Prognostic and Clinicopathological Significance of the Aberrant Expression of β-Catenin in Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Study Selection Process
2.5. Data Extraction
2.6. Evaluation of Quality and Risk of Bias
2.7. Statistical Analysis
2.8. Validation of Methodological Quality
3. Results
3.1. Results of the Literature Search
3.2. Study Characteristics
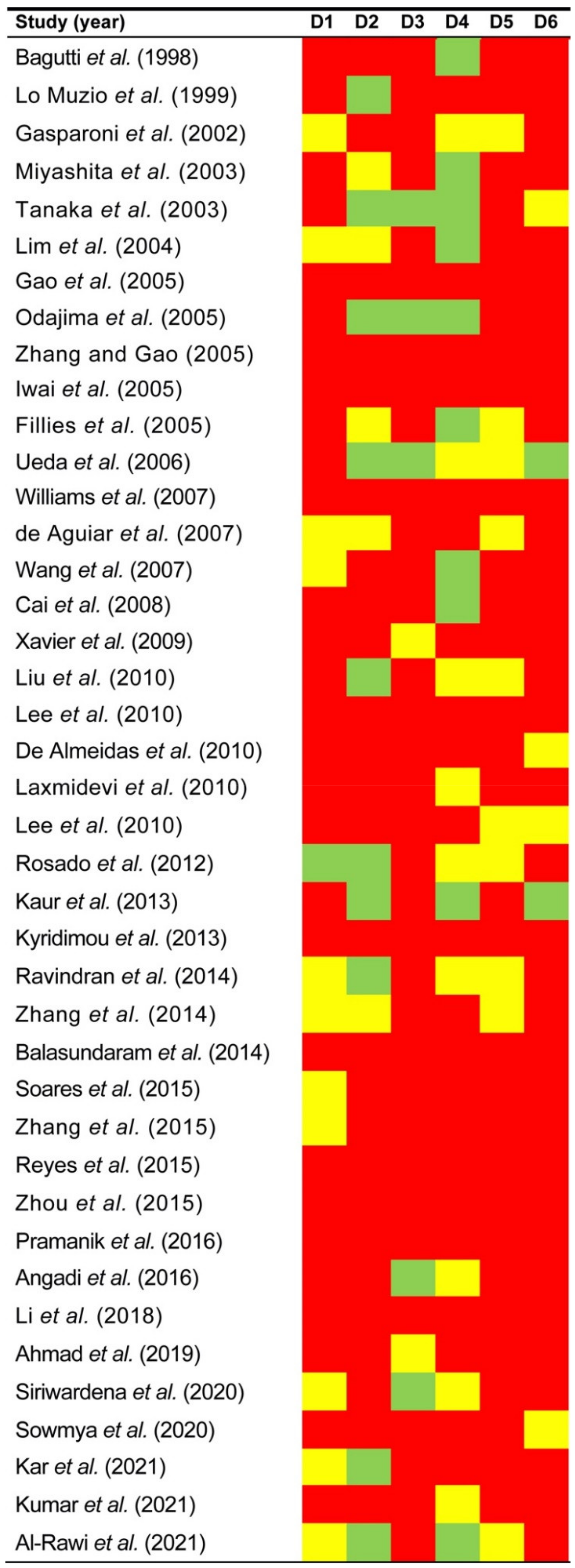
3.3. Qualitative Evaluation
3.3.1. Study Participation
3.3.2. Study Attrition
3.3.3. Prognostic Factor Measurement
3.3.4. Outcome Measurement
3.3.5. Study Confounding
3.3.6. Statistical Analysis and Reporting
3.4. Quantitative Evaluation (Meta-Analysis)
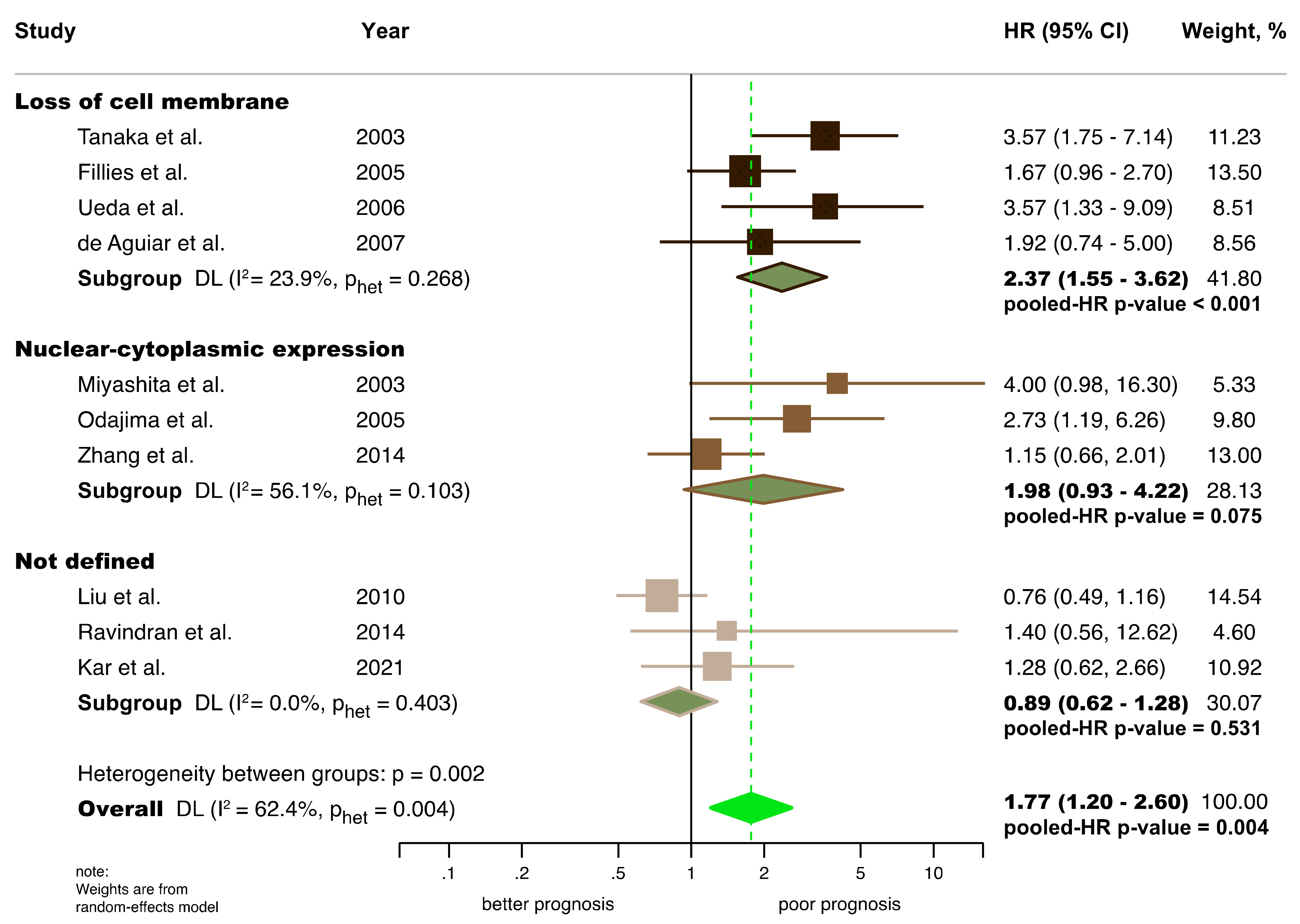
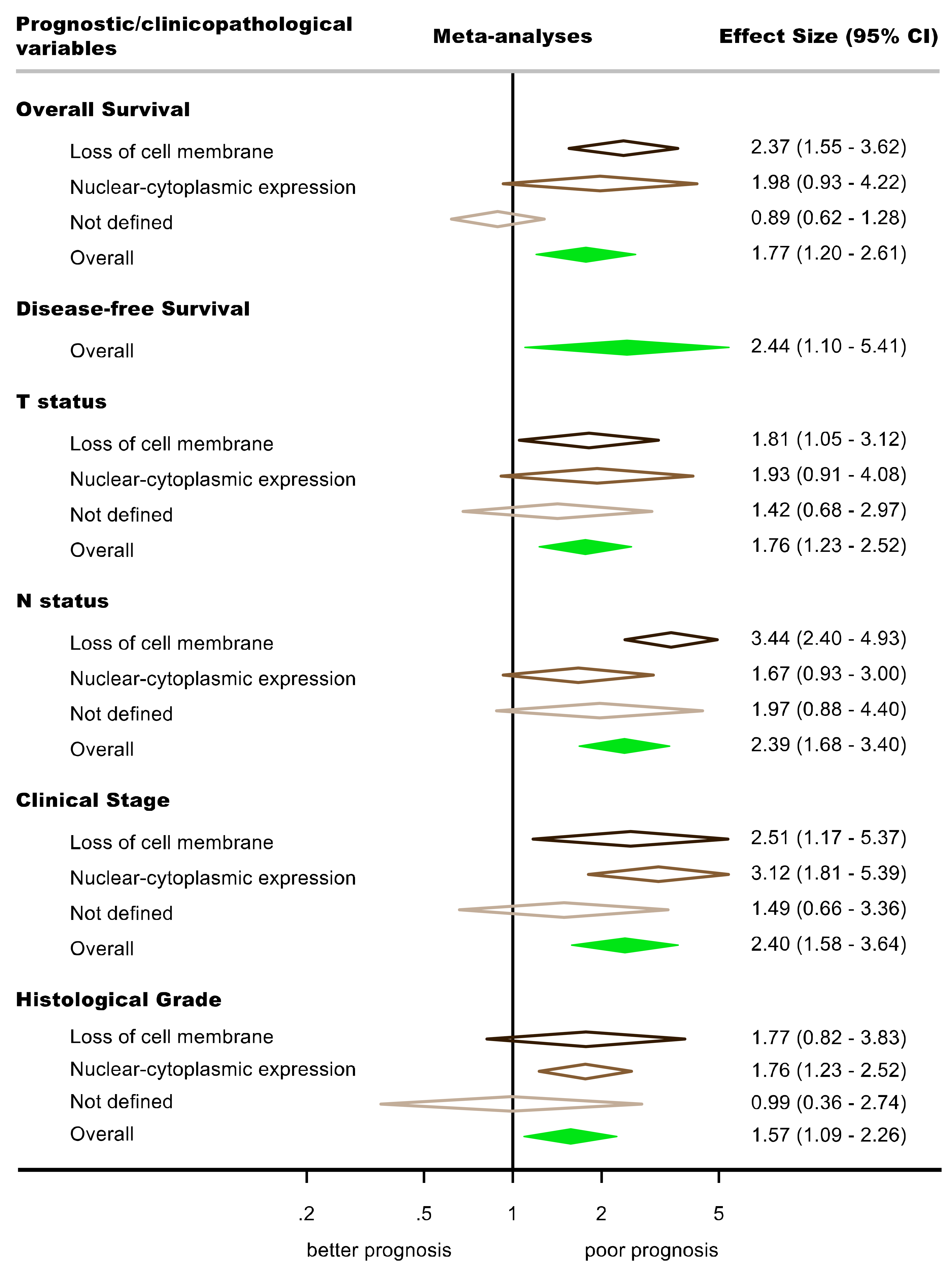
3.4.1. Association between the Aberrant Expression of β-Catenin and Prognostic Variables
Overall Survival (OS)
Disease-Free Survival (DFS)
3.4.2. Association between the Aberrant Expression of β-Catenin and Clinicopathological Variables
T Status
N Status
Clinical Stage
Histological Grade
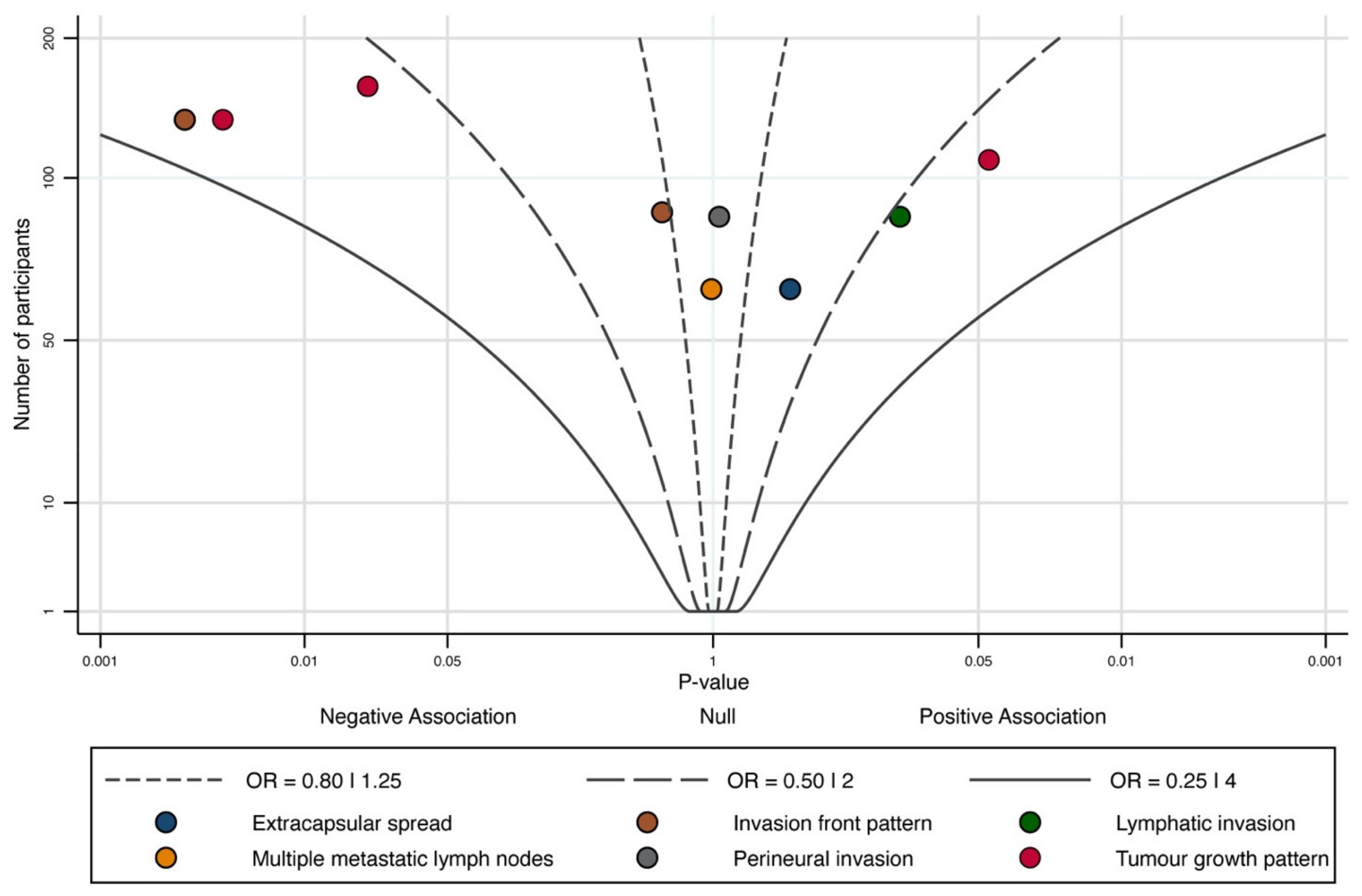
3.5. Quantitative Evaluation (Variables Not Included in Meta-Analysis)
3.6. Quantitative Evaluation (Secondary Analyses)
3.6.1. Analysis of Subgroups
3.6.2. Meta-Regression Analysis
3.6.3. Sensitivity Analysis
3.6.4. Analysis of Small-Study Effects
3.7. Validation of Methodological Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma-an update. CA Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef] [PubMed]
- González-moles, M.A.; Ruiz-ávila, I.; Gil-montoya, J.A.; Plaza-campillo, J.; Scully, C. β-Catenin in oral cancer: An update on current knowledge. Oral Oncol. 2014, 50, 818–824. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Kimelman, D.; Xu, W. Beta-catenin destruction complex: Insights and questions from a structural perspective. Oncogene 2006, 4, 7482–7491. [Google Scholar] [CrossRef] [PubMed]
