Vaccination Therapy for Acute Myeloid Leukemia: Where Do We Stand?
Abstract
Simple Summary
Abstract
1. Introduction
2. Peptide Vaccines
3. DC Vaccines
4. Comparison to Other Immunotherapies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Dinardo, C.D.; Jonas, B.A.; Pullarkat, V.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Pratz, K.W. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; Thomas, X.G.; Dmoszynska, A.; Wierzbowska, A.; Mazur, G.; Mayer, J.; Gau, J.; Chou, W.; Buckstein, R.; Cermak, J.; et al. Multicenter, Randomized, Open-Label, Phase III Trial of Decitabine Versus Patient Choice, With Physician Advice, of Either Supportive Care or Low-Dose Cytarabine for the Treatment of Older Patients With Newly Diagnosed Acute Myeloid Leukemia. J. Clin. Oncol. 2012, 30, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef]
- Altman, J.D.; Moss, P.A.H.; Goulder, P.J.R.; Barouch, D.H.; McHeyzer-Williams, M.G.; Bell, J.I.; McMichael, A.J.; Davis, M.M. Phenotypic analysis of antigen-specific T lymphocytes. Science 1996, 274, 94–96. [Google Scholar] [CrossRef]
- Kuball, J.; Dossett, M.L.; Wolfl, M.; Ho, W.Y.; Voss, R.H.; Fowler, C.; Greenberg, P.D. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood 2007, 109, 2331–2338. [Google Scholar] [CrossRef]
- Slota, M.; Lim, J.-B.; Dang, Y.; Disis, M.L. ELISpot for measuring human immune responses to vaccines. Expert Rev. Vaccines 2011, 10, 299–306. [Google Scholar] [CrossRef]
- Kuball, J.; de Boer, K.; Wagner, E.; Wattad, M.; Antunes, E.; Weeratna, R.D.; Vicari, A.P.; Lotz, C.; van Dorp, S.; Hol, S.; et al. Pitfalls of vaccinations with WT1-, Proteinase3- and MUC1-derived peptides in combination with MontanideISA51 and CpG7909. Cancer Immunol. Immunother. 2011, 60, 161–171. [Google Scholar] [CrossRef]
- Sugiyama, H. Wilms’ tumor gene wt1: Its oncogenic function and clinical application. Int. J. Hematol. 2001, 73, 177–187. [Google Scholar] [CrossRef]
- Scheibenbogen, C.; Letsch, A.; Thiel, E.; Schmittel, A.; Mailaender, V.; Baerwolf, S.; Nagorsen, D.; Keilholz, U. CD8 T-cell responses to Wilms tumor gene product WT1 and proteinase 3 in patients with acute myeloid leukemia. Blood 2002, 100, 2132–2137. [Google Scholar] [CrossRef]
- Brayer, J.; Lancet, J.E.; Powers, J.; List, A.; Balducci, L.; Komrokji, R.; Pinilla-Ibarz, J. WT1 vaccination in AML and MDS: A pilot trial with synthetic analog peptides. Am. J. Hematol. 2015, 9, 602–607. [Google Scholar] [CrossRef]
