Coxsackievirus Group B3 Has Oncolytic Activity against Colon Cancer through Gasdermin E-Mediated Pyroptosis
Abstract
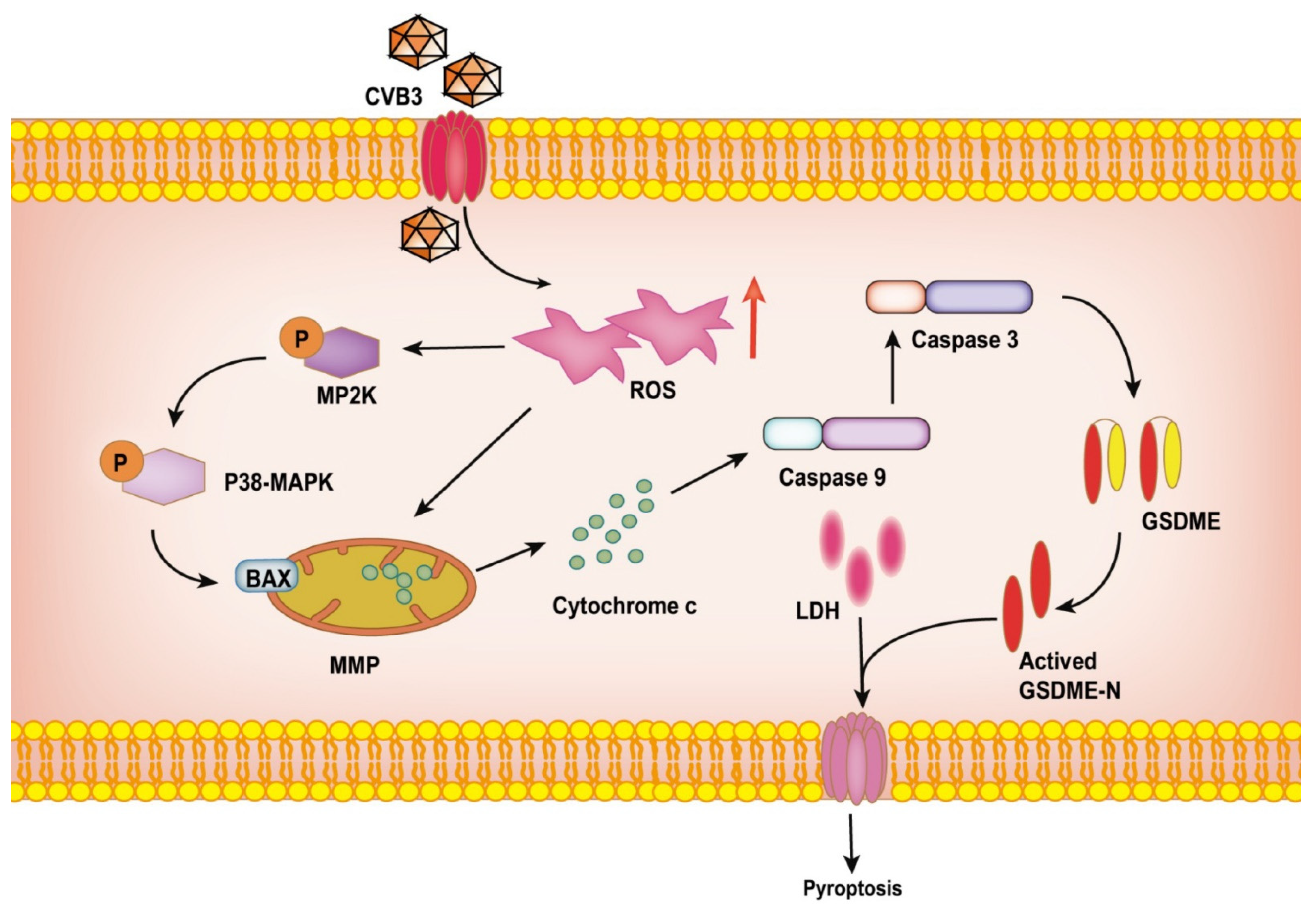
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cell Culture, Virus, and Reagents
2.3. Fluorescence Inverted Microscope Imaging
2.4. Flow Cytometry
2.5. LDH Release Assay
2.6. Cell Viability Assay
2.7. Detection of Reactive Oxygen Species (ROS)
2.8. Western Blot
2.9. Nude Mouse Xenograft Assay
2.10. Immunohistochemical Analysis
2.11. Statistical Analysis
3. Results
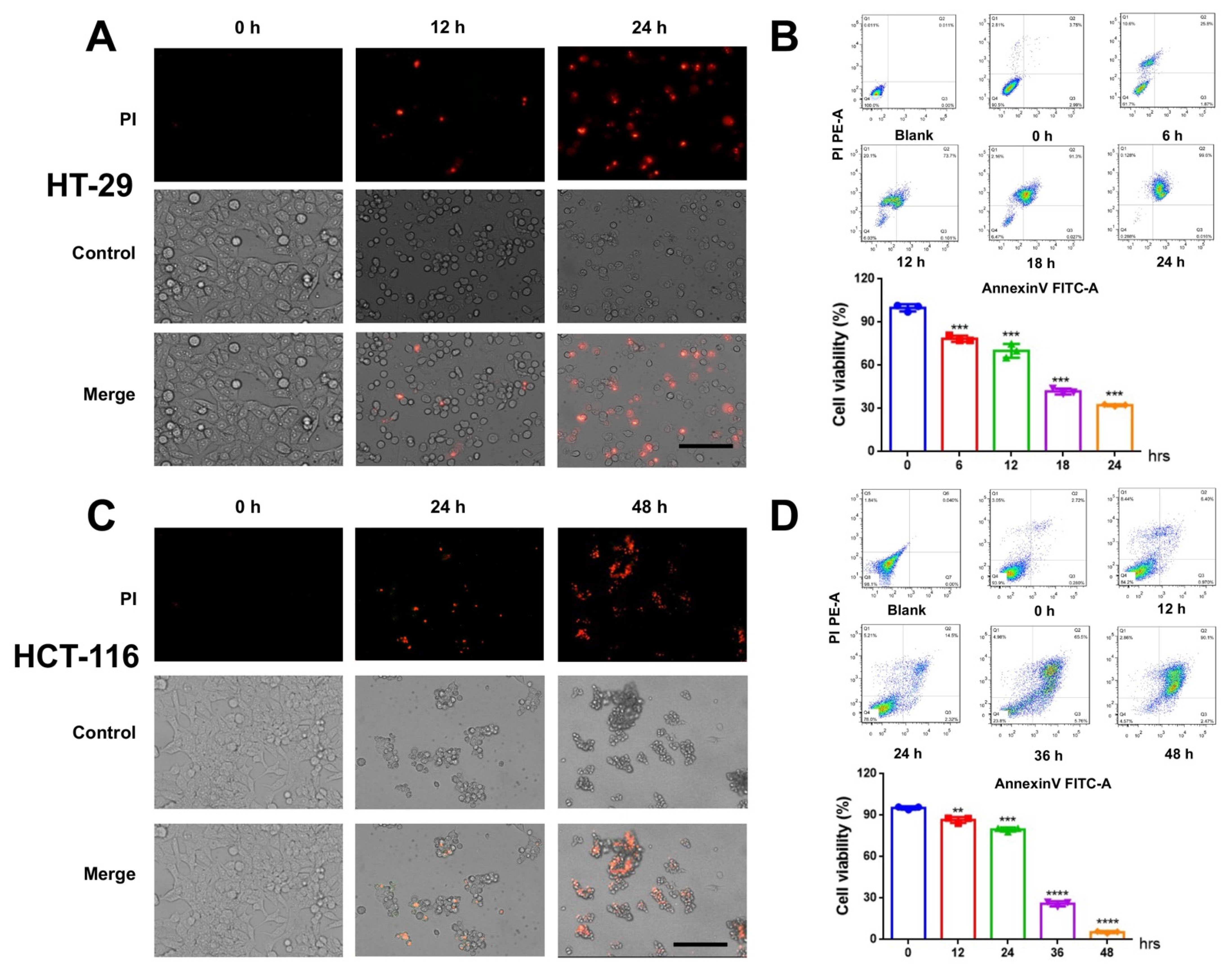
3.1. CVB3 Induces Colon Cancer Cell Death
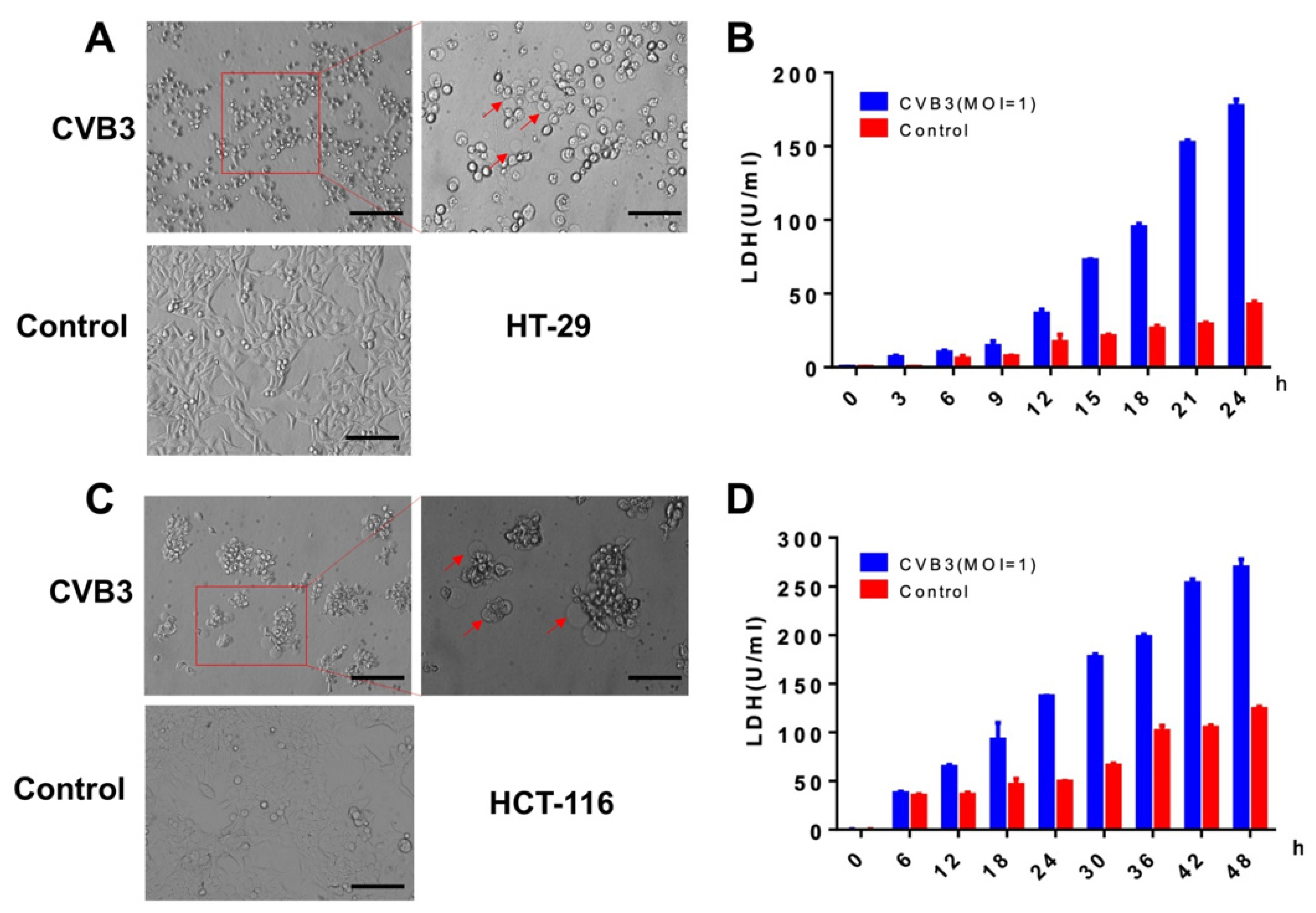
3.2. CVB3 Induces Pyroptosis in Colon Cancer Cells and Causes LDH Release
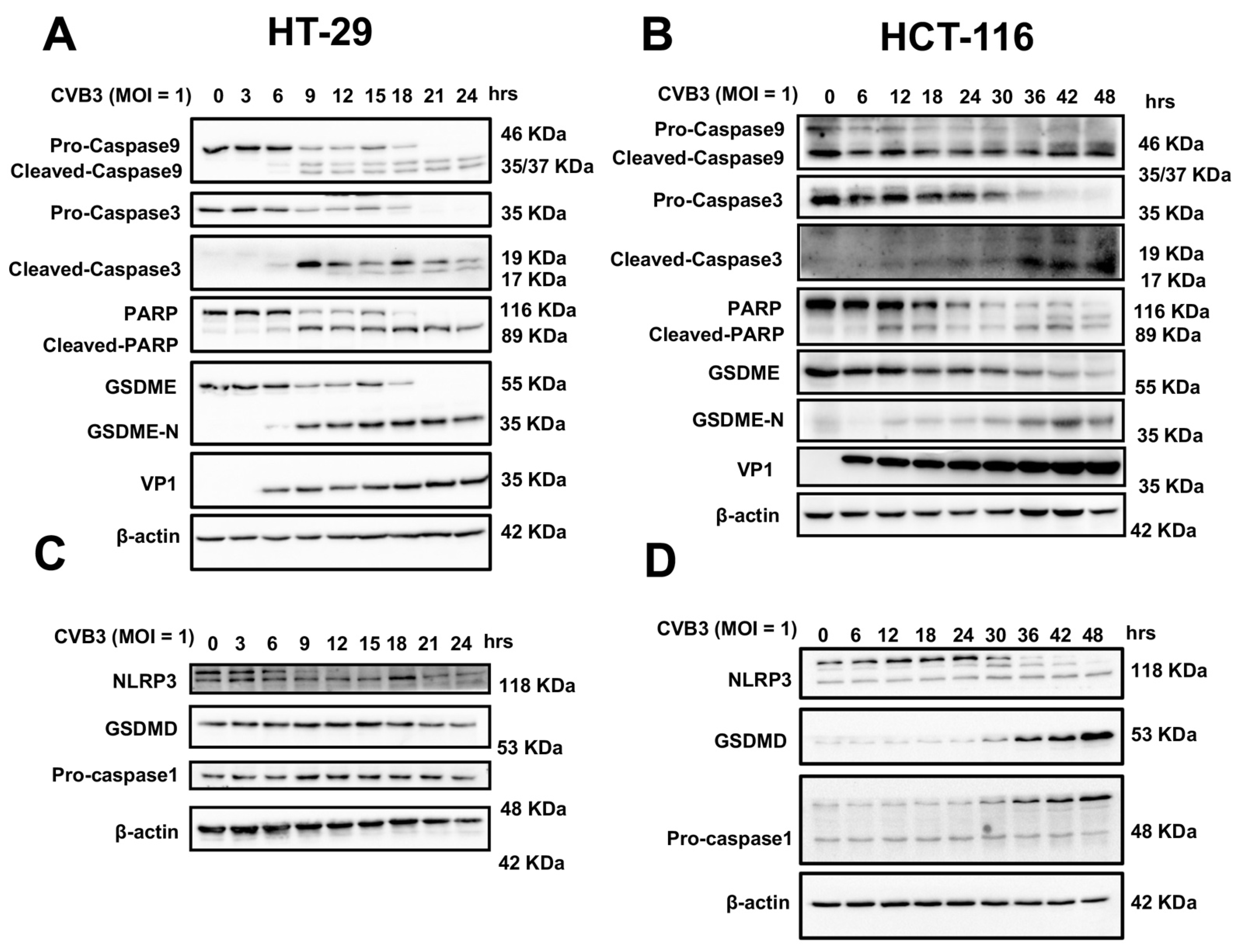
3.3. CVB3 Induces Pyroptosis in Colon Cancer Cell Lines through the GSDME Pathway
3.4. CVB3-Induced GSDME-Dependent Pyroptosis Is Promoted by ROS Signaling
3.5. CVB3-Induced Pyroptosis Is Dependent on Casp-3-Mediated Cleavage of GSDME
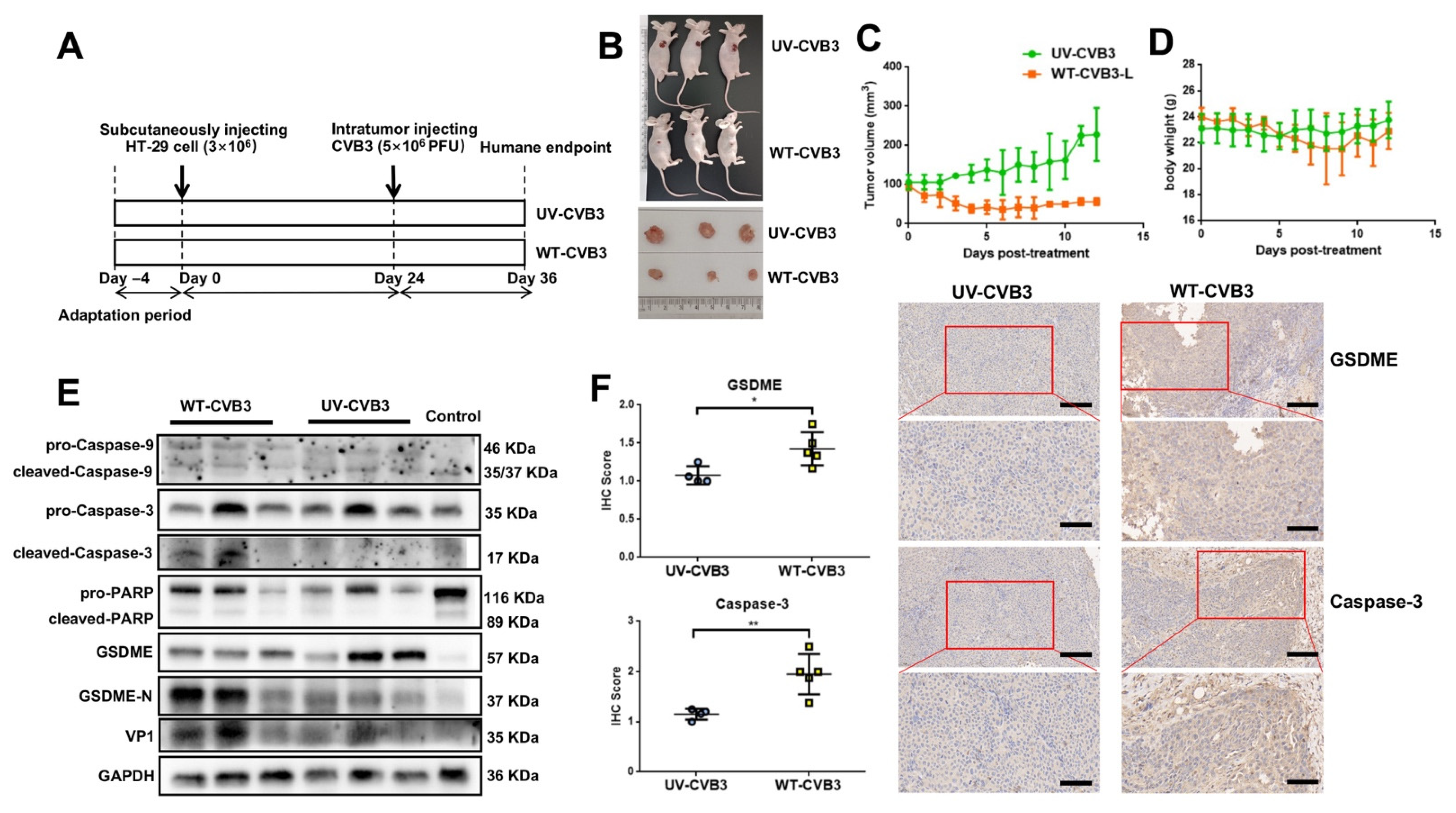
3.6. CVB3-Induced GSDME-Dependent Pyroptosis Inhibits the Growth of Colon Cancer Cells in Mice
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pączek, S.; Łukaszewicz-Zając, M.; Mroczko, B. Granzymes—Their Role in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 5277. [Google Scholar] [CrossRef]
- Li, Q.; Oduro, P.K.; Guo, R.; Li, R.; Leng, L.; Kong, X.; Wang, Q.; Yang, L. Oncolytic Viruses: Immunotherapy Drugs for Gastrointestinal Malignant Tumors. Front. Cell. Infect. Microbiol. 2022, 12, 921534. [Google Scholar] [CrossRef]
