Impact of Mobilization Strategies on Peripheral Blood Stem Cell Collection Efficiency and Product Quality: A Retrospective Single-Center Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Study Design and Methods
2.1. Cohorts and Data Collection
2.2. Stem Cell Mobilization
2.3. Blood Count Parameters
2.4. Apheresis
2.5. Procedure Performance
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duarte, R.F.; Labopin, M.; Bader, P.; Basak, G.W.; Bonini, C.; Chabannon, C.; Corbacioglu, S.; Dreger, P.; Dufour, C.; Gennery, A.R.; et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: Current practice in Europe, 2019. Bone Marrow Transplant. 2019, 54, 1525–1552. [Google Scholar] [CrossRef] [PubMed]
- Al Hamed, R.; Bazarbachi, A.H.; Malard, F.; Harousseau, J.L.; Mohty, M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.; Szabo, A.; Chhabra, S.; Hamadani, M.; D’Souza, A.; Usmani, S.Z.; Sieracki, R.; Gyawali, B.; Jackson, J.L.; Asimakopoulos, F.; et al. Autologous Transplantation for Newly Diagnosed Multiple Myeloma in the Era of Novel Agent Induction: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Ghielmini, M.; Vitolo, U.; Kimby, E.; Montoto, S.; Walewski, J.; Pfreundschuh, M.; Federico, M.; Hoskin, P.; McNamara, C.; Caligaris-Cappio, F.; et al. ESMO Guidelines consensus conference on malignant lymphoma 2011 part 1: Diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL). Ann. Oncol. 2013, 24, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Porfyriou, E.; Letsa, S.; Kosmas, C. Hematopoietic stem cell mobilization strategies to support high-dose chemotherapy: A focus on relapsed/refractory germ cell tumors. World J. Clin. Oncol. 2021, 12, 746–766. [Google Scholar] [CrossRef]
- Karadurmus, N.; Sahin, U.; Bahadir Basgoz, B.; Demirer, T. Is there a role of high dose chemotherapy and autologous stem cell transplantation in the treatment of Ewing’s sarcoma and osteosarcomas? J. BUON 2018, 23, 1235–1241. [Google Scholar]
- Hatzimichael, E.; Tuthill, M. Hematopoietic stem cell transplantation. Stem Cells Cloning 2010, 3, 105–117. [Google Scholar] [CrossRef]
- Körbling, M.; Freireich, E.J. Twenty-five years of peripheral blood stem cell transplantation. Blood 2011, 117, 6411–6416. [Google Scholar] [CrossRef]
- Karpova, D.; Rettig, M.P.; DiPersio, J.F. Mobilized peripheral blood: An updated perspective. F1000Research 2019, 8, 2125. [Google Scholar] [CrossRef]
- Bensinger, W.; Appelbaum, F.; Rowley, S.; Storb, R.; Sanders, J.; Lilleby, K.; Gooley, T.; Demirer, T.; Schiffman, K.; Weaver, C.; et al. Factors that influence collection and engraftment of autologous peripheral-blood stem cells. J. Clin. Oncol. 1995, 13, 2547–2555. [Google Scholar] [CrossRef]