- Maslak, P.G.; Dao, T.; Krug, L.M.; Chanel, S.; Korontsvit, T.; Zakhaleva, V.; Zhang, R.; Wolchok, J.D.; Yuan, J.; Pinilla-Ibarz, J.; et al. Vaccination with synthetic analog peptides derived from WT1 oncoprotein induces T-cell responses in patients with complete remission from acute myeloid leukemia. Blood 2010, 116, 171–179. [Google Scholar] [CrossRef]
- Di Stasi, A.; Jimenez, A.M.; Eminagawa, K.; Al-Obaidi, M.; Rezvani, K. Review of the results of WT1 peptide vaccination strategies for myelodysplastic syndromes and acute myeloid leukemia from nine different studies. Front. Immunol. 2015, 6, 36. [Google Scholar] [CrossRef]
- Tsuboi, A.; Oka, Y.; Kyo, T.; Katayama, Y.; Elisseeva, O.A.; Kawakami, M.; Nishida, S.; Morimoto, S.; Murao, A.; Nakajima, H.; et al. Long-term WT1 peptide vaccination for patients with acute myeloid leukemia with minimal residual disease. Leukemia 2012, 26, 1410–1413. [Google Scholar] [CrossRef][Green Version]
- Keilholz, U.; Letsch, A.; Busse, A.; Asemissen, A.M.; Bauer, S.; Blau, I.W.; Hofmann, W.K.; Uharek, L.; Thiel, E.; Scheibenbogen, C. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood 2009, 113, 6541–6548. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Takezako, N.; Kiguchi, T.; Miyawaki, S.; Heike, Y.; Mitsuki, K.; Yoshida, T.; Liew, E.L.; Naoe, T. Phase II Trial of a Peptide Vaccine, Ocv-501 in Elderly Patients with Acute Myeloid Leukemia. Blood 2018, 132, 29. [Google Scholar] [CrossRef]
- Uttenthal, B.; Martinez-Davila, I.; Ivey, A.; Craddock, C.; Chen, F.; Virchis, A.; Kottaridis, P.; Grimwade, D.; Khwaja, A.; Stauss, H.; et al. Wilms’ Tumour 1 (WT1) peptide vaccination in patients with acute myeloid leukaemia induces short-lived WT1-specific immune responses. Br. J. Haematol. 2014, 164, 366–375. [Google Scholar] [CrossRef]
- Maslak, P.G.; Dao, T.; Bernal, Y.; Chanel, S.M.; Zhang, R.; Frattini, M.; Rosenblat, T.; Jurcic, J.G.; Brentjens, R.J.; Arcila, M.E.; et al. Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia. Blood Adv. 2018, 2, 224–234. [Google Scholar] [CrossRef]
- Schmitt, M.; Schmitt, A.; Rojewski, M.T.; Chen, J.; Giannopoulos, K.; Fei, F.; Yu, Y.; Götz, M.; Heyduk, M.; Ritter, G.; et al. RHAMM-R3 peptide vaccination in patients with acute myeloid leukemia, myelodysplastic syndrome, and multiple myeloma elicits immunologic and clinical responses. Blood 2008, 111, 1357–1365. [Google Scholar] [CrossRef]
- Wang, M.-M.; Zhuang, L.-K.; Zhang, Y.-T.; Xia, D.; Pan, X.-R.; Tong, J.-H. A novel specific cleavage of IκBα protein in acute myeloid leukemia cells involves protease PR3. Exp. Cell Res. 2019, 382, 111441. [Google Scholar] [CrossRef]