- Lawler, S.E.; Speranza, M.-C.; Cho, C.-F.; Chiocca, E.A. Oncolytic Viruses in Cancer Treatment: A Review. JAMA Oncol. 2017, 3, 841–849. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2016, 15, 660. [Google Scholar] [CrossRef]
- Guo, Z.S.; Lu, B.; Guo, Z.; Giehl, E.; Feist, M.; Dai, E.; Liu, W.; Storkus, W.J.; He, Y.; Liu, Z.; et al. Vaccinia virus-mediated cancer immunotherapy: Cancer vaccines and oncolytics. J. Immunother. Cancer 2019, 7, 6. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, L.; Liu, Q.; Jin, G.; Zhang, J.; Li, Z.; Xu, Y.; Tian, H.; Deng, S.; Shi, Q.; et al. CVB3 VP1 interacts with MAT1 to inhibit cell proliferation by interfering with Cdk-activating kinase complex activity in CVB3-induced acute pancreatitis. PLoS Pathog. 2021, 17, e1008992. [Google Scholar] [CrossRef]
- Sagara, M.; Miyamoto, S.; Itoh, S.; Soda, Y.; Tani, K. Development of New Oncolytic Virotherapy Targeting Breast Cancer Using Coxsackievirus B3. Anticancer Res. 2021, 41, 81–89. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, W.; Wan, J.; Yang, Y.; Fu, W.; Pan, D.; Cai, L.; Cheng, T.; Huang, X.; Wang, Y. Oncolytic activity of a coxsackievirus B3 strain in human endometrial cancer cell lines. Virol. J. 2018, 15, 65. [Google Scholar] [CrossRef]
