Therapeutic Advancements in Metal and Metal Oxide Nanoparticle-Based Radiosensitization for Head and Neck Cancer Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
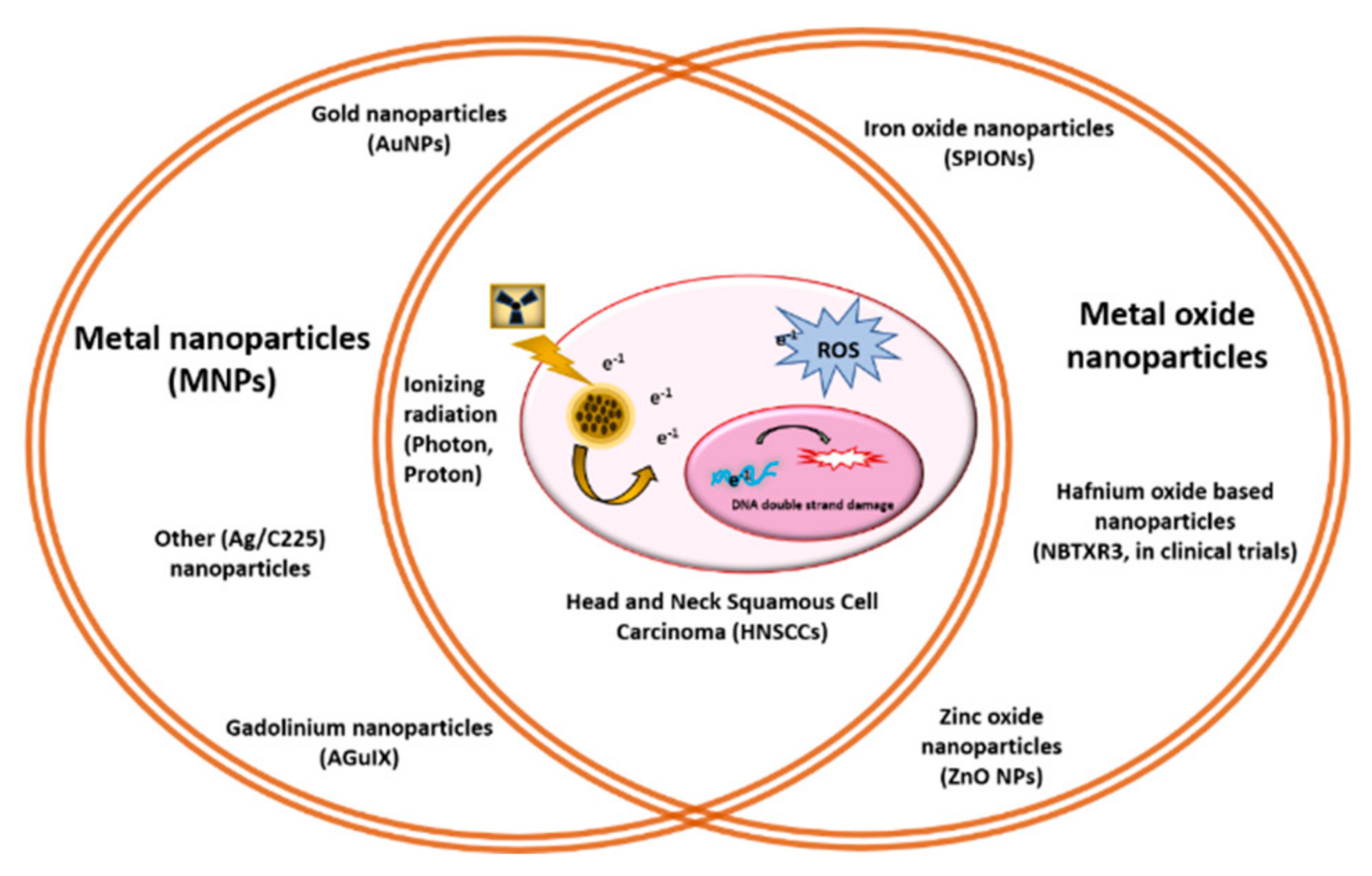
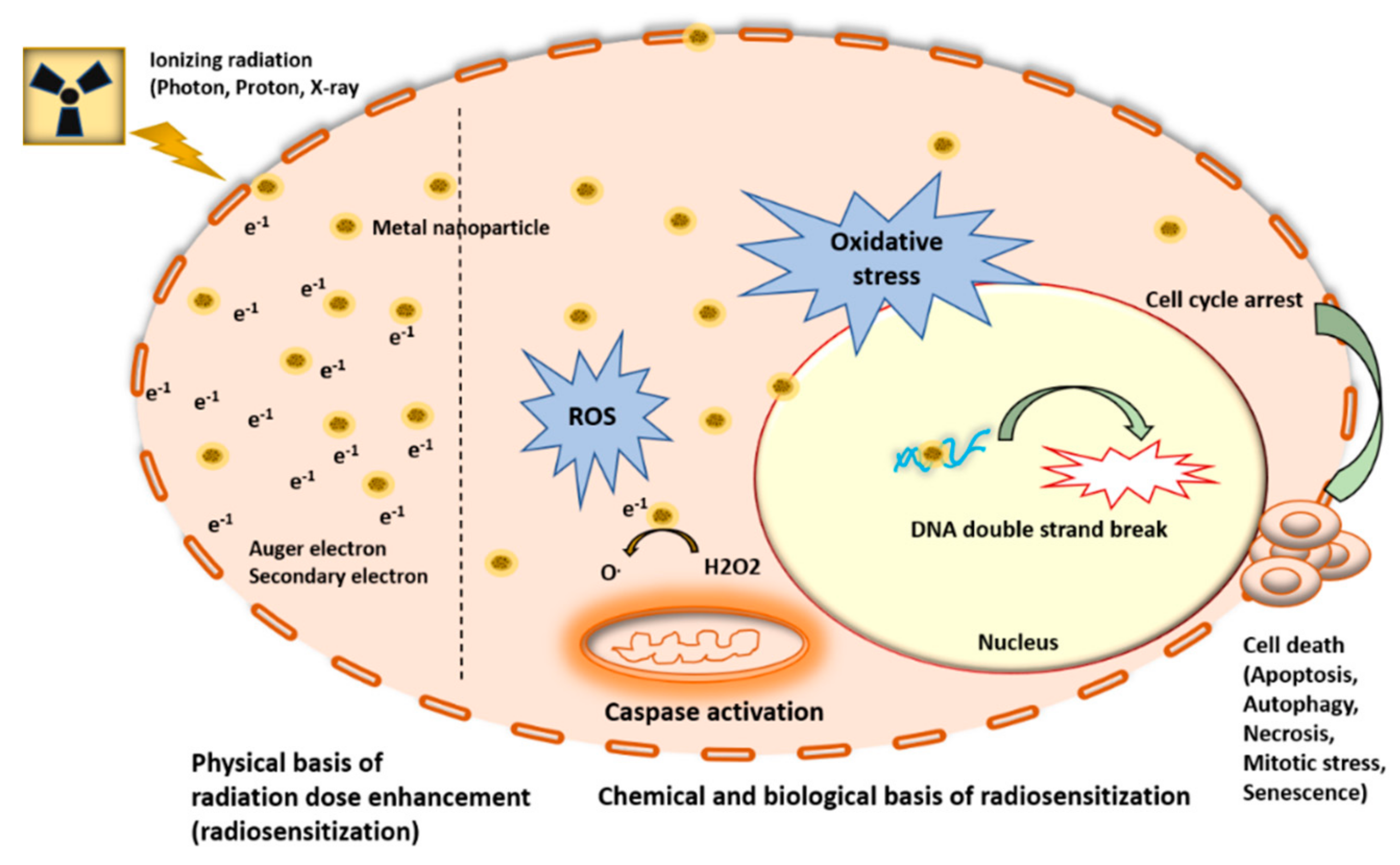
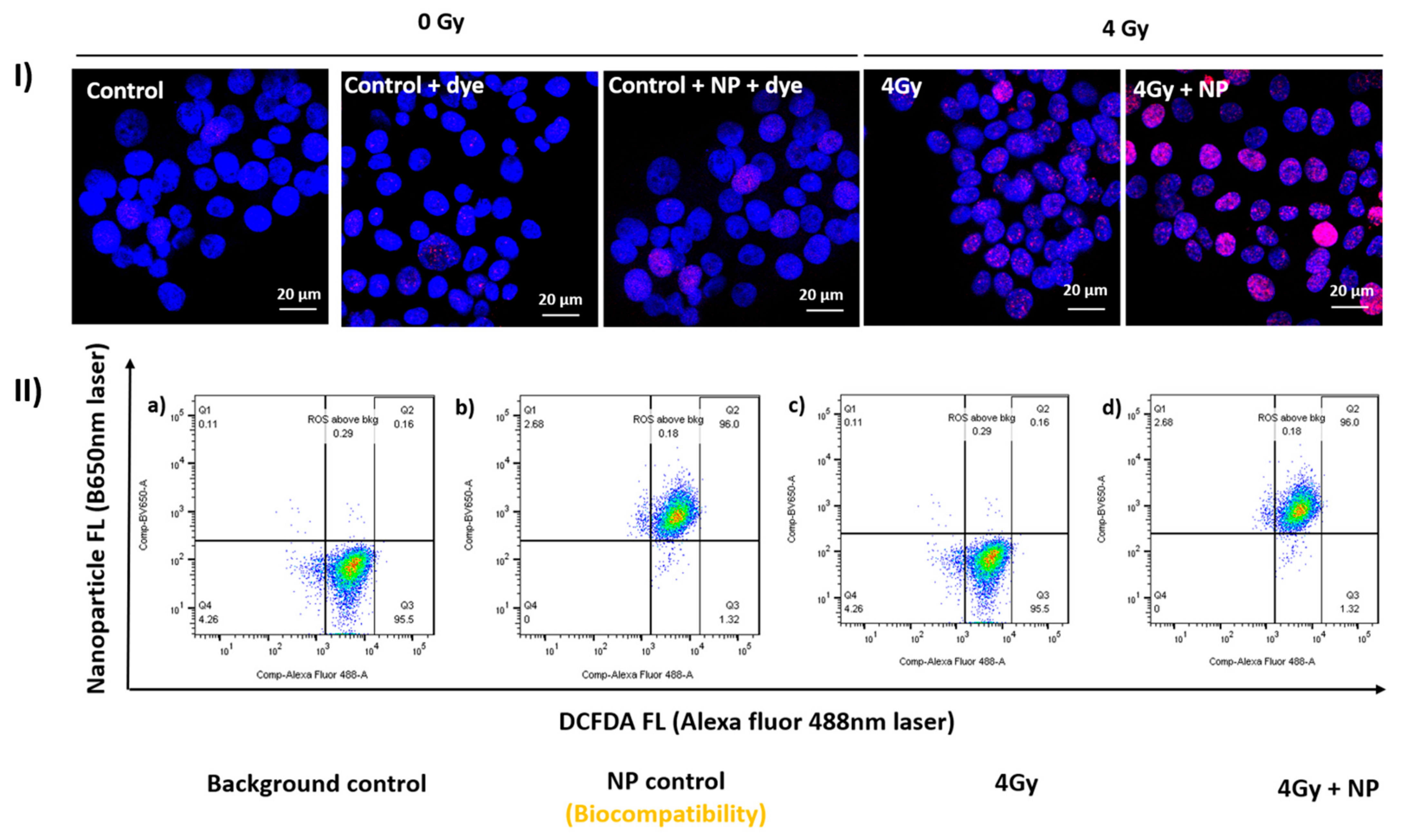
2. Metal Nanoparticles (MNPs) Function as Radiosensitizers
2.1. Proton-Based Radiosensitization by MNPs
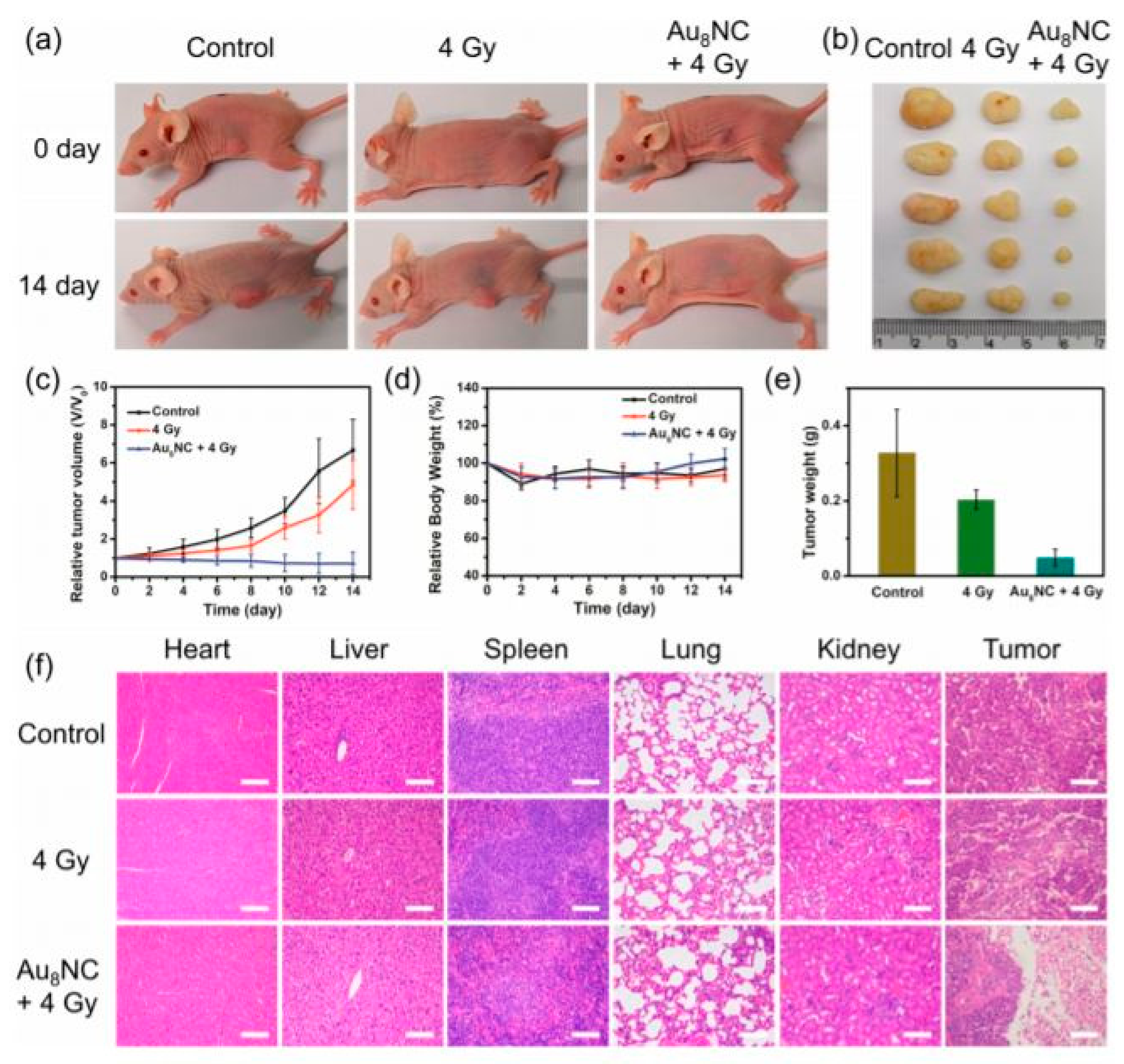
2.2. Gold Nanoparticle Applications
2.3. Gadolinium-Based Nanoparticles
2.4. Miscellaneous MNPs Explored as Radiosensitizer for HNCs
3. Metal Oxide Nanoparticles as Radioenhancers for HNCs
4. MNPs-Based Radiosensitizers in Clinical Trial
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Elkashty, O.A.; Ashry, R.; Tran, S.D. Head and neck cancer management and cancer stem cells implication. Saudi Dent. J. 2019, 31, 395–416. [Google Scholar] [CrossRef] [PubMed]
- Peitzsch, C.; Nathansen, J.; Schniewind, S.I.; Schwarz, F.; Dubrovska, A. Cancer Stem Cells in Head and Neck Squamous Cell Carcinoma: Identification, Characterization and Clinical Implications. Cancers 2019, 11, 616. [Google Scholar] [CrossRef] [Green Version]
- Pisani, E.B.P.; Airoldi, M.; Allais, A.; Valletti, P.A.; Battista, M.; Benazzo, M.; Briatore, R.; Cacciola, S.; Cocuzza, S.; Colombo, A.; et al. Metastatic disease in head & neck oncology. Acta Otorhinolaryngol. Ital. 2020, 40, S1–S86. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K. Intensity modulated radiotherapy: Advantages, limitations and future development. Biomed. Imaging Interv. J. 2006, 2, e19. [Google Scholar] [CrossRef]
- Liauw, S.L.; Connell, P.P.; Weichselbaum, R.R. New Paradigms and Future Challenges in Radiation Oncology: An Update of Biological Targets and Technology. Sci. Transl. Med. 2013, 5, 173sr2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.-Y.; Wu, L.-L.; Zhang, J.-Y.; Zheng, J.; Cheung, M.L.-M.; Ma, C.-C.; Xie, L.-X.; Huang, B.-T. Improving target dose coverage and organ-at-risk sparing in intensity-modulated radiotherapy of advanced laryngeal cancer by a simple optimization technique. Br. J. Radiol. 