- Rezvani, K.; Yong, A.S.; Mielke, S.; Jafarpour, B.; Savani, B.N.; Le, R.Q.; Eniafe, R.; Musse, L.; Boss, C.; Kurlander, R.; et al. Repeated PR1 and WT1 peptide vaccination in montanide-adjuvant fails to induce sustained high-avidity, epitope-specific CD8+ T cells in myeloid malignancies. Haematologica 2011, 96, 432–440. [Google Scholar] [CrossRef]
- Schürch, C.; Riether, C.; Ochsenbein, A.F. Dendritic cell-based immunotherapy for myeloid leukemias. Front. Immunol. 2013, 4, 496. [Google Scholar] [CrossRef]
- Chijioke, O.; Muenz, C. Dendritic cell derived cytokines in human natural killer cell differentiation and activation. Front. Immunol. 2013, 4, 365. [Google Scholar] [CrossRef]
- Houtenbos, I.; Westers, T.M.; Ossenkoppele, G.J.; van de Loosdrecht, A.A. Leukemia-derived dendritic cells: Towards clinical vaccination protocols in acute myeloid leukemia. Haematologica 2006, 91, 348–355. [Google Scholar]
- Zhou, L.J.; Tedder, T.F. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc. Natl. Acad. Sci. USA 1996, 93, 2588–2592. [Google Scholar] [CrossRef]
- Bigalke, I.; Fløisand, Y.; Solum, G.; Hønnåshagen, K.; Lundby, M.; Anderson, K.; Sæbøe-Larssen, S.; Inderberg, E.-M.; Eckl, J.; Schendel, D.J.; et al. AML Patients in Minimal Residual Disease Vaccinated with a Novel Generation of Fast Dendritic Cells Expressing WT-1 and PRAME Mount Specific Immune Responses That Relate to Clinical Outcome. Blood 2015, 126, 3798. [Google Scholar] [CrossRef]
- Lichtenegger, F.S.; Schnorfeil, F.M.; Rothe, M.; Dieser, K.; Altmann, T.; Bücklein, V.L.; Köhnke, T.; Augsberger, C.; Konstandin, N.P.; Spiekermann, K.; et al. Toll-like receptor 7/8-matured RNA-transduced dendritic cells as post-remission therapy in acute myeloid leukaemia: Results of a phase I trial. Clin. Transl. Immunol. 2020, 9, e1117. [Google Scholar] [CrossRef]
- Khoury, H.J.; Collins, R.H., Jr.; Blum, W.; Stiff, P.S.; Elias, L.; Lebkowski, J.S.; Reddy, A.; Nishimoto, K.P.; Sen, D.; Wirth, E.D., III; et al. Immune responses and long-term disease recurrence status after telomerase-based dendritic cell immunotherapy in patients with acute myeloid leukemia. Cancer 2017, 123, 3061–3072. [Google Scholar] [CrossRef]
- Chevallier, P.; Saiagh, S.; Dehame, V.; Guillaume, T.; Peterlin, P.; Bercegeay, S.; Le Bris, Y.; Bossard, C.; Gauvrit, I.; Dreno, B.; et al. A phase I/II feasibility vaccine study by autologous leukemic apoptotic corpse-pulsed dendritic cells for elderly AML patients. Hum. Vaccines Immunother. 2021, 17, 3511–3514. [Google Scholar] [CrossRef]