- Weaver, C.H.; Hazelton, B.; Birch, R.; Palmer, P.; Allen, C.; Schwartzberg, L.; West, W. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood 1995, 86, 3961–3969. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Simón, J.A.; Martín, A.; Caballero, D.; Corral, M.; Nieto, M.J.; Gonzalez, M.; Vazquez, L.; López-Berges, C.; Cañizo, M.C.; Mateos, M.V.; et al. Clinical significance of CD34+ cell dose in long-term engraftment following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 1999, 24, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Kurnaz, F.; Kaynar, L. Peripheral blood stem cell mobilization failure. Transfus. Apher. Sci. 2015, 53, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Bojanic, I.; Besson, N.; Vidovic, I.; Cepulic, B.G. Performance prediction algorithm for autologous PBSC collection in adults and pediatric patients using large volume leukapheresis. J. Clin. Apher. 2019, 34, 407–415. [Google Scholar] [CrossRef]
- Cousins, A.F.; Sinclair, J.E.; Alcorn, M.J.; Green, R.H.A.; Douglas, K.W. HPC-A dose prediction on the Optia® cell separator based on a benchmark CE2 collection efficiency: Promoting clinical efficiency, minimizing toxicity, and allowing quality control. J. Clin. Apher. 2015, 30, 321–328. [Google Scholar] [CrossRef]
- Hosing, C.; Saliba, R.M.; Hamerschlak, N.; Kutner, J.M.; Sakashita, A.M.; Kondo, A.T.; Rodrigues, M.; Fernande, J.F.; Chiattone, A.; Chiattone, V.C.; et al. Peripheral blood stem cell yield calculated using preapheresis absolute CD34+ cell count, peripheral blood volume processed, and donor body weight accurately predicts actual yield at multiple centers. Transfusion 2014, 54, 1081–1087. [Google Scholar] [CrossRef]
- Leberfinger, D.L.; Badman, K.L.; Roig, J.M.; Loos, T. Improved planning of leukapheresis endpoint with customized prediction algorithm: Minimizing collection days, volume of blood processed, procedure time, and citrate toxicity. Transfusion 2017, 57, 685–693. [Google Scholar] [CrossRef]
- Pierelli, L.; Maresca, M.; Piccirillo, N.; Pupella, S.; Gozzer, M.; Foddai, M.L.; Vacca, M.; Adorno, G.; Coppetelli, U.; Paladini, U. Accurate prediction of autologous stem cell apheresis yields using a double variable-dependent method assures systematic efficiency control of continuous flow collection procedures. Vox Sang. 2006, 91, 126–134. [Google Scholar] [CrossRef]
- Rosenbaum, E.R.; O’Connell, B.; Cottler-Fox, M. Validation of a formula for predicting daily CD34(+) cell collection by leukapheresis. Cytotherapy 2012, 14, 461–466. [Google Scholar] [CrossRef]