- Atcha, F.A.; Syed, A.; Wu, B.; Hoverter, N.P.; Yokoyama, N.N.; Ting, J.-H.T.; Munguia, J.E.; Mangalam, H.J.; Marsh, J.L.; Waterman, M.L. A Unique DNA Binding Domain Converts T-Cell Factors into Strong Wnt Effectors. Mol. Cell. Biol. 2007, 27, 8352–8363. [Google Scholar] [CrossRef] [PubMed]
- Auersperg, N.; Pan, J.; Grove, B.D.; Peterson, T.; Fisher, J.; Maines-Bandiera, S.; Somasiri, A.; Roskelley, C.D. E-cadherin induces mesenchymal-to-epithelial transition in human ovarian surface epithelium. Proc. Natl. Acad. Sci. USA 1999, 96, 6249–6254. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.A.; Scully, C.; Ruiz-Ávila, I.; Plaza-Campillo, J.J. The cancer stem cell hypothesis applied to oral carcinoma. Oral Oncol. 2013, 49, 738–746. [Google Scholar] [CrossRef]
- Bailey, T.; Biddlestone, L.; Shepherd, N.; Barr, H.; Warner, P.; Jankowski, J. Altered cadherin and catenin complexes in the Barrett’s esophagus- dysplasia-adenocarcinoma sequence: Correlation with disease progression and dedifferentiation. Am. J. Pathol. 1998, 152, 135–144. [Google Scholar]
- Hao, X.; Palazzo, J.P.; Ilyas, M.; Tomlinson, I.; Talbot, I.C. Reduced expression of molecules of the cadherin/catenin complex in the transition from colorectal adenoma to carcinoma. Anticancer Res. 1997, 17, 2241–2247. [Google Scholar] [PubMed]
- Psyrri, A.; Kotoula, V.; Fountzilas, E.; Alexopoulou, Z.; Bobos, M.; Televantou, D.; Karayannopoulou, G.; Krikelis, D.; Markou, K.; Karasmanis, I.; et al. Prognostic significance of the Wnt pathway in squamous cell laryngeal cancer. Oral Oncol. 2014, 50, 298–305. [Google Scholar] [CrossRef]
- Williams, H.K.; Sanders, D.S.A.; Jankowski, J.A.Z.; Landini, G.; Brown, A.M.S. Expression of cadherins and catenins in oral epithelial dysplasia and squamous cell carcinoma. J. Oral Pathol. Med. 1998, 27, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Bankfalvi, A.; Krabort, M.; Buchwalow, I.; Vegh, A.; Felszeghy, E.; Piffko, J. Gains and losses of adhesion molecules (CD44, E-cadherin, and beta-catenin) during oral carcinogenesis and tumour progression. J. Pathol. 2002, 198, 343–351. [Google Scholar] [CrossRef]