- Van De Loosdrecht, A.A.; Van Wetering, S.; Santegoets, S.J.A.M.; Singh, S.K.; Eeltink, C.M.; den Hartog, Y.; Koppes, M.; Kaspers, J.; Ossenkoppele, G.J.; Kruisbeek, A.M.; et al. A novel allogeneic off-the-shelf dendritic cell vaccine for post-remission treatment of elderly patients with acute myeloid leukemia. Cancer Immunol. Immunother. 2018, 67, 1505–1518. [Google Scholar] [CrossRef]
- Yang, J.; Bernier, S.M.; Ichim, T.E.; Li, M.; Xia, X.; Zhou, D.; Huang, X.; Strejan, G.H.; White, D.J.; Zhong, R.; et al. LF15-0195 generates tolerogenic dendritic cells by suppression of NF-kappaB signaling through inhibition of IKK activity. J. Leukoc. Biol. 2003, 74, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Curti, A.; Pandolfi, S.; Aluigi, M.; Isidori, A.; Alessandrini, I.; Chiodoni, C.; Testoni, N.; Colombo, M.P.; Baccarani, M.; Lemoli, R.M. Interleukin-12 production by leukemia-derived dendritic cells counteracts the inhibitory effect of leukemic microenvironment on T cells. Exp. Hematol. 2005, 33, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Draube, A.; Beyer, M.; Wolf, J. Activation of autologous leukemia-specific T cells in acute myeloid leukemia: Monocyte-derived dendritic cells cocultured with leukemic blasts compared with leukemia-derived dendritic cells. Eur. J. Haematol. 2008, 81, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Anguille, S.; Van de Velde, A.L.; Smits, E.L.; Van Tendeloo, V.F.; Juliusson, G.; Cools, N.; Nijs, G.; Stein, B.; Lion, E.; Van Driessche, A.; et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood 2017, 130, 1713–1721. [Google Scholar] [CrossRef]
- Dagvadorj, N.; Deuretzbacher, A.; Weisenberger, D.; Baumeister, E.; Trebing, J.; Lang, I.; Köchel, C.; Kapp, M.; Kapp, K.; Beilhack, A.; et al. Targeting of the WT191–138 fragment to human dendritic cells improves leukemia-specific T-cell responses providing an alternative approach to WT1-based vaccination. Cancer Immunol. Immunother. 2017, 66, 319–332. [Google Scholar] [CrossRef]
- Eckl, J.; Raffegerst, S.; Schnorfeil, F.; Prinz-Schulz, P.; Fingerhut, C.; Floisand, Y.; Josefsen, D.; Bigalke, I.; Pinkernell, K.; Kvalheim, G.; et al. DC Vaccination Induces Antigen Specific Immune Responses in AML Patients: A 1-Year Interim Assessment. Blood 2019, 134, 3923. [Google Scholar] [CrossRef]