- Deng, H.; Liu, H.; de Silva, T.; Xue, Y.; Mohamud, Y.; Ng, C.S.; Qu, J.; Zhang, J.; Jia, W.W.; Lockwood, W.W.; et al. Coxsackievirus Type B3 Is a Potent Oncolytic Virus against KRAS-Mutant Lung Adenocarcinoma. Mol. Ther. Oncolytics 2019, 14, 266–278. [Google Scholar] [CrossRef]
- Hazini, A.; Dieringer, B.; Pryshliak, M.; Knoch, K.-P.; Heimann, L.; Tolksdorf, B.; Pappritz, K.; El-Shafeey, M.; Solimena, M.; Beling, A.; et al. miR-375- and miR-1-Regulated Coxsackievirus B3 Has No Pancreas and Heart Toxicity But Strong Antitumor Efficiency in Colorectal Carcinomas. Hum. Gene Ther. 2021, 32, 216–230. [Google Scholar] [CrossRef]
- Hazini, A.; Pryshliak, M.; Brückner, V.; Klingel, K.; Sauter, M.; Pinkert, S.; Kurreck, J.; Fechner, H. Heparan Sulfate Binding Coxsackievirus B3 Strain PD: A Novel Avirulent Oncolytic Agent Against Human Colorectal Carcinoma. Hum. Gene Ther. 2018, 29, 1301–1314. [Google Scholar] [CrossRef]
- Li, W.; Sun, J.; Zhou, X.; Lu, Y.; Cui, W.; Miao, L. Mini-Review: GSDME-Mediated Pyroptosis in Diabetic Nephropathy. Front. Pharmacol. 2021, 12, 780790. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, H.; Weng, C.; Jiang, H.; Chen, J. Caspase 3/GSDME-dependent pyroptosis contributes to chemotherapy drug-induced nephrotoxicity. Cell Death Dis. 2021, 12, 186. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef]
- Li, X.; Xia, Y.; Huang, S.; Liu, F.; Ying, Y.; Xu, Q.; Liu, X.; Jin, G.; Papasian, C.J.; Chen, J.; et al. Identification of the interaction of VP1 with GM130 which may implicate in the pathogenesis of CVB3-induced acute pancreatitis. Sci. Rep. 2015, 5, 13324. [Google Scholar] [CrossRef]
