Wirth, L.J.; Carter, M.R.; Jänne, P.A.; Johnson, B.E. Outcome of patients with pulmonary carcinoid tumors receiving chemotherapy or chemoradiotherapy. Lung Cancer 2004, 44, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.A.; Stuart, K.; Earle, C.C.; Clark, J.W.; Bhargava, P.; Miksad, R.; Blaszkowsky, L.; Enzinger, P.C.; Meyerhardt, J.A.; Zheng, H.; et al. Prospective Study of Bevacizumab Plus Temozolomide in Patients with Advanced Neuroendocrine Tumors. J. Clin. Oncol. 2012, 30, 2963–2968. [Google Scholar] [CrossRef] [PubMed]
- Al-Toubah, T.; Morse, B.; Strosberg, J. Capecitabine and Temozolomide in Advanced Lung Neuroendocrine Neoplasms. Oncologist 2020, 25, e48–e52. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Lipsitz, S.; Catalano, P.; Mailliard, J.A.; Haller, D.G. Phase II/III Study of Doxorubicin with Fluorouracil Compared with Streptozocin With Fluorouracil or Dacarbazine in the Treatment of Advanced Carcinoid Tumors: Eastern Cooperative Oncology Group Study E1281. J. Clin. Oncol. 2005, 23, 4897–4904. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Strauss, S.J.; Sarker, D.; Gillmore, R.; Kirkwood, A.; Hackshaw, A.; Papadopoulou, A.; Bell, J.; Kayani, I.; Toumpanakis, C.; et al. Chemotherapy with 5-fluorouracil, cisplatin and streptozocin for neuroendocrine tumours. Br. J. Cancer 2010, 102, 1106–1112. [Google Scholar] [CrossRef]
- Meyer, T.; Qian, W.; Caplin, M.E.; Armstrong, G.; Lao-Sirieix, S.-H.; Hardy, R.; Valle, J.; Talbot, D.C.; Cunningham, D.; Reed, N.; et al. Capecitabine and streptozocin±cisplatin in advanced gastroenteropancreatic neuroendocrine tumours. Eur. J. Cancer 2014, 50, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; Planchard, D.; Bouledrak, K.; Scoazec, J.; Souquet, P.; Dussol, A.; Guigay, J.; Hervieu, V.; Berdelou, A.; Ducreux, M.; et al. Evaluation of the combination of oxaliplatin and 5-fluorouracil or gemcitabine in patients with sporadic metastatic pulmonary carcinoid tumors. Lung Cancer 2016, 96, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Spada, F.; Antonuzzo, L.; Marconcini, R.; Radice, D.; Antonuzzo, A.; Ricci, S.; Di Costanzo, F.; Fontana, A.; Gelsomino, F.; Luppi, G.; et al. Oxaliplatin-Based Chemotherapy in Advanced Neuroendocrine Tumors: Clinical Outcomes and Preliminary Correlation with Biological Factors. Neuroendocrinology 2016, 103, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.L.; Balise, R.R.; Fehrenbacher, L.; Pan, M.; Venook, A.P.; Fisher, G.A.; Tempero, M.A.; Ko, A.H.; Korn, W.M.; Hwang, J.; et al. Oxaliplatin–Fluoropyrimidine Chemotherapy Plus Bevacizumab in Advanced Neuroendocrine Tumors: An Analysis of 2 Phase II Trials. Pancreas 2016, 45, 1394–1400. [Google Scholar] [CrossRef]
- Sherman, S.; Rotem, O.; Shochat, T.; Zer, A.; Moore, A.; Dudnik, E. Efficacy of immune check-point inhibitors (ICPi) in large cell neuroendocrine tumors of lung (LCNEC). Lung Cancer 2020, 143, 40–46. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Al Baghdadi, T.; et al. A Phase II Basket Trial of Dual Anti–CTLA-4 and Anti–PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef]
- Capdevila, J.; Teule, A.; López, C.; García-Carbonero, R.; Benavent, M.; Custodio, A.; Cubillo, A.; Alonso, V.; Gordoa, T.A.; Carmona-Bayonas, A.; et al. 1157O A multi-cohort phase II study of durvalumab plus tremelimumab for the treatment of patients (pts) with advanced neuroendocrine neoplasms (NENs) of gastroenteropancreatic or lung origin: The DUNE trial (GETNE 1601). Ann. Oncol. 2020, 31, S770–S771. [Google Scholar] [CrossRef]