- Bánkfalvi, A.; Krassort, M.; Végh, A.; Felszeghy, E.; Piffkó, J. Deranged expression of the E-cadherin/beta-catenin complex and the epidermal growth factor receptor in the clinical evolution and progression of oral squamous cell carcinomas. J. Oral Pathol. Med. 2002, 31, 450–457. [Google Scholar] [CrossRef]
- Ishida, K.; Ito, S.; Wada, N.; Deguchi, H.; Hata, T.; Hosoda, M.; Nohno, T. Nuclear localization of beta-catenin involved in precancerous change in oral leukoplakia. Mol. Cancer 2007, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Muzio, L.L.; Russo, L.L.; Falaschini, S.; Ciavarella, D.; Pentenero, M.; Arduino, P.; Favia, G.; Maiorano, E.; Rubini, C.; Pieramici, T.; et al. Beta- and Gamma-Catenin Expression in Oral Dysplasia. Oral Oncol. 2009, 45, 501–504. [Google Scholar] [CrossRef]
- Moles, M.A.G.; Montoya, J.A.G.; Salvago, M.D.M.; Ávila, I.R.; Campillo, J.J.P.; Bravo, M. Implications of differential expression of catenin in oral carcinoma. Anticancer Res. 2016, 36, 1599–1604. [Google Scholar] [PubMed]
- Garcia, N.G.; González-Moles, M.A.; Ruiz-Ávila, I.; Bravo, M.; Ramos-García, P.; Minicucci, E.M.; Domingues, M.A.C.; Oliveira, D.T. Asymmetrical proliferative pattern loss linked to cyclin D1 overexpression during malignant transformation of the lip epithelium. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.D.; Ridley, G.; Williams, K.; Altman, D.G.; Hayden, J.; de Vet, H.C.W. Prognosis research: Toward evidence-based results and a Cochrane methods group. J. Clin. Epidemiol. 2007, 60, 863–865. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Available online: http://handbook.cochrane.org/ (accessed on 28 December 2021).