- Wen, S.; Wang, Z.-H.; Zhang, C.-X.; Yang, Y.; Fan, Q.-L. Caspase-3 Promotes Diabetic Kidney Disease Through Gasdermin E-Mediated Progression to Secondary Necrosis During Apoptosis. Diabetes Metab. Syndr. Obes. 2020, 13, 313–323. [Google Scholar] [CrossRef]
- Knoblach, S.M.; Alroy, D.A.; Nikolaeva, M.; Cernak, I.; Stoica, B.A.; Faden, A.I. Caspase Inhibitor z-DEVD-fmk Attenuates Calpain and Necrotic Cell Death in Vitro and after Traumatic Brain Injury. J. Cereb. Blood Flow Metab. 2004, 24, 1119–1132. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Liu, X.-L.; Zhao, R. Induction of Pyroptosis and Its Implications in Cancer Management. Front. Oncol. 2019, 9, 971. [Google Scholar] [CrossRef]
- E Kagan, V.; Tyurin, V.; Jiang, J.; Tyurina, Y.; Ritov, V.B.; Amoscato, A.; Osipov, A.N.; A Belikova, N.; Kapralov, O.; Kini, V.; et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 2005, 1, 223–232. [Google Scholar] [CrossRef]
- Huang, J.; Wang, F.; Sun, X.; Chu, X.; Jiang, R.; Wang, Y.; Pang, L. Myocardial infarction cardiomyocytes-derived exosomal miR-328-3p promote apoptosis via Caspase signaling. Am. J. Transl. Res. 2021, 13, 2365–2378. [Google Scholar]
- Ji, N.; Yang, Y.; Cai, C.-Y.; Lei, Z.-N.; Wang, J.-Q.; Gupta, P.; Shukla, S.; Ambudkar, S.V.; Kong, D.; Chen, Z.