- Kitawaki, T.; Kadowaki, N.; Fukunaga, K.; Kasai, Y.; Maekawa, T.; Ohmori, K.; Kondo, T.; Maekawa, R.; Takahara, M.; Nieda, M.; et al. A phase I/IIa clinical trial of immunotherapy for elderly patients with acute myeloid leukaemia using dendritic cells co-pulsed with WT1 peptide and zoledronate. Br. J. Haematol. 2011, 153, 796–799. [Google Scholar] [CrossRef]
- Rosenblatt, J.; Stone, R.M.; Uhl, L.; Neuberg, D.; Joyce, R.; Levine, J.D.; Arnason, J.; McMasters, M.; Luptakova, K.; Jain, S.; et al. Individualized vaccination of AML patients in remission is associated with induction of antileukemia immunity and prolonged remissions. Sci. Transl. Med. 2016, 8, 368ra171. [Google Scholar] [CrossRef]
- Mac Keon, S.; Ruiz, M.S.; Gazzaniga, S.; Wainstok, R. Dendritic cell-based vaccination in cancer: Therapeutic implications emerging from murine models. Front. Immunol. 2015, 6, 243. [Google Scholar] [CrossRef]
- Van de Loosdrecht, A.A.; Cloos, J.; Wagner, E.M.; Platzbecker, U.; Holderried, T.A.; van Elssen, J.; Giagounidis, A.; Lehmann, S.; van Zeeburg, H.; Rovers, T.; et al. Treatment with an Allogeneic Leukemia-Derived Dendritic Cell Vaccine in AML Patients Shows MRD Conversion and Improved Survival. Blood 2021, 138, 1274. [Google Scholar] [CrossRef]
- Massumoto, C.; Sousa-Canavez, J.M.; Camara-Lopes, L.H.; Leite, K.R.M. Camara-Lopes LH. Stabilization of acute myeloid leukemia with a dendritic cell vaccine. Hematol. Oncol. Stem Cell Ther. 2008, 1, 239–240. [Google Scholar] [CrossRef]
- Li, L.; Giannopoulos, K.; Reinhardt, P.; Tabarkiewicz, J.; Schmitt, A.; Greiner, J.; Rolinski, J.; Hus, I.; Dmoszynska, A.; Wiesneth, M.; et al. Immunotherapy for patients with acute myeloid leukemia using autologous dendritic cells generated from leukemic blasts. Int. J. Oncol. 2006, 28, 855–861. [Google Scholar] [CrossRef]
- Kitawaki, T.; Kadowaki, N.; Fukunaga, K.; Kasai, Y.; Maekawa, T.; Ohmori, K.; Itoh, T.; Shimizu, A.; Kuzushima, K.; Kondo, T.; et al. Cross-priming of CD8+ T cells in vivo by dendritic cells pulsed with autologous apoptotic leukemic cells in immunotherapy for elderly patients with acute myeloid leukemia. Exp. Hematol. 2011, 39, 424–433. [Google Scholar] [CrossRef]
- Dong, M.; Liang, D.; Li, Y.; Li, Y.; Kong, D.; Kang, P.; Li, K.; Ping, C.; Zhang, Y.; Zhou, X.; et al. Autologous dendritic cells combined with cytokine-induced killer cells synergize low-dose chemotherapy in elderly patients with acute myeloid leukaemia. J. Int. Med. Res. 2012, 40, 1265–1274. [Google Scholar] [CrossRef]