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.A.; Côté, P.; Bombardier, C. Evaluation of the quality of prognosis studies in systematic reviews. Ann. Intern. Med. 2006, 144, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Ayén, Á.; González-Ruiz, I.; de Porras-Carrique, T.; González-Ruiz, L.; Ruiz-Ávila, I.; Ramos-García, P. Prognostic and Clinicopathological Significance of FADD Upregulation in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 2393. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Parmar, M.K.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Thompson, S.G.; Higgins, J.P.T. How should meta-regression analyses be undertaken and interpreted? Stat. Med. 2002, 21, 1559–1573. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Controlling the risk of spurious findings from meta-regression. Stat. Med. 2004, 23, 1663–1682. [Google Scholar] [CrossRef]
- Manly, B.F.J. Randomization, Bootstrap and Monte Carlo Methods in Biology; Taylor & Francis Group: New York, NY, USA, 2006; Volume 53. [Google Scholar]
- Viechtbauer, W.; Cheung, M.W.-L. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Harrison, S.; Jones, H.E.; Martin, R.M.; Lewis, S.J.; Higgins, J.P.T. The albatross plot: A novel graphical tool for presenting results of diversely reported studies in a systematic review. Res. Synth. Methods 2017, 8, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Bagutti, C.; Speight, P.M.; Watt, F.M. Comparison of integrin, cadherin, and catenin expression in squamous cell carcinomas of the oral cavity. J. Pathol. 1998, 186, 8–16. [Google Scholar] [CrossRef]
- Iwai, S.; Katagiri, W.; Kong, C.; Amekawa, S.; Nakazawa, M.; Yura, Y. Mutations of the APC, beta-catenin, and axin 1 genes and cytoplasmic accumulation of beta-catenin in oral squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2005, 131, 773–782. [Google Scholar] [CrossRef]
- Fillies, T.; Buerger, H.; Gaertner, C.; August, C.; Brandt, B.; Joos, U.; Werkmeister, R. Catenin expression in T1/2 carcinomas of the floor of the mouth. Int. J. Oral Maxillofac. Surg. 2005, 34, 907–911. [Google Scholar] [CrossRef]
- Ueda, G.; Sunakawa, H.; Nakamori, K.; Shinya, T.; Tsuhako, W.; Tamura, Y.; Kosugi, T.; Sato, N.; Ogi, K.; Hiratsuka, H. Aberrant expression of β- and γ-catenin is an independent prognostic marker in oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2006, 35, 356–361. [Google Scholar] [CrossRef] [PubMed]
- De Aguiar, F.C.A.; Kowalski, L.P.; de Almeida, O.P. Clinicopathological and immunohistochemical evaluation of oral squamous cell carcinoma in patients with early local recurrence. Oral Oncol. 2007, 43, 593–601. [Google Scholar] [CrossRef]
- Wang, L.; Liu, T.; Wang, Y.; Cao, L.; Nishioka, M.; Aguirre, R.L.; Ishikawa, A.; Geng, L.; Okada, N. Altered expression of desmocollin 3, desmoglein 3, and β-catenin in oral squamous cell carcinoma: Correlation with lymph node metastasis and cell proliferation. Virchows Arch. 2007, 451, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.G.; Shi, X.J.; Gao, Y.; Wei, M.J.; Wang, C.Y.; Yu, G.Y. Β-Catenin Expression Pattern in Primary Oral Squamous Cell Carcinoma. Chin. Med. J. 2008, 121, 1866–1870. [Google Scholar] [CrossRef] [PubMed]
- Xavier, F.C.A.; Rodini, C.O.; Ramalho, L.M.P.; Mantesso, A.; Nunes, F.D. WNT-5A, but not matrix metalloproteinase 3 or β-catenin protein, expression is related to early stages of lip carcinogenesis. J. Oral Pathol. Med. 2009, 38, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.K.; Jiang, X.Y.; Zhou, X.X.; Wang, D.M.; Song, X.L.; Jiang, H.B. Upregulation of vimentin and aberrant expression of E-cadherin/Β- catenin complex in oral squamous cell carcinomas: Correlation with the clinicopathological features and patient outcome. Mod. Pathol. 2010, 23, 213–224. [Google Scholar] [CrossRef]
- Lee, C.H.; Hung, H.W.; Hung, P.H.; Shieh, Y.S. Epidermal growth factor receptor regulates β-catenin location, stability, and transcriptional activity in oral cancer. Mol. Cancer 2010, 9, 64. [Google Scholar] [CrossRef]
- Freitas, R.D.A.; Silveira, E.J.D.; Da Silveira, J.P.B.; Da Silva, F.M.; De Amorim, R.F.B. Correlation of β-catenin expresssion and metastasis in tongue squamous cell carcinoma. Acta Cir. Bras. 2010, 25, 513–517. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lo Muzio, L.; Staibano, S.; Pannone, G.; Grieco, M.; Mignogna, M.D.; Cerrato, A.; Testa, N.F.; De Rosa, G. Beta- and gamma-catenin expression in oral squamous cell carcinomas. Anticancer Res. 1999, 19, 3817–3826. [Google Scholar]
- Laxmidevi, L.B.; Angadi, P.V.; Pillai, R.K.; Chandreshekar, C. Aberrant β-catenin expression in the histologic differentiation of oral squamous cell carcinoma and verrucous carcinoma: An immunohistochemical study. J. Oral Sci. 2010, 52, 633–640. [Google Scholar] [CrossRef][Green Version]
- Lee, S.S.; Tsai, C.H.; Tsai, L.L.; Chou, M.C.; Chou, M.Y.; Chang, Y.C. Β-Catenin Expression in Areca Quid Chewing-Associated Oral Squamous Cell Carcinomas and Upregulated By Arecoline in Human Oral Epithelial Cells. J. Formos. Med. Assoc. 2012, 111, 194–200. [Google Scholar] [CrossRef]
- Rosado, P.; Lequerica-Fernández, P.; Fernández, S.; Allonca, E.; Villallaín, L.; De Vicente, J.C. E-cadherin and β-catenin expression in well-differentiated and moderately-differentiated oral squamous cell carcinoma: Relations with clinical variables. Br. J. Oral Maxillofac. Surg. 2013, 51, 149–156. [Google Scholar] [CrossRef]