-S. Selonsertib (GS-4997), an ASK1 inhibitor, antagonizes multidrug resistance in ABCB1- and ABCG2-overexpressing cancer cells. Cancer Lett. 2019, 440–441, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Min, X.; Shen, M.; Hua, Q.; Han, Y.; Zhao, L.; Liu, L.; Huang, G.; Liu, J.; Zhao, X. ACLY facilitates colon cancer cell metastasis by CTNNB1. J. Exp. Clin. Cancer Res. 2019, 38, 401. [Google Scholar] [CrossRef] [PubMed]
- Fidelle, M.; Yonekura, S.; Picard, M.; Cogdill, A.; Hollebecque, A.; Roberti, M.P.; Zitvogel, L. Resolving the Paradox of Colon Cancer Through the Integration of Genetics, Immunology, and the Microbiota. Front. Immunol. 2020, 11, 600886. [Google Scholar] [CrossRef] [PubMed]
- Raja, J.; Ludwig, J.M.; Gettinger, S.N.; Schalper, K.A.; Kim, H.S. Oncolytic virus immunotherapy: Future prospects for oncology. J. Immunother. Cancer 2018, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Planès, R.; Pinilla, M.; Santoni, K.; Hessel, A.; Passemar, C.; Lay, K.; Paillette, P.; Valadão, A.-L.C.; Robinson, K.S.; Bastard, P.; et al. Human NLRP1 is a sensor of pathogenic coronavirus 3CL proteases in lung epithelial cells. Mol. Cell 2022, 82, 2385–2400.e9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Zhu, Y.; Li, N.; Li, W.; Shang, C.; Song, G.; Li, S.; Cong, J.; Li, T.; et al. Apoptin induces pyroptosis of colorectal cancer cells via the GSDME-dependent pathway. Int. J. Biol. Sci. 2022, 18, 717–730. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, J.-Y.; Liu, X.-S.; Chen, H.-Z.; Ai, Y.-L.; Cheng, K.; Sun, R.-Y.; Zhou, D.; Han, J.; Wu, Q. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 2018, 28, 1171–1185. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Y.; Ma, X.-F.; Hou, D.; Zhang, Y.-H.; Sun, Y.; Shi, S.-S.; Forouzanfar, T.; Lin, H.-Y.; Fan, J.; et al. Triclabendazole Induces Pyroptosis by Activating Caspase-3 to Cleave GSDME in Breast Cancer Cells. Front. Pharmacol. 2021, 12, 670081. [Google Scholar] [CrossRef]
- Rogers, C.; Fernandes-Alnemri, T.; Mayes, L.; Alnemri, D.; Cingolani, G.; Alnemri, E.S. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 2017, 8, 14128. [Google Scholar] [CrossRef]
- Geisler, A.; Hazini, A.; Heimann, L.; Kurreck, J.; Fechner, H. Coxsackievirus B3—Its Potential as an Oncolytic Virus. Viruses 2021, 13, 718. [Google Scholar] [CrossRef]
- Erttmann, S.F.; Swacha, P.; Aung, K.M.; Brindefalk, B.; Jiang, H.; Härtlova, A.; Uhlin, B.E.; Wai, S.N.; Gekara, N.O. The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis. Immunity 2022, 55, 847–861.e10. [Google Scholar] [CrossRef]
- Miyamoto, S.; Inoue, H.; Nakamura, T.; Yamada, M.; Sakamoto, C.; Urata, Y.; Okazaki, T.; Marumoto, T.; Takahashi, A.; Takayama, K.; et al. Coxsackievirus B3 Is an Oncolytic Virus with Immunostimulatory Properties That Is Active against Lung Adenocarcinoma. Cancer Res 2012, 72, 2609–2621. [Google Scholar] [CrossRef]
- Ebauzon, M.; Ehermiston, T. Armed Therapeutic Viruses—A Disruptive Therapy on the Horizon of Cancer Immunotherapy. Front. Immunol. 2014, 5, 74. [Google Scholar] [CrossRef]