- Sheppard, D.; Tay, J.; Palmer, D.; Xenocostas, A.; Doulaverakis, C.; Huebsch, L.; McDiarmid, S.; Tinmouth, A.; Mallick, R.; Martin, L.; et al. Improved Prediction of CD34+ Cell Yield before Peripheral Blood Hematopoietic Progenitor Cell Collection Using a Modified Target Value-Tailored Approach. Biol. Blood Marrow Transplant. 2016, 22, 763–767. [Google Scholar] [CrossRef]
- Verlinden, A.; Van de Velde, A.; Verpooten, G.A.; Janssen van Doorn, K. Determining factors predictive of CD34+ cell collection efficiency in an effort to avoid extended and repeated apheresis sessions. J. Clin. Apher. 2013, 28, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Babic, A.; Trigoso, E. Cell Source and Apheresis. In The European Blood and Marrow Transplantation Textbook for Nurses: Under the Auspices of EBMT; Kenyon, M., Babic, A., Eds.; EBMT and the Author(s): Cham, Switzerland, 2018; pp. 71–87. [Google Scholar]
- DiPersio, J.F.; Stadtmauer, E.A.; Nademanee, A.; Micallef, I.N.; Stiff, P.J.; Kaufman, J.L.; Maziarz, R.T.; Hosing, C.; Früehauf, S.; Horwitz, M.; et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood 2009, 113, 5720–5726. [Google Scholar] [CrossRef] [PubMed]
- Giralt, S.; Costa, L.; Schriber, J.; Dipersio, J.; Maziarz, R.; McCarty, J.; Shaughnessy, P.; Snyder, E.; Bensinger, W.; Copelan, E.; et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: Consensus guidelines and recommendations. Biol. Blood Marrow Transplant. 2014, 20, 295–308. [Google Scholar] [CrossRef]
- Humpe, A.; Riggert, J.; Meineke, I.; Kurz, M.; Eil, A.; Storkebaum, B.; Binder, C.; Munzel, U.; Funke, I.; Höcker, P.; et al. A cell-kinetic model of CD34+ cell mobilization and harvest: Development of a predictive algorithm for CD34+ cell yield in PBPC collections. Transfusion 2000, 40, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.M.; Nikolaisen, K.; Gaarsdal, E.; Johnsen, H.E. Kinetic studies during peripheral blood stem cell collection show CD34+ cell recruitment intra-apheresis. J. Clin. Apher. 2001, 16, 114–119. [Google Scholar] [CrossRef]
- Worel, N. Plerixafor--the magic bullet in stem cell mobilization failure? Leuk. Res. 2011, 35, 701–702. [Google Scholar] [CrossRef]
- Worel, N.; Fritsch, G.; Agis, H.; Böhm, A.; Engelich, G.; Leitner, G.C.; Geissler, K.; Gleixner, K.; Kalhs, P.; Buxhofer-Ausch, V.; et al. Plerixafor as preemptive strategy results in high success rates in autologous stem cell mobilization failure. J. Clin. Apher. 2017, 32, 224–234. [Google Scholar] [CrossRef]