- Kaur, J.; Sawhney, M.; DattaGupta, S.; Shukla, N.K.; Srivastava, A.; Walfish, P.G.; Ralhan, R. Clinical Significance of Altered Expression of β-Catenin and E-Cadherin in Oral Dysplasia and Cancer: Potential Link with ALCAM Expression. PLoS ONE 2013, 8, e67361. [Google Scholar] [CrossRef]
- Kyrodimou, M.; Andreadis, D.; Drougou, A.; Amanatiadou, E.; Angelis, L.; Barbatis, C.; Epivatianos, A.; Vizirianakis, I. Desmoglein-3/γ-catenin and E-cadherin/β-catenin differential expression in oral leukoplakia and squamous cell carcinoma. Clin. Oral Investig. 2014, 18, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, G.; Sawant, S.S.; Hague, A.; Kingsley, K.; Devaraj, H. Association of differential β-catenin expression with Oct-4 and Nanog in oral squamous cell carcinoma and their correlation with clinicopathological factors and prognosis. Head Neck 2015, 37, 982–993. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, X.; Wang, B.; Zhang, K.; Liu, S.; Yue, K.; Zhang, L.; Wang, X. Loss of VHL expression contributes to epithelial-mesenchymal transition in oral squamous cell carcinoma. Oral Oncol. 2014, 50, 809–817. [Google Scholar] [CrossRef]
- Balasundaram, P.; Singh, M.K.; Dinda, A.K.; Thakar, A.; Yadav, R. Study of β-catenin, E-cadherin and vimentin in oral squamous cell carcinoma with and without lymph node metastases. Diagn. Pathol. 2014, 9, 145. [Google Scholar] [CrossRef]
- Soares, M.Q.S.; Mendonça, J.A.; Morais, M.O.; Leles, C.R.; Batista, A.C.; Mendonça, E.F. E-cadherin, β-catenin, and α2β1 and α3β1 integrin expression in primary oral squamous cell carcinoma and its regional metastasis. Histol. Histopathol. 2015, 30, 1213–1222. [Google Scholar] [CrossRef]
- Zhang, P.; Cao, H.Y.; Bai, L.L.; Li, W.N.; Wang, Y.; Chen, S.Y.; Zhang, L.; Yang, L.H.; Xu, H.T.; Wang, E.H. The high expression of TC1 (C8orf4) was correlated with the expression of β-catenin and cyclin D1 and the progression of squamous cell carcinomas of the tongue. Tumor Biol. 2015, 36, 7061–7067. [Google Scholar] [CrossRef] [PubMed]
- Gasparoni, A.; Chaves, A.; Fonzi, L.; Johnson, G.K.; Schneider, G.B.; Squier, C.A. Subcellular localization of beta-catenin in malignant cell lines and squamous cell carcinomas of the oral cavity. J. Oral Pathol. Med. 2002, 31, 385–394. [Google Scholar] [CrossRef]
- Reyes, M.; Rojas-Alcayaga, G.; Maturana, A.; Aitken, J.P.; Rojas, C.; Ortega, A.V. Increased nuclear β-catenin expression in oral potentially malignant lesions: A marker of epithelial dysplasia. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e540–e546. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chen, L.; Mashrah, M.; Zhu, Y.; Liu, J.; Yang, X.; He, Z.; Wang, L.; Xiang, T.; Yao, Z.; et al. Deregulation of secreted frizzled-related proteins is associated with aberrant β-catenin activation in the carcinogenesis of oral submucous fibrosis. Onco. Targets. Ther. 2015, 8, 2923–2931. [Google Scholar] [CrossRef]
- Angadi, P.V.; Patil, P.V.; Angadi, V.; Mane, D.; Shekar, S.; Hallikerimath, S.; Kale, A.D.; Kardesai, S.G. Immunoexpression of Epithelial Mesenchymal Transition Proteins E-Cadherin, β-Catenin, and N-Cadherin in Oral Squamous Cell Carcinoma. Int. J. Surg. Pathol. 2016, 24, 696–703. [Google Scholar] [CrossRef]
- Pramanik, K.K.; Singh, A.K.; Alam, M.; Kashyap, T.; Mishra, P.; Panda, A.K.; Dey, R.K.; Rana, A.; Nagini, S.; Mishra, R. Reversion-inducing cysteine-rich protein with Kazal motifs and its regulation by glycogen synthase kinase 3 signaling in oral cancer. Tumor Biol. 2016, 37, 15253–15264. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Z.; Li, J.; Ban, S.; Duan, C.; Liu, W. Interleukin-18 expression in oral squamous cell carcinoma: Its role in tumor cell migration and invasion, and growth of tumor cell xenografts. FEBS Open Bio 2018, 8, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Asif, M.; Ali, A.; Jamal, S.; Khan, M.Z.; Khadim, M.T. Expression of Ki-67 and beta-catenin in pseudoepitheliomatous hyperplasia and squamous cell carcinoma in oral mucosal biopsies: An immunohistochemical study. Asian Pac. J. Cancer Prev. 2020, 21, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Siriwardena, B.S.M.S.; Karunathilaka, H.D.N.U.; Kumarasiri, P.V.R.; Tilakaratne, W.M. Impact of Histological and Molecular Parameters on Prognosis of Oral Squamous Cell Carcinoma: Analysis of 290 Cases. Biomed Res. Int. 2020, 2020, 2059240. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, S.; Rao, R.; Prasad, K. Prediction of metastasis in oral squamous cell carcinoma through phenotypic evaluation and gene expression of E-cadherin, β-catenin, matrix metalloproteinase-2, and matrix metalloproteinase-9 biomarkers with clinical correlation. J. Carcinog. 2020, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Kar, M.; Sultania, M.; Roy, S.; Padhi, S.; Banerjee, B. β-Catenin—A Possible Prognostic Molecular Marker for Recurrence in Histopathologically Negative Surgical Margin of Oral Cancer. Indian J. Surg. Oncol. 2021, 12, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Panda, A.; Dash, K.; Bhuyan, L.; Mahapatra, N.; Mishra, P. Immunohistochemical Expression of the Epithelial to Mesenchymal Transition Proteins E-cadherin and β-catenin in Grades of Oral Squamous Cell Carcinoma. J. Pharm. Bioallied Sci. 2021, 13, S555–S560. [Google Scholar] [CrossRef]
- Miyashita, H.; Mori, S.; Motegi, K.; Fukumoto, M.; Uchida, T. Pin1 is overexpressed in oral squamous cell carcinoma and its levels correlate with cyclin D1 overexpression. Oncol. Rep. 2003, 10, 455–461. [Google Scholar] [CrossRef]