2015, 88, 20140654. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Hevia, D.; Patchva, S.; Park, B.; Koh, W.; Aggarwal, B.B. Upsides and Downsides of Reactive Oxygen Species for Cancer: The Roles of Reactive Oxygen Species in Tumorigenesis, Prevention, and Therapy. Antioxidants Redox Signal. 2012, 16, 1295–1322. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-C.; Chiang, C.-S. Challenges of Using High-Dose Fractionation Radiotherapy in Combination Therapy. Front. Oncol. 2016, 6, 165. [Google Scholar] [CrossRef] [Green Version]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Haddad, R. Current and future directions in the treatment of squamous cell carcinoma of the head and neck: Multidisciplinary symposium on head and neck cancer. Expert Opin. Ther. Targets 2006, 10, 333–336. [Google Scholar] [CrossRef]
- Pinna, R.; Campus, G.; Cumbo, E.; Mura, I.; Milia, E. Xerostomia induced by radiotherapy: An overview of the physiopathology, clinical evidence, and management of the oral damage. Ther. Clin. Risk Manag. 2015, 11, 171–188. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Wolfe, T.; Lee, J.; Brown, A.P.; Singh, P.K.; Bhattarai, S.R.; Diagaradjane, P.; Krishnan, S. Convergence of nanotechnology with radiation therapy—insights and implications for clinical translation. Transl. Cancer Res. 2013, 2, 256–268. [Google Scholar] [PubMed]
- De Lima, J.M.; Bonan, P.R.; da Cruz Perez, D.E.; Hier, M.; Alaoui-Jamali, M.A.; da Silva, S.D. Nanoparticle-based chemotherapy formulations for head and neck cancer: A systematic review and perspectives. Nanomaterials 2020, 10, 1938. [Google Scholar] [CrossRef] [PubMed]
- Björnmalm, M.; Thurecht, K.J.; Michael, M.; Scott, A.; Caruso, F. Bridging Bio–Nano Science and Cancer Nanomedicine. ACS Nano 2017, 11, 9594–9613. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Wang, K.; Oppong-Gyebi, A.; Hu, J. Application of Nanotechnology in Cancer Diagnosis and Therapy—A Mini-Review. Int. J. Med. Sci. 2020, 17, 2964–2973. [Google Scholar] [CrossRef]