- Worel, N.; Rosskopf, K.; Neumeister, P.; Kasparu, H.; Nachbaur, D.; Russ, G.; Namberger, K.; Witt, V.; Schloegl, E.; Zojer, N.; et al. Plerixafor and granulocyte-colony-stimulating factor (G-CSF) in patients with lymphoma and multiple myeloma previously failing mobilization with G-CSF with or without chemotherapy for autologous hematopoietic stem cell mobilization: The Austrian experience on a named patient program. Transfusion 2011, 51, 968–975. [Google Scholar] [CrossRef]
- Bartnik, K.; Maciejewska, M.; Farhan, R.; Urbanowska, E.; Król, M.; Król, M.; Feliksbrot, M.; Wiktor-Jędrzejczak, W.; Snarski, E. Continuous Mononuclear Cell Collection (cMNC) protocol impact on hematopoietic stem cell collections in donors with negative collection predictors. Transfus. Apher. Sci. 2018, 57, 401–405. [Google Scholar] [CrossRef]
- Karafin, M.S.; Graminske, S.; Erickson, P.; Walters, M.C.; Scott, E.P.; Carter, S.; Padmanabhan, A. Evaluation of the Spectra Optia apheresis system for mononuclear cell (MNC) collection in G-CSF mobilized and nonmobilized healthy donors: Results of a multicenter study. J. Clin. Apher. 2014, 29, 273–280. [Google Scholar] [CrossRef]
- Sanderson, F.; Poullin, P.; Smith, R.; Nicolino-Brunet, C.; Philip, P.; Chaib, A.; Costello, R. Peripheral blood stem cells collection on spectra optia apheresis system using the continuous mononuclear cell collection protocol: A single center report of 39 procedures. J. Clin. Apher. 2017, 32, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Solmaz, S.; Kahraman, S.; Sevindik, O.G.; Acar, C.; Turkyilmaz, M.; Alacacioglu, I.; Piskin, O.; Ozcan, M.A.; Ozsan, H.G.; Undar, B.; et al. A Comparison of Fresenius Com.Tec Cell and Spectra Optia Cell Separators for Autologous and Allogeneic Stem Cell Collections: Single Center Experience. Indian J. Hematol. Blood Transfus. 2018, 34, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Spoerl, S.; Wäscher, D.; Nagel, S.; Peschel, C.; Verbeek, M.; Götze, K.; Krackhardt, A.M. Evaluation of the new continuous mononuclear cell collection protocol versus an older version on two different apheresis machines. Transfusion 2018, 58, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Long, G.; Waller, E.K.; Gregurek, S.; Tricot, G.; Marschner, S.; Bill, J. Evaluation of the spectra Optia® mononuclear cell collection procedure in multiple myeloma patients. J. Clin. Apher. 2015, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wuchter, P.; Hundemer, M.; Schmitt, A.; Witzens-Harig, M.; Pavel, P.; Hillengass, J.; Goldschmidt, H.; Ho, A.D.; Lisenko, K. Performance assessment and benchmarking of autologous peripheral blood stem cell collection with two different apheresis devices. Transfus. Med. 2017, 27, 36–42. [Google Scholar] [CrossRef]