- Al-Rawi, N.; Al Ani, M.; Quadri, A.; Hamdoon, Z.; Awwad, A.; Al Kawas, S.; Al Nuaimi, A. Prognostic Significance of E-Cadherin, B-Catenin and Cyclin D1 in Oral Squamous Cell Carcinoma: A tissue microarray study. Histol. Histopathol. 2021, 36, 18363. [Google Scholar] [CrossRef]
- Tanaka, N.; Odajima, T.; Ogi, K.; Ikeda, T.; Satoh, M. Expression of E-cadherin, α-catenin, and β-catenin in the process of lymph node metastasis in oral squamous cell carcinoma. Br. J. Cancer 2003, 89, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.C.; Zhang, S.; Ishii, G.; Endoh, Y.; Kodama, K.; Miyamoto, S.; Hayashi, R.; Ebihara, S.; Cho, J.S.; Ochiai, A. Predictive Markers for Late Cervical Metastasis in Stage I and II Invasive Squamous Cell Carcinoma of the Oral Tongue. Clin. Cancer Res. 2004, 10, 166–172. [Google Scholar] [CrossRef]
- Gao, S.; Eiberg, H.; Krogdahl, A.; Liu, C.J.; Sørensen, J.A. Cytoplasmic expression of E-cadherin and β-catenin correlated with LOH and hypermethylation of the APC gene in oral squamous cell carcinomas. J. Oral Pathol. Med. 2005, 34, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Odajima, T.; Sasaki, Y.; Tanaka, N.; Kato-Mori, Y.; Asanuma, H.; Ikeda, T.; Satoh, M.; Hiratsuka, H.; Tokino, T.; Sawada, N. Abnormal β-catenin expression in oral cancer with no gene mutation: Correlation with expression of cyclin D1 and epidermal growth factor receptor, Ki-67 labeling index, and clinicopathological features. Hum. Pathol. 2005, 36, 234–241. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, Y. Roles of Wnt-1, beta-catenin and adenomatous polyposis coli in the differentiation and proliferation of oral squamous cell carcinoma. Zhonghua Kou Qiang Yi Xue Za Zhi 2005, 40, 491–494. [Google Scholar] [PubMed]
- Hirohashi, S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am. J. Pathol. 1998, 153, 333–339. [Google Scholar] [CrossRef]
- Kudo, Y.; Kitajima, S.; Ogawa, I.; Hiraoka, M.; Sargolzaei, S.; Keikhaee, M.R.; Sato, S.; Miyauchi, M.; Takata, T. Invasion and metastasis of oral cancer cells require methylation of E-cadherin and/or degradation of membranous beta-catenin. Clin. Cancer Res. 2004, 10, 5455–5463. [Google Scholar] [CrossRef]
- González-Moles, M.A.; Bravo, M.; Ruiz-Avila, I.; Gil-Montoya, J.A.; Acebal, F.; Esteban, F. E-cadherin in non-tumor epithelium adjacent to oral cancer as risk marker for the development of multiple tumors. Br. J. Oral Maxillofac. Surg. 2013, 51, 157–163. [Google Scholar] [CrossRef]
- Pastushenko, I.; Brisebarre, A.; Sifrim, A.; Fioramonti, M.; Revenco, T.; Boumahdi, S.; Van Keymeulen, A.; Brown, D.; Moers, V.; Lemaire, S.; et al. Identification of the tumour transition states occurring during EMT. Nature 2018, 556, 463–468. [Google Scholar] [CrossRef]
- Liao, C.; Wang, Q.; An, J.; Long, Q.; Wang, H.; Xiang, M.; Xiang, M.; Zhao, Y.; Liu, Y.; Liu, J.; et al. Partial EMT in squamous cell carcinoma: A snapshot. Int. J. Biol. Sci. 2021, 17, 3036–3047. [Google Scholar] [CrossRef]
- Wangmo, C.; Charoen, N.; Jantharapattana, K.; Dechaphunkul, A.; Thongsuksai, P. Epithelial–Mesenchymal Transition Predicts Survival in Oral Squamous Cell Carcinoma. Pathol. Oncol. Res. 2020, 26, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, K.; Suzuki, K.; Yanamoto, S.; Naruse, T.; Tsuchihashi, H.; Yamashita, S.; Umeda, M. Anaplastic transition within the cancer microenvironment in early-stage oral tongue squamous cell carcinoma is associated with local recurrence. Int. J. Oncol. 2018, 53, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Ramos-García, P.; Gil-Montoya, J.A.; Scully, C.; Ayén, A.; González-Ruiz, L.; Navarro-Triviño, F.J.; González-Moles, M.A. An update on the implications of cyclin D1 in oral carcinogenesis. Oral Dis. 2017, 23, 897–912. [Google Scholar] [CrossRef] [PubMed]
- Ramos-García, P.; González-Moles, M.Á.; González-Ruiz, L.; Ayén, Á.; Ruiz-Ávila, I.; Bravo, M.; Gil-Montoya, J.A. Clinicopathological significance of tumor cyclin D1 expression in oral cancer. Arch. Oral Biol. 2019, 99, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Ramos-García, P.; González-Moles, M.Á.; Ayén, Á.; González-Ruiz, L.; Ruiz-Ávila, I.; Lenouvel, D.; Gil-Montoya, J.A.; Bravo, M. Asymmetrical proliferative pattern loss linked to cyclin D1 overexpression in adjacent non-tumour epithelium in oral squamous cell carcinoma. Arch. Oral Biol. 2019, 97, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [CrossRef]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef]
- Guerra, E.N.S.; Rêgo, D.F.; Elias, S.T.; Coletta, R.D.; Mezzomo, L.A.M.; Gozal, D.; De Luca Canto, G. Diagnostic accuracy of serum biomarkers for head and neck cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2016, 101, 93–118. [Google Scholar] [CrossRef]
- Ramos-García, P.; González-Moles, M.; González-Ruiz, L.; Ruiz-Ávila, I.; Ayén, Á.; Gil-Montoya, J.A. Prognostic and clinicopathological significance of cyclin D1 expression in oral squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2018, 83, 96–106. [Google Scholar] [CrossRef]
- Xie, S.; Xu, H.; Shan, X.; Liu, B.; Wang, K.; Cai, Z. Clinicopathological and prognostic significance of survivin expression in patients with oral squamous cell carcinoma: Evidence from a meta-analysis. PLoS ONE 2015, 10, e0116517. [Google Scholar] [CrossRef] [PubMed]