- Kuncic, Z.; Lacombe, S. Nanoparticle radio-enhancement: Principles, progress and application to cancer treatment. Phys. Med. Biol. 2017, 63, 02TR01. [Google Scholar] [CrossRef]
- Kwatra, D.; Venugopal, A.; Anant, S. Nanoparticles in radiation therapy: A summary of various approaches to enhance radiosensitization in cancer. Transl. Cancer Res. 2013, 2, 330–342. [Google Scholar]
- Zhang, X.-Q.; Xu, X.; Bertrand, N.; Pridgen, E.; Swami, A.; Farokhzad, O.C. Interactions of nanomaterials and biological systems: Implications to personalized nanomedicine. Adv. Drug Deliv. Rev. 2012, 64, 1363–1384. [Google Scholar] [CrossRef] [PubMed]
- Pottier, A.; Borghi, E.; Levy, L. The future of nanosized radiation enhancers. Br. J. Radiol. 2015, 88, 20150171. [Google Scholar] [CrossRef]
- Jin, J.; Zhao, Q. Engineering nanoparticles to reprogram radiotherapy and immunotherapy: Recent advances and future challenges. J. Nanobiotechnol. 2020, 18, 75. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Caro, C.; Gámez, F.; Quaresma, P.; Páez-Muñoz, J.; Domínguez, A.; Pearson, J.; Leal, M.P.; Beltrán, A.; Fernandez-Afonso, Y.; De la Fuente, J.; et al. Fe3O4-Au Core-Shell Nanoparticles as a Multimodal Platform for In Vivo Imaging and Focused Photothermal Therapy. Pharmaceutics 2021, 13, 416. [Google Scholar] [CrossRef]
- Caro, C.; Pozo, D. Polysaccharide Colloids as Smart Vehicles in Cancer Therapy. Curr. Pharm. Des. 2015, 21, 4822–4836. [Google Scholar] [CrossRef]
- Pottier, A.; Borghi, E.; Levy, L. New use of metals as nanosized radioenhancers. Anticancer. Res. 2014, 34, 443–453. [Google Scholar]
- Teske, S.S.; Detweiler, C.S. The Biomechanisms of Metal and Metal-Oxide Nanoparticles’ Interactions with Cells. Int. J. Environ. Res. Public Health 2015, 12, 1112–1134. [Google Scholar] [CrossRef] [Green Version]