- Brauninger, S.; Bialleck, H.; Thorausch, K.; Felt, T.; Seifried, E.; Bonig, H. Allogeneic donor peripheral blood “stem cell” apheresis: Prospective comparison of two apheresis systems. Transfusion 2012, 52, 1137–1145. [Google Scholar] [CrossRef]
- Baertsch, M.A.; Kriegsmann, K.; Pavel, P.; Bruckner, T.; Hundemer, M.; Kriegsmann, M.; Ho, A.D.; Goldschmidt, H.; Wuchter, P. Platelet Count before Peripheral Blood Stem Cell Mobilization Is Associated with the Need for Plerixafor but Not with the Collection Result. Transfus. Med. Hemotherapy 2018, 45, 24–31. [Google Scholar] [CrossRef]
- Abrahamsen, J.F.; Stamnesfet, S.; Liseth, K.; Hervig, T.; Bruserud, O. Large-volume leukapheresis yields more viable CD34+ cells and colony-forming units than normal-volume leukapheresis, especially in patients who mobilize low numbers of CD34+ cells. Transfusion 2005, 45, 248–253. [Google Scholar] [CrossRef]
- Gasová, Z.; Marinov, I.; Vodvárková, S.; Böhmová, M.; Bhuyian-Ludvíková, Z. PBPC collection techniques: Standard versus large volume leukapheresis (LVL) in donors and in patients. Transfus. Apher. Sci. 2005, 32, 167–176. [Google Scholar] [CrossRef]
- Besson, N.; Topholm Bruun, M.; Stauffer Larsen, T.; Nielsen, C. Impact of apheresis automation on procedure quality and predictability of CD34+ cell yield. J. Clin. Apher. 2018, 33, 494–504. [Google Scholar] [CrossRef]
- Drezet, A.; Granata, A.; Lemarie, C.; Calmels, B.; Chabannon, C. An intra-patient comparison of blood cell separators Spectra and Optia in patients and donors undergoing blood mononuclear cell collections at a single institution for subsequent autologous or allogeneic hematopoietic cell transplantation reveals comparable collection efficiencies. Bone Marrow Transplant. 2016, 51, 1007–1009. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, S.N.; Sohn, J.Y.; Kong, J.H.; Eom, H.S.; Lee, H.; Kong, S.Y. Comparison of Two Apheresis Systems of COBE and Optia for Autologous Peripheral Blood Stem Cell Collection. Ann. Lab. Med. 2017, 37, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Cottler-Fox, M. Optia® continuous mononuclear collection (CMNC) system is a safe and efficient system for hematopoietic progenitor cells-apheresis (HPC-a) collection and yields a lower product hematocrit (HCT%) than the COBE® spectra system: A retrospective study. J. Clin. Apher. 2018, 33, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Punzel, M.; Kozlova, A.; Quade, A.; Schmidt, A.H.; Smith, R. Evolution of MNC and lymphocyte collection settings employing different Spectra Optia® Leukapheresis systems. Vox Sang. 2017, 112, 586–594. [Google Scholar] [CrossRef]