- Mello, F.W.; Kammer, P.V.; Silva, C.A.B.; Parkinson, E.K.; Monteiro, L.; Warnakulasuriya, S.; Rivero, E.R.C. Prognostic and clinicopathological significance of podoplanin immunoexpression in oral and oropharyngeal squamous cell carcinoma: A systematic review. J. Oral Pathol. Med. 2021, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ramos-García, P.; González-Moles, M.Á.; Ayén, Á.; González-Ruiz, L.; Ruiz-Ávila, I.; Gil-Montoya, J.A. Prognostic and clinicopathological significance of CTTN/cortactin alterations in head and neck squamous cell carcinoma: Systematic review and meta-analysis. Head Neck 2018, 41, 1963–1978. [Google Scholar] [CrossRef] [PubMed]
- Lenouvel, D.; González-Moles, M.Á.; Ruiz-Ávila, I.; Gonzalez-Ruiz, L.; Gonzalez-Ruiz, I.; Ramos-García, P. Prognostic and clinicopathological significance of PD-L1 overexpression in oral squamous cell carcinoma: A systematic review and comprehensive meta-analysis. Oral Oncol. 2020, 106, 104722. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yang, P.; Ma, L.; Tarantino, G. Prognostic and clinical implications of c-erbB-2 expression in patients with oral cancer: A meta-analysis. Medicine 2020, 99, e20575. [Google Scholar] [CrossRef]
- Marques, A.E.M.; Elias, S.T.; Porporatti, A.L.; Castilho, R.M.; Squarize, C.H.; De Luca Canto, G.; Guerra, E.N.S. mTOR pathway protein immunoexpression as a prognostic factor for survival in head and neck cancer patients: A systematic review and meta-analysis. J. Oral Pathol. Med. 2016, 45, 319–328. [Google Scholar] [CrossRef]
- Lippert, B.M.; Knauer, S.K.; Fetz, V.; Mann, W.; Stauber, R.H. Dynamic survivin in head and neck cancer: Molecular mechanism and therapeutic potential. Int. J. Cancer 2007, 121, 1169–1174. [Google Scholar] [CrossRef]
- Swain, N.; Kumar, S.V.; Routray, S.; Pathak, J.; Patel, S. Podoplanin—a novel marker in oral carcinogenesis. Tumor Biol. 2014, 35, 8407–8413. [Google Scholar] [CrossRef]
- Ramos-García, P.; González-Moles, M.Á.; González-Ruiz, L.; Ayén, Á.; Ruiz-Ávila, I.; Navarro-Triviño, F.J.; Gil-Montoya, J.A. An update of knowledge on cortactin as a metastatic driver and potential therapeutic target in oral squamous cell carcinoma. Oral Dis. 2019, 25, 949–971. [Google Scholar] [CrossRef]
- Yilmaz, M.; Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009, 28, 15–33. [Google Scholar] [CrossRef]
- Krisanaprakornkit, S.; Iamaroon, A. Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma. ISRN Oncol. 2012, 2012, 681469. [Google Scholar] [CrossRef]
- Kyzas, P.A.; Denaxa-Kyza, D.; Ioannidis, J.P.A. Almost all articles on cancer prognostic markers report statistically significant results. Eur. J. Cancer 2007, 43, 2559–2579. [Google Scholar] [CrossRef] [PubMed]

| Total | 41 Studies |
|---|---|
| Year of publication | 1998–2021 |
| Total patients (range) | 2746 (12–290) |
| β-catenin aberrant subcellular location | |
| Cell membrane loss | 11 studies |
| Cytoplasmic-nuclear expression | 21 studies |
| Not defined in primary-level studies | 9 studies |
| Study design | |
| Retrospective cohort | 41 studies |
| Experimental methods for β-catenin expression determination | |
| Immunohistochemistry | 41 studies |
| Anti-β-catenin antibody | |
| Clone 14 | 6 studies |
| sc-7963 | 2 studies |
| C19220 | 2 studies |
| Other | 10 studies |
| Not reported | 21 studies |
| Anti-β-catenin antibody dilution | |
| <1:250 | 22 studies |
| 1:500–1000 | 7 studies |
| Not reported | 12 studies |
| Anti- β-catenin antibody incubation time | |
| Overnight | 15 studies |
| 1 h | 13 studies |
| Not reported | 13 studies |
| Anti-β-catenin antibody incubation temperature | |
| 4 °C | 13 studies |
| Room temperature or higher | 13 studies |
| Not reported | 15 studies |
| Geographical region | |
| Asian countries | 29 studies |
| Non-Asian countries | 12 studies |
| Meta-Analyses | No. of Studies | No. of Patients | Stat. Model | Wt | Pooled Data | Heterogeneity | Supplementary Materials a | ||
|---|---|---|---|---|---|---|---|---|---|
| ES (95% CI) | p-Value | Phet | I2 (%) | ||||||
| Survival Parameters | |||||||||
| Overall Survival | |||||||||
| Aberrant β-catenin expression (all) b | 10 | 938 | REM | DL | HR = 1.77 (1.20–2.60) | 0.004 | 0.004 | 62.4 | Manuscript, Figure 3 |
| Subgroup analysis by differential subcellular location c | Figure 3 | ||||||||
| Loss of cell membrane | 4 | 460 | REM | DL | HR = 2.37 (1.55–3.62) | <0.001 | 0.27 | 23.9 | |
| Nuclear-cytoplasmic expression | 3 | 255 | REM | DL | HR = 1.98 (0.93–4.22) | 0.07 | 0.10 | 56.1 | |
| Not defined in primary-level studies | 3 | 223 | REM | DL | HR = 0.89 (0.62–1.28) | 0.53 | 0.40 | 0.0 | |
| Subgroup analysis by geographical area c | Figure S1 | ||||||||
| Asian | 8 | 772 | REM | DL | HR = 1.81 (1.10–3.00) | 0.02 | 0.001 | 70.1 | |
| Non-Asian | 2 | 166 | REM | DL | HR = 1.72 (1.09–2.71) | 0.02 | 0.80 | 0.0 | |
| Subgroup analysis by anti-β-catenin antibody c | Figure S2 | ||||||||
| Clone-14 | 2 | 294 | REM | DL | HR = 3.57 (2.03–6.29) | <0.001 | 1.00 | 0.0 | |
| Sc-7963 | 2 | 143 | REM | DL | HR = 0.79 (0.52–1.20) | 0.27 | 0.46 | 0.0 | |
| Other | 2 | 190 | REM | DL | HR = 1.82 (0.87–3.81) | 0.11 | 0.18 | 44.7 | |
| Not reported | 4 | 311 | REM | DL | HR = 1.56 (1.10–2.22) | 0.01 | 0.37 | 3.9 | |
| Subgroup analysis by anti-β-catenin antibody dilution c | Figure S3 | ||||||||
| <1:250 | 5 | 545 | REM | DL | HR = 2.81 (1.87–4.22) | <0.001 | 0.72 | 0.0 | |
| 1:500–1000 | 3 | 230 | REM | DL | HR = 1.65 (1.10–2.47) | 0.02 | 0.37 | 0.0 | |
| Not reported | 2 | 163 | REM | DL | HR = 0.90 (0.60–1.34) | 0.60 | 0.25 | 26.0 | |