- Schuemann, J.; Bagley, A.F.; Berbeco, R.; Bromma, K.; Butterworth, K.T.; Byrne, H.L.; Chithrani, B.D.; Cho, S.H.; Cook, J.R.; Favaudon, V.; et al. Roadmap for metal nanoparticles in radiation therapy: Current status, translational challenges, and future directions. Phys. Med. Biol. 2020, 65, 21RM02. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Li, F.; Jin, X.; Li, J.; Chen, W.; Li, Q. Metal-based NanoEnhancers for Future Radiotherapy: Radiosensitizing and Synergistic Effects on Tumor Cells. Theranostics 2018, 8, 1824–1849. [Google Scholar] [CrossRef]
- Liu, T.; Yang, K.; Liu, Z. Recent advances in functional nanomaterials for X-ray triggered cancer therapy. Prog. Nat. Sci. 2020, 30, 567–576. [Google Scholar] [CrossRef]
- Wälzlein, C.; Scifoni, E.; Krämer, M.; Durante, M. Simulations of dose enhancement for heavy atom nanoparticles irradiated by protons. Phys. Med. Biol. 2014, 59, 1441–1458. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Coulter, J.; Jain, S.; Butterworth, K.; Schettino, G.; Dickson, G.R.; Hounsell, A.R.; O’Sullivan, J.M.; et al. Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci. Rep. 2011, 1, 18. [Google Scholar] [CrossRef] [Green Version]
- Rashid, R.A.; Abidin, S.Z.; Anuar, M.A.K.; Tominaga, T.; Akasaka, H.; Sasaki, R.; Kie, K.; Razak, K.A.; Pham, B.T.; Hawkett, B.; et al. Radiosensitization effects and ROS generation by high Z metallic nanoparticles on human colon carcinoma cell (HCT116) irradiated under 150 MeV proton beam. OpenNano 2019, 4, 100027. [Google Scholar] [CrossRef]
- Martínez-Rovira, I.; Seksek, O.; Dokic, I.; Brons, S.; Abdollahi, A.; Yousef, I. Study of the intracellular nanoparticle-based radiosensitization mechanisms in F98 glioma cells treated with charged particle therapy through synchrotron-based infrared microspectroscopy. Analyst 2020, 145, 2345–2356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbull, T.; Douglass, M.; Williamson, N.H.; Howard, D.; Bhardwaj, R.; Lawrence, M.; Paterson, D.J.; Bezak, E.; Thierry, B.; Kempson, I.M. Cross-Correlative Single-Cell Analysis Reveals Biological Mechanisms of Nanoparticle Radiosensitization. ACS Nano 2019, 13, 5077–5090. [Google Scholar] [CrossRef] [PubMed]