- Thorausch, K.; Schulz, M.; Bialleck, H.; Luxembourg, B.; Seifried, E.; Bonig, H. Granulocyte collections: Comparison of two apheresis systems. Transfusion 2013, 53, 3262–3268. [Google Scholar] [CrossRef]
- Yang, X.; Wan, M.; Yu, F.; Wang, Z. Efficacy and safety of plerixafor for hematopoietic stem cell mobilization for autologous transplantation in patients with non-Hodgkin lymphoma and multiple myeloma: A systematic review and meta-analysis. Exp. Ther. Med. 2019, 18, 1141–1148. [Google Scholar] [CrossRef]
- Chen, K.Y.; Bucci, T.G.; Shaw, J.R.; Alexander, M.D.; Grgic, T.; Riches, M.; Ptachcinski, J.R. Plerixafor strategies for autologous hematopoietic cell transplant mobilization: A comparison of efficacy and cost. Transfus. Apher. Sci. 2022, 61, 103303. [Google Scholar] [CrossRef]
- Prakash, V.S.; Malik, P.S.; Sahoo, R.K.; Pramanik, R.; Choudhary, P.; Varshney, A.N.; Kumar, L. Multiple Myeloma: Risk Adapted Use of Plerixafor for Stem Cell Mobilization Prior to Autologous Stem Cell Transplantation is Effective and Cost Efficient. Clin. Lymphoma Myeloma Leuk. 2022, 22, 44–51. [Google Scholar] [CrossRef]

| Variables | Mobilization without Plerixafor | Mobilization with Plerixafor | p-Value |
|---|---|---|---|
| Number of collections, n (%) * | 210 (68.0%) | 99 (32.0%) | |
| G-CSF | 97 (31.4% **) | 64 (20.7% **) | 0.02 |
| G-CSF + CT | 113 (36.6% **) | 35 (11.3% **) | 0.02 |
| Gender (male), n (%) | 143 (68.1%) | 68 (68.7%) | 0.917 |
| G-CSF | 67 (69.1% ***) | 48 (75.0% ***) | |
| G-CSF + CT | 76 (67.3% ***) | 20 (57.1% ***) | |
| Age (years), median (range) | 54 (19–74) | 58 (23–76) | 0.016 |
| G-CSF | 56 (19–74) | 61 (24–75) | |
| G-CSF + CT | 51 (20–73) | 52 (23–76) | |
| Weight (kg), median (range) | 80 (42–150) | 82 (48–120) | 0.588 |
| G-CSF | 83 (52–124) | 82 (54–117) | |
| G-CSF + CT | 76 (42–150) | 79 (48–120) | |
| TBV (L), median (range) | 5.1 (2.7–7.4) | 5.1 (3.1–6.9) | 0.689 |
| G-CSF | 5.1 (3.3–6.9) | 5.2 (3.4–6.5) | |
| G-CSF + CT | 5.0 (2.7–7.4) | 4.6 (3.1–6.9) | |
| Diagnosis, n (%) | |||
| Multiple myeloma | 112 (53.3%) | 65 (65.7%) | |
| Non-Hodgkin’s lymphoma | 69 (32.9%) | 21 (21.2%) | |
| Hodgkin’s disease | 6 (2.9%) | 7 (7.1%) | |
| Other carcinoma | 22 (10.5%) | 6 (6.1%) | |
| Non-malignant disease | 1 (0.5%) | 0 (0.0%) | |
| Pre-apheresis peripheral blood counts | |||
| White blood cells (109/L) | 36.1 (6.5–132.0) | 42.8 (6.2–77.1) | 0.001 |
| G-CSF | 44.8 (17.1–88.0) | 44.5 (21.6–77.1) | |
| G-CSF + CT | 25.0 (6.5–132.0) | 37.2 (6.2–74.7) | |
| Platelets (109/L) | 134 (15–562) | 104 (20–296) | 0.042 |