- Chen, D.; Wang, R.; Long, M.; Li, W.; Xiao, B.; Deng, H.; Weng, K.; Gong, D.; Liu, F.; Luo, S.; et al. Identification of in vitro and in vivo oncolytic effect in colorectal cancer cells by Orf virus strain NA1/11. Oncol. Rep. 2020, 45, 535–546. [Google Scholar] [CrossRef]
- Garmaroudi, G.A.; Karimi, F.; Naeini, L.G.; Kokabian, P.; Givtaj, N. Therapeutic Efficacy of Oncolytic Viruses in Fighting Cancer: Recent Advances and Perspective. Oxidative Med. Cell. Longev. 2022, 2022, 3142306. [Google Scholar] [CrossRef]
- Longo, S.L.; Griffith, C.; Glass, A.; Shillitoe, E.J.; E Post, D. Development of an oncolytic herpes simplex virus using a tumor-specific HIF-responsive promoter. Cancer Gene Ther. 2011, 18, 123–134. [Google Scholar] [CrossRef]
- Ruiz, A.J.; Hadac, E.M.; Nace, R.A.; Russell, S.J. MicroRNA-Detargeted Mengovirus for Oncolytic Virotherapy. J. Virol. 2016, 90, 4078–4092. [Google Scholar] [CrossRef]
- Shayestehpour, M.; Moghim, S.; Salimi, V.; Jalilvand, S.; Yavarian, J.; Romani, B.; Mokhtari-Azad, T. Targeting human breast cancer cells by an oncolytic adenovirus using microRNA-targeting strategy. Virus Res. 2017, 240, 207–214. [Google Scholar] [CrossRef]
- Holland, J.; Spindler, K.; Horodyski, F.; Grabau, E.; Nichol, S.; VandePol, S. Rapid Evolution of RNA Genomes. Science 1982, 215, 1577–1585. [Google Scholar] [CrossRef]