- Shah, N.N.; Loeb, D.M.; Khuu, H.; Stroncek, D.; Ariyo, T.; Raffeld, M.; Delbrook, C.; Mackall, C.L.; Wayne, A.S.; Fry, T.J. Induction of Immune Response after Allogeneic Wilms’ Tumor 1 Dendritic Cell Vaccination and Donor Lymphocyte Infusion in Patients with Hematologic Malignancies and Post-Transplantation Relapse. Biol. Blood Marrow Transplant. 2016, 22, 2149–2154. [Google Scholar] [CrossRef]
- Wang, D.; Huang, X.F.; Hong, B.; Song, X.-T.; Hu, L.; Jiang, M.; Zhang, B.; Ning, H.; Li, Y.; Xu, C.; et al. Efficacy of intracellular immune checkpoint-silenced DC vaccine. JCI Insight 2018, 3, 1–14. [Google Scholar] [CrossRef]
- Isidori, A.; Cerchione, C.; Daver, N.; DiNardo, C.; Garcia-Manero, G.; Konopleva, M.; Jabbour, E.; Ravandi, F.; Kadia, T.; de la Fuente Burguera, A.; et al. Immunotherapy in Acute Myeloid Leukemia: Where We Stand. Front. Oncol. 2021, 11, 1558. [Google Scholar] [CrossRef]
- Lambert, J.; Pautas, C.; Terré, C.; Raffoux, E.; Turlure, P.; Caillot, D.; Legrand, O.; Thomas, X.; Gardin, C.; Gogat-Marchant, K.; et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: Final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 2019, 104, 113–119. [Google Scholar] [CrossRef]
- Jen, E.Y.; Gao, X.; Li, L.; Zhuang, L.; Simpson, N.E.; Aryal, B.; Wang, R.; Przepiorka, D.; Shen, Y.L.; Leong, R.; et al. FDA approval summary: Tagraxofusp-erzs for Treatment of Blastic Plasmacytoid Dendritic Cell Neoplasm. Clin. Cancer Res. 2020, 26, 532–536. [Google Scholar] [CrossRef]
- Lu, C.; Redd, P.S.; Lee, J.R.; Savage, N.; Liu, K. The expression profiles and regulation of PD-L1 in tumor-induced myeloid-derived suppressor cells. Oncoimmunology 2016, 5, e1247135. [Google Scholar] [CrossRef]
- Yang, H.; Bueso-Ramos, C.; Dinardo, C.; Estecio, M.R.; Davanlou, M.; Geng, Q.R.; Fang, Z.; Nguyen, M.; Pierce, S.; Wei, Y.; et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia 2014, 28, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, A.; Khan, A.Y.; Malik, S.U.; Faridi, W.; Fraz, M.A.; Usman, M.; Tariq, M.J.; Durer, S.; Durer, C.; Russ, A.; et al. Significant Risk of Graft-versus-Host Disease with Exposure to Checkpoint Inhibitors Before and After Allogeneic transplantation. Biol. Blood Marrow Transplant. 2019, 25, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Alotaibi, A.S.; Bücklein, V.; Subklewe, M. T-cell-based immunotherapy of acute myeloid leukemia: Current concepts and future developments. Leukemia 2021, 35, 1843–1863. [Google Scholar] [CrossRef]
- Hoseini, S.S.; Cheung, N.K. Acute myeloid leukemia targets for bispecific antibodies. Blood Cancer J. 2017, 7, e522. [Google Scholar] [CrossRef] [PubMed]