| G-CSF | 198 (57–562) | 136 (52–296) | |
| G-CSF + CT | 80 (15–304) | 64.0 (20–246) | |
| Hematocrit (%) | 34.8 (20.4–49.9) | 33.2 (22.1–45.1) | 0.206 |
| G-CSF | 38.0 (24.0–50.0) | 36.8 (25.1–45.1) | |
| G-CSF + CT | 31.2 (20.4–44.3) | 29.7 (22.1–42.2) | |
| Granulocytes (%) | 85 (50–95) | 84 (58–95) | 0.372 |
| G-CSF | 86 (71–93) | 83 (69–95) | |
| G-CSF + CT | 84 (50–95) | 87 (58–95) | |
| CD34+ cells (×106/L) | 47.4 (3.8–663.0) | 28.2 (2.5–80.8) | <0.001 |
| G-CSF | 33.2 (3.8–162.8) | 30.6 (2.5–80.8) | |
| G-CSF + CT | 75.4 (8.6–663.0) | 20.3 (4.7–69.6) | |
| CD34+ cells (%) | 0.1 (0.0–2.7) | 0.1 (0.0–0.3) | <0.001 |
| G-CSF | 0.1 (0.0–0.3) | 0.1 (0.0–0.2) | |
| G-CSF + CT | 0.3 (0.0–2.7) | 0.1 (0.0–0.3) | |
| Variables | Mobilization Without Plerixafor | Mobilization with Plerixafor | p-Value |
|---|---|---|---|
| Whole blood processed (L) | 17.7 (6.4–29.4) | 19.4 (10.7–28.9) | 0.004 |
| TBV processed (× times) | 3.9 (1.4–5.7) | 4.0 (2.0–5.3) | <0.001 |
| White blood cells (×109/L) | 194.0 (44.0–633.0) | 214.0 (48.1–569.0) | 0.021 |
| Platelets (×109/L) | 1015 (29–4980) | 765 (71–4505) | 0.034 |
| Hematocrit (%) | 1.4 (0.0–5.3) | 1.1 (0.0–4.2) | 0.069 |
| Granulocytes (%) | 7 (0–67) | 13 (0–71) | 0.010 |
| CD34+ cells total in bag (106) | 400 (20–2800) | 270 (30–1200) | 0.001 |
| CD34+ cells collected (106/kg b.w.) | 5.7 (0.2–34.9) | 3.1 (0.5–11.3) | <0.001 |
| Post-apheresis CD34+ cells (×106/L) | 22.1 (1.0–588.0) | 12.5 (1.5–73.6) | <0.001 |
| G-CSF | 14.1 (2.0–103.0) | 11.7 (1.7–73.6) | |
| G-CSF + CT | 41.1 (1.0–588.0) | 12.5 (1.5–63.0) | |
| CD34+ change (%) | 51.1 (−153.7–96.5) | 51.0 (−127.4–90.7) | 0.384 |
| G-CSF | 57.4 (−153.7–89.7) | 53.0 (−127.4–90.7) | |
| G-CSF + CT | 48.6 (−86.5–96.5) | 41.4 (−10.1–73.5) | |
| Apheresis duration (minutes) | 314 (140–510) | 333 (186–480) | 0.002 |
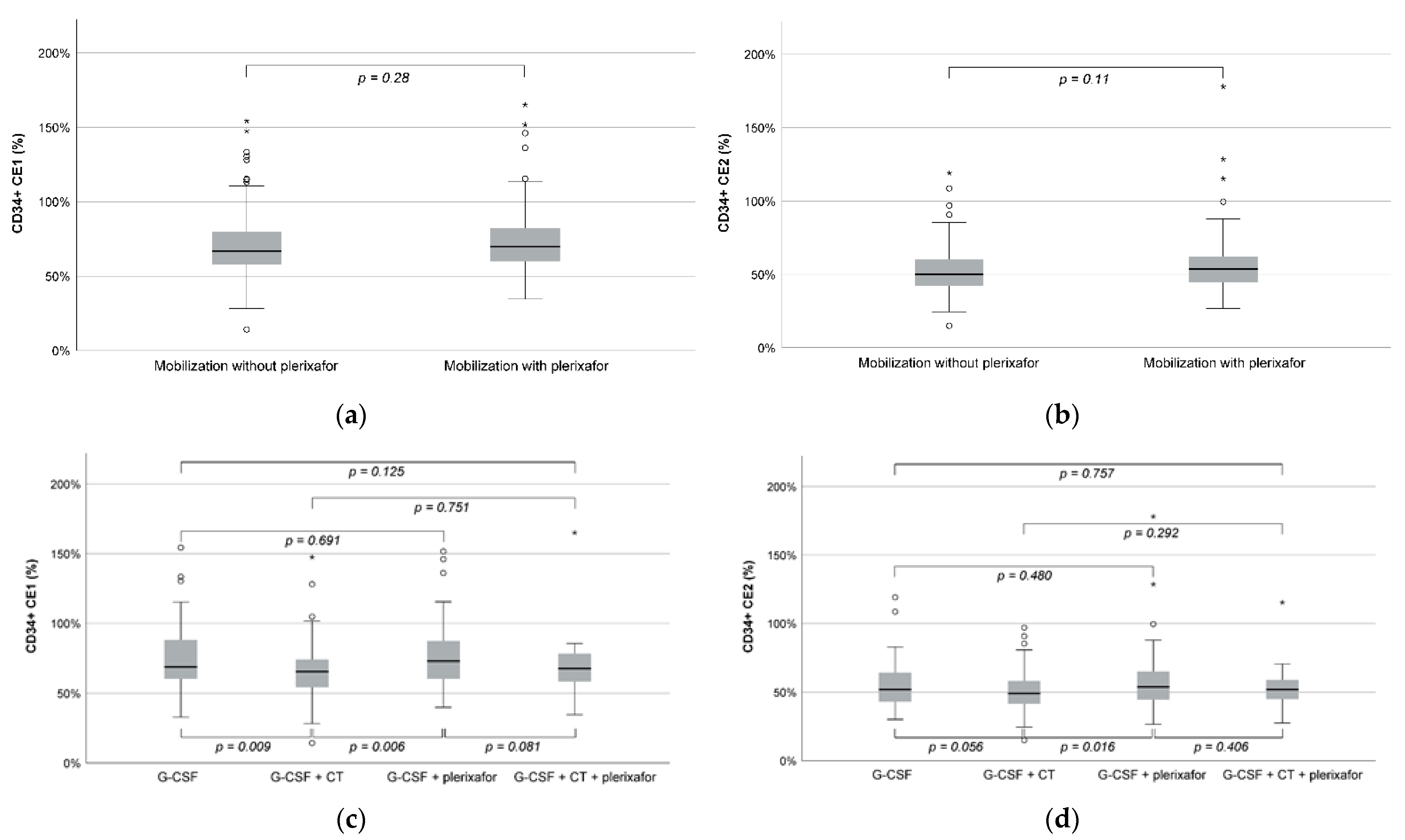
| CD34+ CE2 (%) | 50.1 (15.0–119.1) | 53.0 (26.7–178.0) | 0.11 |
| CD34+ CE1 (%) | 66.9 (14.2–154.4) | 69.9 (34.6–165.1) | 0.28 |
| CD34+ TP2 | 3.1 (0.8–7.1) | 3.3 (1.5–9.5) | 0.084 |
| CD34+ TP1 | 3.9 (0.8–9.2) | 4.1 (1.8–10.2) | 0.291 |
| CD34+ recruitment factor | 2.3 (1.1–5.5) | 2.7 (1.3–9.3) | <0.001 |
| Platelet loss (%) | 35.2 (0.0–60.8) | 34.6 (3.9–61.3) | 0.491 |
| Platelet CE1 (%) | 13.9 (2.9–57.6) | 14.1 (5.6–70.2) | 0.510 |
| Variables | Mobilization without Plerixafor | Mobilization with Plerixafor | ||
|---|---|---|---|---|
| G-CSF (N = 97) | G-CSF + CT (N = 113) | G-CSF (N = 64) | G-CSF + CT (N = 35) | |
| White blood cells (109/L) | 216.5 (77.4–438.0) | 171.0 (44.0–633.0) | 268.0 (117.0–569.0) | 185.0 (48.1–460.1) |