- Lim, B.-K.; Shin, J.-O.; Lee, S.-C.; Kim, D.-K.; Choi, N.-J.; Choe, S.-C.; Knowlton, K.U.; Jeon, E.-S. Long-term cardiac gene expression using a coxsackieviral vector. J. Mol. Cell. Cardiol. 2005, 38, 745–751. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, X.X.; Dai, J.P.; Zhao, X.F.; Xin, G.; Su, Y.; Wang, G.F.; Li, R.; Yan, Y.X.; Su, J.H.; et al. An Attenuated Coxsackievirus B3 Vector: A Potential Tool for Viral Tracking Study and Gene Delivery. PLoS ONE 2013, 8, e83753. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Kanneganti, T.-D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xu, T.; Tian, H.; Wu, J.; Yu, X.; Zeng, L.; Liu, F.; Liu, Q.; Huang, X. Coxsackievirus Group B3 Has Oncolytic Activity against Colon Cancer through Gasdermin E-Mediated Pyroptosis. Cancers 2022, 14, 6206. https://doi.org/10.3390/cancers14246206
Zhang Y, Xu T, Tian H, Wu J, Yu X, Zeng L, Liu F, Liu Q, Huang X. Coxsackievirus Group B3 Has Oncolytic Activity against Colon Cancer through Gasdermin E-Mediated Pyroptosis. Cancers. 2022; 14(24):6206. https://doi.org/10.3390/cancers14246206
Chicago/Turabian StyleZhang, Yejia, Tian Xu, Huizhen Tian, Jianfeng Wu, Xiaomin Yu, Lingbing Zeng, Fadi Liu, Qiong Liu, and Xiaotian Huang. 2022. "Coxsackievirus Group B3 Has Oncolytic Activity against Colon Cancer through Gasdermin E-Mediated Pyroptosis" Cancers 14, no. 24: 6206. https://doi.org/10.3390/cancers14246206
APA StyleZhang, Y., Xu, T., Tian, H., Wu, J., Yu, X., Zeng, L., Liu, F., Liu, Q., & Huang, X. (2022). Coxsackievirus Group B3 Has Oncolytic Activity against Colon Cancer through Gasdermin E-Mediated Pyroptosis. Cancers, 14(24), 6206. https://doi.org/10.3390/cancers14246206