| Subgroup analysis by anti-β-catenin antibody incubation time c | Figure S4 | ||||||||
| 1 h | 3 | 273 | REM | DL | HR = 1.23 (0.64–2.36) | 0.53 | 0.03 | 72.9 | |
| Overnight | 6 | 585 | REM | DL | HR = 2.31 (1.65–3.23) | <0.001 | 0.43 | 0.0 | |
| Not reported | 1 | 80 | - | - | HR = 1.28 (0.62–2.65) | 0.51 | - | - | |
| Subgroup analysis by anti-β-catenin antibody incubation temperature c | Figure S5 | ||||||||
| 4 °C | 6 | 585 | REM | DL | HR = 2.31 (1.65–3.23) | <0.001 | 0.43 | 0.0 | |
| Room temperature or higher | 3 | 273 | REM | DL | HR = 1.23 (0.64–2.36) | 0.53 | 0.03 | 72.9 | |
| Not reported | 1 | 80 | - | - | HR = 1.28 (0.62–2.65) | 0.51 | - | - | |
| Subgroup analysis by overall risk of bias in primary-level studies c | Figure S6 | ||||||||
| Low RoB | 3 | 404 | REM | DL | HR = 3.28 (2.05–5.24) | <0.001 | 0.87 | 0.0 | |
| Moderate-High RoB | 7 | 534 | REM | DL | HR = 1.29 (0.91–1.83) | 0.15 | 0.14 | 38.5 | |
| Univariable meta-regressions by study design and patients’ characteristics d | |||||||||
| Follow up (months, mean) | 7 | 618 | random-effects meta-regression | Coef = 0.016 (−0.033 to 0.065) | 0.47 ± 0.005 e | hetexplained = −1.02% f | Figure S7 | ||
| Sex (proportion of males, %) | 10 | 938 | random-effects meta-regression | Coef = −0.005 (−0.035 to 0.024) | 0.69 ± 0.005 e | hetexplained = −17.95% f | Figure S8 | ||
| Age (years, mean) | 9 | 858 | random-effects meta-regression | Coef = 0.132 (0.034 to 0.229) | 0.02 ± 0.002 e | hetexplained = 79.62% f | Figure S9 | ||
| Clinical stage (proportion of stage-III/IV patients, %) | 5 | 469 | random-effects meta-regression | Coef = 0.001 (−0.078 to 0.079) | 0.99 ± 0.001 e | hetexplained = −38.30% f | Figure S10 | ||
| Tobacco consumption (proportion of smokers, %) | 2 | 141 | random-effects meta-regression | - | - | - | - | ||
| Areca nut/Betel quid consumption (proportion of chewers, %) | 0 | 0 | - | - | - | - | - | ||
| Alcohol consumption (% of patients with positive habits) | 2 | 141 | - | - | - | - | - | ||
| Disease-free survival | |||||||||
| Aberrant β-catenin expression (all) b | 5 | 379 | REM | DL | HR = 2.44 (1.10–5.40) | 0.03 | <0.001 | 88.6 | Figure S11 |
| Clinicopathological Characteristics | |||||||||
| T Status | |||||||||
| Aberrant β-catenin expression (all) b | 10 | 1418 | REM | DL | OR = 1.76 (1.23–2.53) | 0.004 | 0.06 | 37.1 | Figure S12 |
| Subgroup analysis by differential subcellular location c | Figure S12 | ||||||||
| Loss of cell membrane | 7 | 673 | REM | DL | OR = 1.81 (1.05–3.11) | 0.03 | 0.08 | 47.7 | |
| Nuclear-cytoplasmic expression | 7 | 542 | REM | DL | OR = 1.93 (0.91–4.06) | 0.09 | 0.05 | 53.0 | |
| Not defined in primary-level studies | 4 | 203 | REM | DL | OR = 1.42 (0.68–2.97) | 0.35 | 0.50 | 0.0 | |
| N Status | |||||||||
| Aberrant β-catenin expression (all) b | 23 | 1881 | REM | DL | OR = 2.39 (1.68–3.40) | <0.001 | 0.002 | 53.0 | Figure S13 |
| Subgroup analysis by differential subcellular location c | Figure S13 | ||||||||
| Loss of cell membrane | 9 | 769 | REM | DL | OR = 3.44 (2.40–4.93) | <0.001 | 0.44 | 0.0 | |
| Nuclear-cytoplasmic expression | 9 | 849 | REM | DL | OR = 1.67 (0.93–3.00) | 0.08 | 0.01 | 59.1 | |
| Not defined in primary-level studies | 5 | 263 | REM | DL | OR = 1.97 (0.88–4.38) | 0.10 | 0.18 | 36.7 | |
| Clinical Stage | |||||||||
| Aberrant β-catenin expression (all) b | 15 | 1165 | REM | DL | OR = 2.40 (1.58–3.63) | <0.001 | 0.03 | 45.6 | Figure S14 |
| Subgroup analysis by differential subcellular location c | Figure S14 | ||||||||
| Loss of cell membrane | 6 | 514 | REM | DL | OR = 2.51 (1.17–5.35) | 0.02 | 0.01 | 66.7 | |
| Nuclear-cytoplasmic expression | 5 | 426 | REM | DL | OR = 3.12 (1.81–5.40) | <0.001 | 0.50 | 0.0 | |
| Not defined in primary-level studies | 4 | 225 | REM | DL | OR = 1.49 (0.66–3.36) | 0.33 | 0.21 | 33.5 | |
| Histological Grade | |||||||||
| Aberrant β-catenin expression (all) b | 32 | 1974 | REM | DL | OR = 1.57 (1.09–2.25) | 0.02 | <0.001 | 55.1 | Figure S15 |
| Subgroup analysis by differential subcellular location c | Figure S15 | ||||||||
| Loss of cell membrane | 7 | 604 | REM | DL | OR = 1.77 (0.82–3.83) | 0.14 | 0.002 | 71.9 | |
| Nuclear-cytoplasmic expression | 17 | 986 | REM | DL | OR = 1.76 (1.23–2.53) | 0.002 | 0.33 | 10.2 | |
| Not defined in primary-level studies | 8 | 384 | REM | DL | OR = 0.99 (0.36–2.76) | 0.99 | <0.001 | 73.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-García, P.; González-Moles, M.Á. Prognostic and Clinicopathological Significance of the Aberrant Expression of β-Catenin in Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 479. https://doi.org/10.3390/cancers14030479
Ramos-García P, González-Moles MÁ. Prognostic and Clinicopathological Significance of the Aberrant Expression of β-Catenin in Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(3):479. https://doi.org/10.3390/cancers14030479
Chicago/Turabian StyleRamos-García, Pablo, and Miguel Á. González-Moles. 2022. "Prognostic and Clinicopathological Significance of the Aberrant Expression of β-Catenin in Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis" Cancers 14, no. 3: 479. https://doi.org/10.3390/cancers14030479
APA StyleRamos-García, P., & González-Moles, M. Á. (2022). Prognostic and Clinicopathological Significance of the Aberrant Expression of β-Catenin in Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers, 14(3), 479. https://doi.org/10.3390/cancers14030479