- Damasco, J.A.; Ohulchanskyy, T.Y.; Mahajan, S.; Chen, G.; Singh, A.; Kutscher, H.L.; Huang, H.; Turowski, S.G.; Spernyak, J.A.; Singh, A.K.; et al. Excretable, ultrasmall hexagonal NaGdF4:Yb50% nanoparticles for bimodal imaging and radiosensitization. Cancer Nanotechnol. 2021, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, G.; Bin Cho, S.; Im, H.-J. Radiosensitizing high-Z metal nanoparticles for enhanced radiotherapy of glioblastoma multiforme. J. Nanobiotechnol. 2020, 18, 122. [Google Scholar] [CrossRef]
- Butterworth, K.T.; McMahon, S.J.; Currell, F.J.; Prise, K.M. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 2012, 4, 4830–4838. [Google Scholar] [CrossRef]
- Lin, A.; Swisher-McClure, S.; Millar, L.B.; Kirk, M.; Yeager, C.; Kassaee, A.; Teo, B.K.K.; Hahn, S.M. Proton therapy for head and neck cancer: Current applications and future directions. Transl. Cancer Res. 2012, 1, 28. [Google Scholar]
- Brada, M.; Pijls-Johannesma, M.; De Ruysscher, D. Proton Therapy in Clinical Practice: Current Clinical Evidence. J. Clin. Oncol. 2007, 25, 965–970. [Google Scholar] [CrossRef] [Green Version]
- Lowe, M.; Gosling, A.; Nicholas, O.; Underwood, T.; Miles, E.; Chang, Y.-C.; Amos, R.; Burnet, N.; Clark, C.; Patel, I.; et al. Comparing Proton to Photon Radiotherapy Plans: UK Consensus Guidance for Reporting Under Uncertainty for Clinical Trials. Clin. Oncol. 2020, 32, 459–466. [Google Scholar] [CrossRef]
- Peukert, D.; Kempson, I.; Douglass, M.; Bezak, E. Gold Nanoparticle Enhanced Proton Therapy: Monte Carlo Modeling of Reactive Species’ Distributions Around a Gold Nanoparticle and the Effects of Nanoparticle Proximity and Clustering. Int. J. Mol. Sci. 2019, 20, 4280. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; McMahon, S.; Scarpelli, M.; Paganetti, H.; Schuemann, J. Comparing gold nano-particle enhanced radiotherapy with protons, megavoltage photons and kilovoltage photons: A Monte Carlo simulation. Phys. Med. Biol. 2014, 59, 7675–7689. [Google Scholar] [CrossRef] [PubMed]
- Gold-Decorated Platinum and Palladium Nanoparticles as Modern Nanocomplexes to Improve the Effectiveness of Simulated Anticancer Proton Therapy. Pharmaceutics 2021, 13, 1–14.