- Cho, H.-S.; Suzuki, T.; Dohmae, N.; Hayami, S.; Unoki, M.; Yoshimatsu, M.; Toyokawa, G.; Takawa, M.; Chen, T.; Kurash, J.K.; et al. Demethylation of RB Regulator MYPT1 by Histone Demethylase LSD1 Promotes Cell Cycle Progression in Cancer Cells. Cancer Res. 2010, 71, 655–660. [Google Scholar] [CrossRef]
- Fanciulli, G.; Nike, O.B.O.; Ruggeri, R.M.; Grossrubatscher, E.; Calzo, F.L.; Wood, T.D.; Faggiano, A.; Isidori, A.; Colao, A. Serotonin pathway in carcinoid syndrome: Clinical, diagnostic, prognostic and therapeutic implications. Rev. Endocr. Metab. Disord. 2020, 21, 599–612. [Google Scholar] [CrossRef]
- Møller, J.E.; Connolly, H.M.; Rubin, J.; Seward, J.B.; Modesto, K.; Pellikka, P.A. Factors Associated with Progression of Carcinoid Heart Disease. N. Engl. J. Med. 2003, 348, 1005–1015. [Google Scholar] [CrossRef]
- Oleinikov, K.; Korach, A.; Planer, D.; Gilon, D.; Grozinsky-Glasberg, S. Update in carcinoid heart disease—The heart of the matter. Rev. Endocr. Metab. Disord. 2021, 22, 553–561. [Google Scholar] [CrossRef]
- Hofland, J.; Herrera-Martínez, A.D.; Zandee, W.T.; De Herder, W.W. Management of carcinoid syndrome: A systematic review and meta-analysis. Endocr.-Relat. Cancer 2019, 26, R145–R156. [Google Scholar] [CrossRef] [PubMed]
- Filosso, P.L.; Ruffini, E.; Oliaro, A.; Papalia, E.; Donati, G.; Rena, O. Long-term survival of atypical bronchial carcinoids with liver metastases, treated with octreotide. Eur. J. Cardio-Thoracic Surg. 2002, 21, 913–917. [Google Scholar] [CrossRef][Green Version]
- Pavel, M.E.; Gross, D.J.; Benavent, M.; Perros, P.; Srirajaskanthan, R.; Warner, R.R.P.; Kulke, M.H.; Anthony, L.B.; Kunz, P.L.; Horsch, D.; et al. Telotristat ethyl in carcinoid syndrome: Safety and efficacy in the TELECAST phase 3 trial. Endocr.-Relat. Cancer 2018, 25, 309–322. [Google Scholar] [CrossRef]
- Zandee, W.T.; Brabander, T.; Blažević, A.; Minczeles, N.S.; Feelders, R.A.; de Herder, W.W.; Hofland, J. Peptide Receptor Radionuclide Therapy With 177Lu-DOTATATE for Symptomatic Control of Refractory Carcinoid Syndrome. J. Clin. Endocrinol. Metab. 2021, 106, e3665–e3672. [Google Scholar] [CrossRef]
- Castro, M.A.; Azpiroz, M.M. Two types of ectopic Cushing syndrome or a continuum? Review. Pituitary 2018, 21, 535–544. [Google Scholar] [CrossRef]
- Castro, M.A.; García, N.P.; Pardo, J.A.; Alvarez, C.I.; Grao, L.A.; García, J.E. Síndrome de Cushing ectópico: Descripción de 9 casos. Endocrinol. Diabetes Nutr. 2018, 65, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Öberg, K.; Hellman, P.; Ferolla, P.; Papotti, M. Neuroendocrine bronchial and thymic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23, vii120–vii123. [Google Scholar] [CrossRef] [PubMed]
- Young, J.; Haissaguerre, M.; Viera-Pinto, O.; Chabre, O.; Baudin, E.; Tabarin, A. Management of Endocrine Disease: Cushing’s syndrome due to ectopic ACTH secretion: An expert operational opinion. Eur. J. Endocrinol. 2020, 182, R29–R58. [Google Scholar] [CrossRef]
- Dimitriadis, G.K.; Angelousi, A.; Weickert, M.O.; Randeva, H.S.; Kaltsas, G.; Grossman, A. Paraneoplastic endocrine syndromes. Endocr.-Relat. Cancer 2017, 24, R173–R190. [Google Scholar] [CrossRef]
| Trial | R | Previous Treatment | Number of Patients | Treatment | Comparator | Main Results |
|---|---|---|---|---|---|---|