- Salvaris, R.; Ong, J.; Gregory, G. Bispecific antibodies: A review of development, clinical efficacy and toxicity in B-cell lymphomas. J. Pers. Med. 2021, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.A.; Kersten, M.J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 640–654. [Google Scholar] [CrossRef]
- Mardiana, S.; Gill, S. CAR T Cells for Acute Myeloid Leukemia: State of the Art and Future Directions. Front Oncol. 2020, 10, 697. [Google Scholar] [CrossRef]
- Marnell, C.S.; Bick, A.; Natarajan, P. Clonal hematopoiesis of indeterminate potential (CHIP): Linking somatic mutations, hematopoiesis, chronic inflammation and cardiovascular disease. J. Mol. Cell. Cardiol. 2021, 161, 98–105. [Google Scholar] [CrossRef]
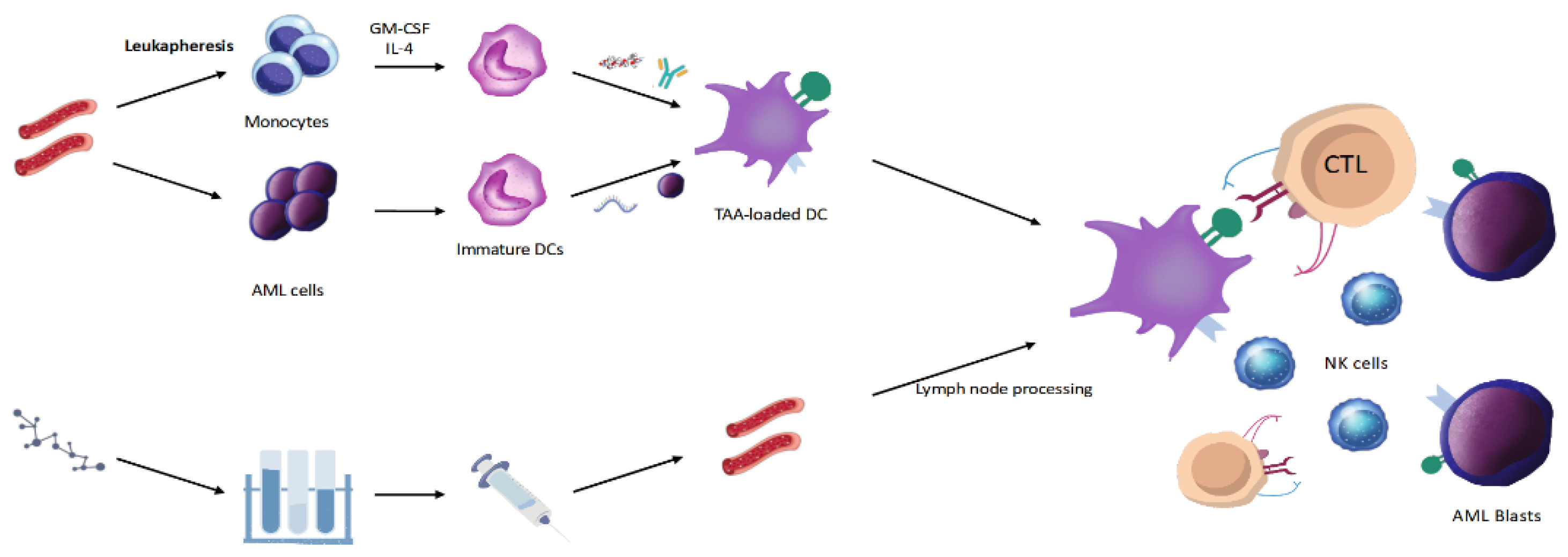
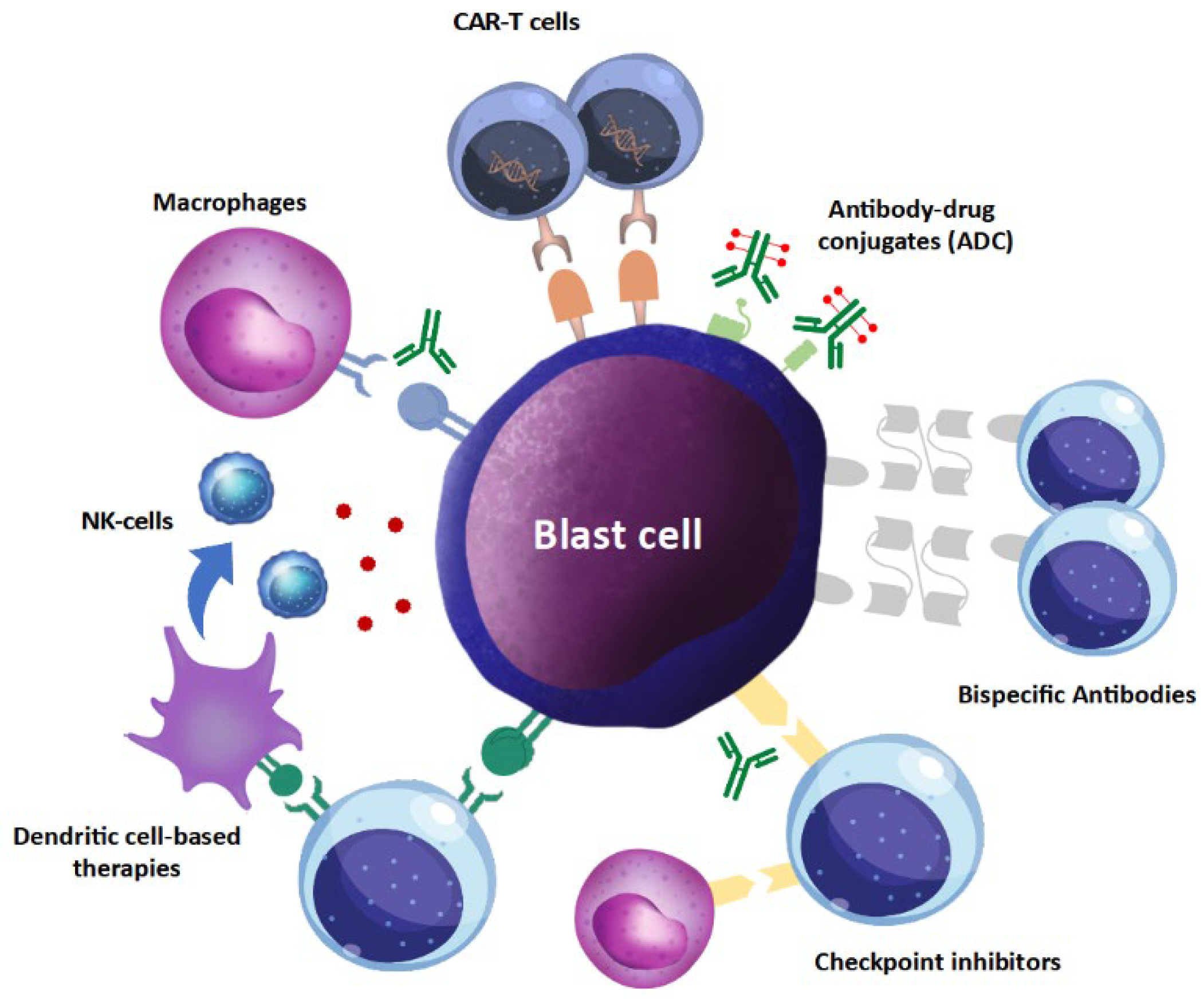
| Refs. | Vaccine Type | Vaccine Antigen | Clinical Setting | N of pts | Doses | Endpoint | Results |
|---|---|---|---|---|---|---|---|
| Brayer 2015 [11] | P | WT1 | CR high risk AML | 16 | 12 | Phase I—safety | No toxicity |
| Maslak 2010 [12] | P | WT1 | CR AML, WT1 + | 9 | 6 | Phase I—Immune response | 7 out of 8 evaluable pts |
| Keilholz 2009 [15] | P | WT1 (+GCSF) | R/R AML | 17 | Median 11 | Response rate | 4 PR, 1 CR, 3 SD |
| Uttenthal 2014 [17] | P | WT1 | R/R AML | 8 | 8 | Response rate and immune response | No correlation |
| Maslak 2018 [18] | P | WT1 | CR1 AML | 22 | 12 | Clinical outcome | DFS 16,9 months |
| Schmitt 2008 [19] | P | RHAMM | Active AML and MM | 10 | 4 | Response rate | 3 PR in AML and 2 PR in MM |
| Kuball 2011 [8] | P | WT1, PR3, PADRE | Active AML and MM | 9 | 4 | Response rate and immune response | No clinical or immune response, |
| Anguille 2017 [34] | DC | WT1 | CR AML | 30 | 4, then every 2 mo | Relapse rate | relapse reduction rate of 25% |
| Eckl 2019 [36] | DC | WT1, PRAME | R/R AML | 20 | 4, then every 6 w | Response rate | SD 60% |
| Lichtenegger 2020 [27] | DC | WT1, PRAME, CMVpp65 | CR high-risk AML | 10 | 10 | Immune response | higher in CMVpp65 |
| Chevallier 2021 [29] | moDC | - | CR high-risk AML | 5 | 5 | Clinical outcome | prolonged OS vs. historical cohorts (16 vs. 8 mo) |
| Rosenblatt 2016 [38] | DC | - | CR high-risk AML | 17 | 3 | Immune response | leukemic reactive CD8+ T cells |
| Loosdrecht 2018 [30] | alloDC | WT1, PRAME | CR / SD AML | 12 | 4 | Clinical outcome | 1 CR, prolonged mOS in CR pts (36 mo) |
| Khoury 2017 [28] | DC | hTERT, LAMP | CR int-risk AML | 22 | 17 | Clinical outcome | DFS 58%—mFU 52 mo |