- Gadoue, S.M.; Toomeh, D. Enhancement of linear energy transfer in gold nanoparticles mediated radiation therapy. Phys. Medica 2019, 60, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Schlatholter, T.; Eustache, P.; Porcel, E.; Salado, D.; Stefancikova, L.; Tillement, O.; Lux, F.; Mowat, P.; Biegun, A.K.; van Goethem, M.-J.; et al. Improving proton therapy by metal-containing nanoparticles: Nanoscale insights. Int. J. Nanomed. 2016, 11, 1549–1556. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, Y.; Fukumitsu, N.; Ishikawa, H.; Nakai, K.; Sakurai, H. A Critical Review of Radiation Therapy: From Particle Beam Therapy (Proton, Carbon, and BNCT) to Beyond. J. Pers. Med. 2021, 11, 825. [Google Scholar] [CrossRef] [PubMed]
- Penninckx, S.; Heuskin, A.C.; Michiels, C.; Lucas, S. Gold nanoparticles as a potent radiosensitizer: A transdisciplinary approach from physics to patient. Cancers 2020, 12, 2021. [Google Scholar] [CrossRef]
- Peng, J.; Liang, X. Progress in research on gold nanoparticles in cancer management. Medicine 2019, 98, e15311. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Herold, D.M.; Das, I.J.; Stobbe, C.C.; Iyer, R.V.; Chapman, J.D. Gold microspheres: A selective technique for producing biologically effective dose enhancement. Int. J. Radiat. Biol. 2000, 76, 1357–1364. [Google Scholar]
- Hainfeld, J.F.; Dilmanian, F.A.; Zhong, Z.; Slatkin, D.N.; Kalef-Ezra, J.A.; Smilowitz, H.M. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys. Med. Biol. 2010, 55, 3045–3059. [Google Scholar] [CrossRef]
- Koonce, N.A.; Quick, C.M.; Hardee, M.E.; Jamshidi-Parsian, A.; Dent, J.A.; Paciotti, G.F.; Nedosekin, D.; Dings, R.; Griffin, R.J. Combination of Gold Nanoparticle-Conjugated Tumor Necrosis Factor-α and Radiation Therapy Results in a Synergistic Antitumor Response in Murine Carcinoma Models. Int. J. Radiat. Oncol. 2015, 93, 588–596. [Google Scholar] [CrossRef] [Green Version]
- Teraoka, S.; Kakei, Y.; Akashi, M.; Iwata, E.; Hasegawa, T.; Miyawaki, D.; Sasaki, R.; Komori, T. Gold nanoparticles enhance X-ray irradiation-induced apoptosis in head and neck squamous cell carcinoma in vitro. Biomed. Rep. 2018, 9, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Popovtzer, A.; Mizrachi, A.; Motiei, M.; Bragilovski, D.; Lubimov, L.; Levi, M.; Hilly, O.; Ben-Aharon, I.; Popovtzer, R. Actively targeted gold nanoparticles as novel radiosensitizer agents: An in vivo head and neck cancer model. Nanoscale 2016, 8, 2678–2685. [Google Scholar] [CrossRef]
- Davidi, E.S.; Dreifuss, T.; Motiei, M.; Shai, E.; Bragilovski, D.; Lubimov, L.; Kindler, M.J.J.; Popovtzer, A.; Don, J.; Popovtzer, R. Cisplatin-conjugated gold nanoparticles as a theranostic agent for head and neck cancer. Head Neck 2018, 40, 70–78. [Google Scholar] [CrossRef]
- Kashin, M.; Kakei, Y.; Teraoka, S.; Hasegawa, T.; Yamaguchi, A.; Fukuoka, T.; Sasaki, R.; Akashi, M. Gold Nanoparticles Enhance EGFR Inhibition and Irradiation Effects in Head and Neck Squamous Carcinoma Cells. BioMed Res. Int. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.-T.; Yang, G.; Mo, S.-J.; Wang, Z.-Y.; Li, B.-J.; Ma, W.; Guo, Y.-X.; Chen, X.; Zhao, X.; Liu, J.-Q.; et al. Atomically Precise Gold–Levonorgestrel Nanocluster as a Radiosensitizer for Enhanced Cancer Therapy. ACS Nano 2019, 13, 8320–8328. [Google Scholar] [CrossRef]
- Butterworth, K.T.; McMahon, S.J.; Taggart, L.E.; Prise, K.M. Radiosensitization by gold nanoparticles: Effective at megavoltage energies and potential role of oxidative stress. Transl. Cancer Res. 2013, 2, 269–279. [Google Scholar]
- Anijdan, S.H.M.; Mahdavi, S.R.; Shirazi, A.; Zarrinfard, M.A.; Hajati, J. Megavoltage X-ray Dose Enhancement with Gold Nanoparticles in Tumor Bearing Mice. Int. J. Mol. Cell. Med. 2013, 2, 118–123. [Google Scholar]