| SPINET Phase III/ NCT02683941 [89] | R | First line | 77 (early closed accrual) | Lanreotide autogel 120 mg/28 d | Placebo | mPFS = 16.6 (95% CI 12.8, 21.9) ORR = 14.0% vs. 0% |
| LUNA Phase II [14] | R | First line | 124 | Pasireotide 60 mg/28 d | Everolimus 10 mg/d Pasireotide 60 mg/28 d+ Everolimus 10 mg/d | PFS rate at 9 months = 39% (95% CI 24·2–55·5) vs. 33.3% (95%CI 19.6–49.5) vs. 58.5% (95% CI 42.1–73.7) |
| ATLANT Trial Phase II [88] | R | ≤3 prior lines | 40 | Lanreotide autogel 120 mg/28 d and temozolomide 250 mg/m2 every 5 of 28 days | No control arm | DCR at 9 m = 35% (95% CI 20.63; 51.68) |
| Phase II | NR | SSA (84%), CT (38%), PRRT (26%) | 34 | 177LuDOTATATE | - | DCR = 62% ORR = 15% mPFS = 18.5 m |
| RADIANT 4 Phase III/NCT01524783 [17] | R | SSA (53%); CT (26%); RT/PRRT (22%) | 90 * (30%) | Everolimus 10 mg/day | Placebo | mPFS = 9.2 vs. 3.6 m (HR = 0.50) |
| RADIANT-2 Phase III/ NCT00412061 [90] | R | SSA (67%); CT (39%); IT (12%); TT (15%) | 44 * (10%) | Everolimus 10 mg/day + Octreotide LAR | Placebo + Octreotide LAR | mPFS = 13.6 vs. 5.6 m (HR = 0.72) |
| EP SANET/ Phase III/ [91] | R | SSA (34%); CT (40%); Everolimus (8%) | 23 * (11.6%) | Surufatinib 300 mg/day | Placebo | PFS * = 7.6 vs. 3.7 m (HR = 0.33) |
| AXINET/ Phase III/NCT [92] | R | SSA (48%); CT (14%); PRRT (4%); Everolimus (13%) | 71 (28%) | Axitinib 5 mg/12 h + Octreotide LAR 30 mg/28 d | Placebo + Octreotide LAR 30 mg/28 d | mPFS = 14.36 vs. 11.72 (HR = 0.84) |
| Study | Treatment | Comparator | n | Primary ENDPOINT | Secondary Endpoints | Reccruitment | NCT Identifier |
|---|---|---|---|---|---|---|---|
| Phase I/II | PEN-221 | - | 89 | MTD | CBR, ORR | 2016–2020 | NCT02936323 |
| NCI-2020-12905 Phase II | 177LuDOTATATE | Everolimus | 108 | PFS | OS, ORR, safety | 2021–2024 | NCT04665739 |
| PUTNET Phase II | 177LuDOTATOC | - | 50 | ORR | PFS | 2021–2022 | NCT04276597 |
| CABINET Phase III | Cabozantinib | Placebo | 395 | PFS | OS, ORR, safety | 2018–2025 | NCT03375320 |
| CABOTEM Phase II | Cabozantinib + Temozolomide | - | 35 | ORR | PFS, CBR, OS | 2021–2023 | NCT04893785 |
| 2020-012-00EU1 Phase II | Surufatinib | - | 76 | DCR | PFS, DoR, Safety | 2021–2022 | NCT04579679 |
| Phase II HMH008 | Nivolumab + 177LuDOTATATE | - | 30 | ORR | ORR, PFS, Safety | 2020–2020 | NCT04525638 |
| Phase II | Nivolumab + Temozolomide | - | 55 | ORR | Safety, PFS, OS | 2018–2021 | NCT03728361 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araujo-Castro, M.; Pascual-Corrales, E.; Molina-Cerrillo, J.; Moreno Mata, N.; Alonso-Gordoa, T. Bronchial Carcinoids: From Molecular Background to Treatment Approach. Cancers 2022, 14, 520. https://doi.org/10.3390/cancers14030520
Araujo-Castro M, Pascual-Corrales E, Molina-Cerrillo J, Moreno Mata N, Alonso-Gordoa T. Bronchial Carcinoids: From Molecular Background to Treatment Approach. Cancers. 2022; 14(3):520. https://doi.org/10.3390/cancers14030520
Chicago/Turabian StyleAraujo-Castro, Marta, Eider Pascual-Corrales, Javier Molina-Cerrillo, Nicolás Moreno Mata, and Teresa Alonso-Gordoa. 2022. "Bronchial Carcinoids: From Molecular Background to Treatment Approach" Cancers 14, no. 3: 520. https://doi.org/10.3390/cancers14030520
APA StyleAraujo-Castro, M., Pascual-Corrales, E., Molina-Cerrillo, J., Moreno Mata, N., & Alonso-Gordoa, T. (2022). Bronchial Carcinoids: From Molecular Background to Treatment Approach. Cancers, 14(3), 520. https://doi.org/10.3390/cancers14030520