| Platelets (×109/L) | 1513 (313–4980) | 523 (29–4350) | 917 (404–4505) | 441 (71–1425) |
| Hematocrit (%) | 1.2 (0.0–5.3) | 1.4 (0.0–4.3) | 1.1 (0.0–4.2) | 1.1 (0.0–3.5) |
| CD34+ cells total in bag (106) | 349 (20–1200) | 570 (90–2790) | 300 (30–1200) | 200 (50–5700) |
| CD34+ cells/μL processed blood | 664 (27–2496) | 1165 (168–8173) | 565 (39–1936) | 379 (94–1241) |
| CD34+ cells collected (106/kg b.w.) | 4.1 (0.2–13.2) | 7.3 (1.1–34.9) | 3.7 (0.5–11.3) | 2.3 (0.7–8.0) |
| Whole blood processed (L) | 19.9 (7.2–29.4) | 14.9 (6.4–25.8) | 19.5 (11.5–28.5) | 18.8 (10.8–29.0) |
| TBV processes (×times) | 4.0 (1.4–5.5) | 3.2 (1.5–5.7) | 4.0 (2.0–5.0) | 4.0 (2.7–5.3) |
| Apheresis duration (minutes) | 346 (155–491) | 270 (140–510) | 334 (186–480) | 329 (220–453) |
| CD34+ CE2 (%) | 52.3 (30.1–119.1) | 49.1 (15.0–97.0) | 54.0 (26.7–178.0) | 52.1 (27.5–115.3) |
| CD34+ CE1 (%) | 69.6 (32.9–154.4) | 65.6 (14.2–147.4) | 72.9 (39.9–151.6) | 67.9 (34.6–165.1) |
| Platelet loss (%) | 42.2 (15.2–60.8) | 29.0 (0.0–59.7) | 36.5 (3.9–61.3) | 29.5 (7.8–50.2) |
| Platelet CE1 (%) | 15.9 (10.4–41.9) | 12.8 (2.9–57.6) | 15.6 (10.0–70.2) | 11.2 (5.6–20.0) |
| CD34+ TP2 (×104) | 3.2 (1.8–7.1) | 2.9 (0.8–6.1) | 3.4 (1.7–9.5) | 3.1 (1.5–7.1) |
| CD34+ TP1 (×104) | 4.3 (1.8–9.2) | 3.6 (0.8–8.9) | 4.2 (2.4–9.5) | 3.8 (1.8–10.2) |
| CD34+ recruitment factor | 2.6 (1.4–5.5) | 2.1 (1.1–4.5) | 2.7 (1.3–9.3) | 2.5 (1.6–5.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajsp, P.; Branka, M.; Besson, N.; Tanzmann, A.; Worel, N. Impact of Mobilization Strategies on Peripheral Blood Stem Cell Collection Efficiency and Product Quality: A Retrospective Single-Center Study. Cancers 2022, 14, 6259. https://doi.org/10.3390/cancers14246259
Rajsp P, Branka M, Besson N, Tanzmann A, Worel N. Impact of Mobilization Strategies on Peripheral Blood Stem Cell Collection Efficiency and Product Quality: A Retrospective Single-Center Study. Cancers. 2022; 14(24):6259. https://doi.org/10.3390/cancers14246259
Chicago/Turabian StyleRajsp, Patricija, Manuela Branka, Nelly Besson, Andreas Tanzmann, and Nina Worel. 2022. "Impact of Mobilization Strategies on Peripheral Blood Stem Cell Collection Efficiency and Product Quality: A Retrospective Single-Center Study" Cancers 14, no. 24: 6259. https://doi.org/10.3390/cancers14246259
APA StyleRajsp, P., Branka, M., Besson, N., Tanzmann, A., & Worel, N. (2022). Impact of Mobilization Strategies on Peripheral Blood Stem Cell Collection Efficiency and Product Quality: A Retrospective Single-Center Study. Cancers, 14(24), 6259. https://doi.org/10.3390/cancers14246259