- Miladi, I.; Aloy, M.-T.; Armandy, E.; Mowat, P.; Kryza, D.; Magné, N.; Tillement, O.; Lux, F.; Billotey, C.; Janier, M.; et al. Combining ultrasmall gadolinium-based nanoparticles with photon irradiation overcomes radioresistance of head and neck squamous cell carcinoma. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lafrasse, C.; Aloy, M.-T.; Miladi, I.; Armandy, E.; Sancey, L.; Berniard, A.; Billotey, C.; Tillement, O.; Lux, F.; Perriat, P.; et al. Gadolinium based nanoparticles for radiosensitization of head and neck squamous cell carcinoma. Radiother. Oncol. 2014, 110, 247–257. [Google Scholar] [CrossRef]
- Lux, F.; Tran, V.L.; Thomas, E.; Dufort, S.; Rossetti, F.; Martini, M.; Truillet, C.; Doussineau, T.; Bort, G.; Denat, F.; et al. AGuIX® from bench to bedside—Transfer of an ultrasmall theranostic gadolinium-based nanoparticle to clinical medicine. Br. J. Radiol. 2018, 92, 20180365. [Google Scholar] [CrossRef] [PubMed]
- Quatre, R.; Jacquet, T.; Atallah, I.; Tillement, O.; Lux, F.; Coll, J.; Dufort, S.; Righini, C. Evaluation of the theranostic properties of gadolinium-based nanoparticles for head and neck cancer. Head Neck 2018, 41, 403–410. [Google Scholar] [CrossRef]
- Wozny, A.-S.; Aloy, M.-T.; Alphonse, G.; Magné, N.; Janier, M.; Tillement, O.; Lux, F.; Beuve, M.; Rodriguez-Lafrasse, C. Gadolinium-based nanoparticles as sensitizing agents to carbon ions in head and neck tumor cells. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2655–2660. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhang, Y.; Lu, H.; Zhao, D. Silver nanoparticles coupled to anti-EGFR antibodies sensitize nasopharyngeal carcinoma cells to irradiation. Mol. Med. Rep. 2017, 16, 9005–9010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thapa, R.; Galoforo, S.; Kandel, S.M.; El-Dakdouki, M.H.; Wilson, T.G.; Huang, X.; Roth, B.J.; Wilson, G.D. Radiosensitizing and Hyperthermic Properties of Hyaluronan Conjugated, Dextran-Coated Ferric Oxide Nanoparticles: Implications for Cancer Stem Cell Therapy. J. Nanomater. 2015, 2015, 400. [Google Scholar] [CrossRef]
- Beola, L.; Grazú, V.; Fernández-Afonso, Y.; Fratila, R.M.; Heras, M.D.L.; de la Fuente, J.M.; Gutiérrez, L.; Asín, L. Critical Parameters to Improve Pancreatic Cancer Treatment Using Magnetic Hyperthermia: Field Conditions, Immune Response, and Particle Biodistribution. ACS Appl. Mater. Interfaces 2021, 13, 12982–12996. [Google Scholar] [CrossRef]
- García-Soriano, D.; Amaro, R.; Lafuente-Gómez, N.; Rois, P.M.; Somoza, Álvaro; Navío, C.; Herranz, F.; Gutiérrez, L.; Salas, G. The influence of cation incorporation and leaching in the properties of Mn-doped nanoparticles for biomedical applications. J. Colloid Interface Sci. 2020, 578, 510–521. [Google Scholar] [CrossRef]
- Beola, L.L.; Asín, L.; Fratila, R.M.M.; Herrero, V.; De La Fuente, J.M.; Grazú, V.; Gutiérrez, L. Dual Role of Magnetic Nanoparticles as Intracellular Hotspots and Extracellular Matrix Disruptors Triggered by Magnetic Hyperthermia in 3D Cell Culture Models. ACS Appl. Mater. Interfaces 2018, 10, 44301–44313. [Google Scholar] [CrossRef]
- Gehrke, T.; Scherzad, A.; Ickrath, P.; Schendzielorz, P.; Hagen, R.; Kleinsasser, N.; Hackenberg, S. Zinc oxide nanoparticles antagonize the effect of Cetuximab on head and neck squamous cell carcinoma in vitro. Cancer Biol. Ther. 2017, 18, 513–518. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, I.H. Nanotechnology in Head and Neck Cancer: The Race Is On. Curr. Oncol. Rep. 2010, 12, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Scher, N.; Bonvalot, S.; Le Tourneau, C.; Chajon, E.; Verry, C.; Thariat, J.; Calugaru, V. Review of clinical applications of radiation-enhancing nanoparticles. Biotechnol. Rep. 2020, 28, e00548. [Google Scholar] [CrossRef]
- Selvaraja, V.K.; Gudipudi, D.K. Fundamentals to clinical application of nanoparticles in cancer immunotherapy and radiotherapy. Ecancermedicalscience 2020, 14, 1095. [Google Scholar] [CrossRef]
- Wu, T.-T.; Zhou, S.-H. Nanoparticle-Based Targeted Therapeutics in Head-And-Neck Cancer. Int. J. Med. Sci. 2015, 12, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Ulldemolins, A.; Seras-Franzoso, J.; Andrade, F.; Rafael, D.; Abasolo, I.; Gener, P.; Jr, S.S. Perspectives of nano-carrier drug delivery systems to overcome cancer drug resistance in the clinics. Cancer Drug Resist. 2021, 4, 44–68. [Google Scholar] [CrossRef]