| Dong 2012 [44] | DC + (CIK + CT) | - | I line AML (vs. CT alone) | 21 | 1 | Clinical outcome | ORR 71 vs. 39% CR 29 vs. 22% |
| Wang 2018 [46] | DC | MUC1, flagellin, SOCS1 | Post-HSCT AML rel | 35 | 1 | ph I (+CIK) vs. DLI, ph II (+CIK) for early mol relapse | higher OS vs. DLI (48.9 vs. 27.5%) molecular CR in 83% in ph II |
| Study | Type | Intervention | Disease Status |
|---|---|---|---|
| NCT01686334 | Randomized phase II | Wilms’ Tumor (WT1) Antigen-targeted Dendritic Cell Vaccination | Prevent Relapse in Adult Patients With Acute Myeloid Leukemia with MRD positivity |
| NCT05000801 | Phase I | WT1/hTERT/Survivin-loaded DCs | Prevent relapse in adult AML patients with MRD at very high risk of relapse |
| NCT03059485 | Randomized phase II | Dendritic Cell/AML Fusion vaccine (DC/AML vaccine | Prevent Relapse in Adult Patients With Acute Myeloid Leukemia with no history of allogeneic transplantation |
| NCT03679650 | Non-randomized phase I | Dendritic Cell/AML Fusion vaccine with or without decitabine | Prevent Relapse in Adult Patients With Acute Myeloid Leukemia with no history of allogeneic transplantation |
| NCT04747002 | Randomized phase II | peptide vaccine DSP-7888 | Prevent relapse in adult AML patients ineligible for HSCT with MRD at very high risk of relapse |
| NCT03761914 | Phase I/II | Wilms Tumor-1 (WT1)-targeting multivalent heteroclitic peptide (Galinpepimut-S) with Pembrolizumab | Active disease |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbullushi, K.; Rampi, N.; Serpenti, F.; Sciumè, M.; Fabris, S.; De Roberto, P.; Fracchiolla, N.S. Vaccination Therapy for Acute Myeloid Leukemia: Where Do We Stand? Cancers 2022, 14, 2994. https://doi.org/10.3390/cancers14122994
Barbullushi K, Rampi N, Serpenti F, Sciumè M, Fabris S, De Roberto P, Fracchiolla NS. Vaccination Therapy for Acute Myeloid Leukemia: Where Do We Stand? Cancers. 2022; 14(12):2994. https://doi.org/10.3390/cancers14122994
Chicago/Turabian StyleBarbullushi, Kordelia, Nicolò Rampi, Fabio Serpenti, Mariarita Sciumè, Sonia Fabris, Pasquale De Roberto, and Nicola Stefano Fracchiolla. 2022. "Vaccination Therapy for Acute Myeloid Leukemia: Where Do We Stand?" Cancers 14, no. 12: 2994. https://doi.org/10.3390/cancers14122994
APA StyleBarbullushi, K., Rampi, N., Serpenti, F., Sciumè, M., Fabris, S., De Roberto, P., & Fracchiolla, N. S. (2022). Vaccination Therapy for Acute Myeloid Leukemia: Where Do We Stand? Cancers, 14(12), 2994. https://doi.org/10.3390/cancers14122994