- Hua, S.; De Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The Use of Alternative Strategies for Enhanced Nanoparticle Delivery to Solid Tumors. Chem. Rev. 2021, 121, 1746–1803. [Google Scholar] [CrossRef] [PubMed]
- Seniwal, B.; Thipe, V.C.; Singh, S.; Fonseca, T.C.F.; Freitas, L.F. Recent Advances in Brachytherapy Using Radioactive Nanoparticles: An Alternative to Seed-Based Brachytherapy. Front. Oncol. 2021, 11, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bayda, S.; Hadla, M.; Palazzolo, S.; Riello, P.; Corona, G.; Toffoli, G.; Rizzolio, F. Inorganic Nanoparticles for Cancer Therapy: A Transition from Lab to Clinic. Curr. Med. Chem. 2018, 25, 4269–4303. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Calugaru, V.; Thariat, J.; Florescu, C.; Mirabel, X.; Jegoux, F.; Jouffroy, T.; Rodriguez, J.; Hoffmann, C.; Dodger, B.; et al. Hafnium Oxide Nanoparticles as a Promising Emergent Treatment for Head and Neck Cancer. Int. J. Radiat. Oncol. 2018, 100, 1377. [Google Scholar] [CrossRef] [Green Version]
| Collaborator | MNP Based Radiosensitizer | Study Phase | Mode of Administration | Age (yrs) | ClinicalTrials.gov Identifier (NCT #) | Study Start Date | Completion Date |
|---|---|---|---|---|---|---|---|
| Nanobiotix and M.D. Anderson Cancer Center Houston, Texas, United States | NBTXR3 (Hafnium oxide NPs) | 1/2 | Single intra-arterial injection | >65 | NCT01946867 | 08/2013 | 08/2017 |
| M.D. Anderson Cancer CenterHouston, Texas, United States | NBTXR3, Radiation Therapy, and Pembrolizumab | 2 | Intratumoral/ Intranodal | >18 | NCT04862455 | 04/2021 | 09/2026 (Active) |
| National Institutes of Health Clinical Center (CC) National Cancer Institute (NCI) | CYT-6091 (TNF-bound colloidal gold) | 1 | Intravenous Administration | >18 | NCT00356980 | 07/2006 | 03/2012 |
| Nanoparticles | Size | Model Studied | Photon Radiation Dose/Energy |
|---|---|---|---|
| Gold nanoparticles (51) | 1.9 nm | In vivo mouse head and neck squamous cell carcinoma model, SCCVII | 42 Gy, 30 Gy, 50.6 Gy |
| Cetuximab-targeted gold nanoparticles (GNPs) (54) | 30 nm | In vivo mouse A431 cells | 25 Gy |
| Glucose and Cisplatin (CG-GNPs) (55) | 20 nm | A431 cells for in vitro and in vivo mice experiments | 6 MV |
| Gadolinium-based nanoparticles (GBNs) (60) | 2.9 ± 0.2 nm | Radioresistant human head and neck squamous cell carcinoma (SQ20B, FaDu and Cal33 cell lines) and SQ20B tumor-bearing mouse model | 10 Gy |
| Gadolinium-based nanoparticles (AGuIX®) (61) | 5 nm | HNC cell lines (SQ20B, FaDu, and Cal33) | 1–4 Gy |
| Gadolinium-based nanoparticles (AGuIX®) (62) | 5 nm | Cal33 Orthotopic female NMRI nude mouse | 10 Gy |
| Nanocomposite Ag/C225, constructed, which consisted of silver nanoparticles (AgNPs) conjugated to an epidermal growth factor receptor-specific antibody (C225) (65) | 20 nm | Nasopharyngeal carcinoma epithelial (CNE) | 6 MV X-ray irradiation (dose rate 200 cGy/min) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubey, P.; Sertorio, M.; Takiar, V. Therapeutic Advancements in Metal and Metal Oxide Nanoparticle-Based Radiosensitization for Head and Neck Cancer Therapy. Cancers 2022, 14, 514. https://doi.org/10.3390/cancers14030514
Dubey P, Sertorio M, Takiar V. Therapeutic Advancements in Metal and Metal Oxide Nanoparticle-Based Radiosensitization for Head and Neck Cancer Therapy. Cancers. 2022; 14(3):514. https://doi.org/10.3390/cancers14030514
Chicago/Turabian StyleDubey, Poornima, Mathieu Sertorio, and Vinita Takiar. 2022. "Therapeutic Advancements in Metal and Metal Oxide Nanoparticle-Based Radiosensitization for Head and Neck Cancer Therapy" Cancers 14, no. 3: 514. https://doi.org/10.3390/cancers14030514
APA StyleDubey, P., Sertorio, M., & Takiar, V. (2022). Therapeutic Advancements in Metal and Metal Oxide Nanoparticle-Based Radiosensitization for Head and Neck Cancer Therapy. Cancers, 14(3), 514. https://doi.org/10.3390/cancers14030514







