Whole-Blood Gene Expression Profiles Correlate with Response to Immune Checkpoint Inhibitors in Patients with Metastatic Renal Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
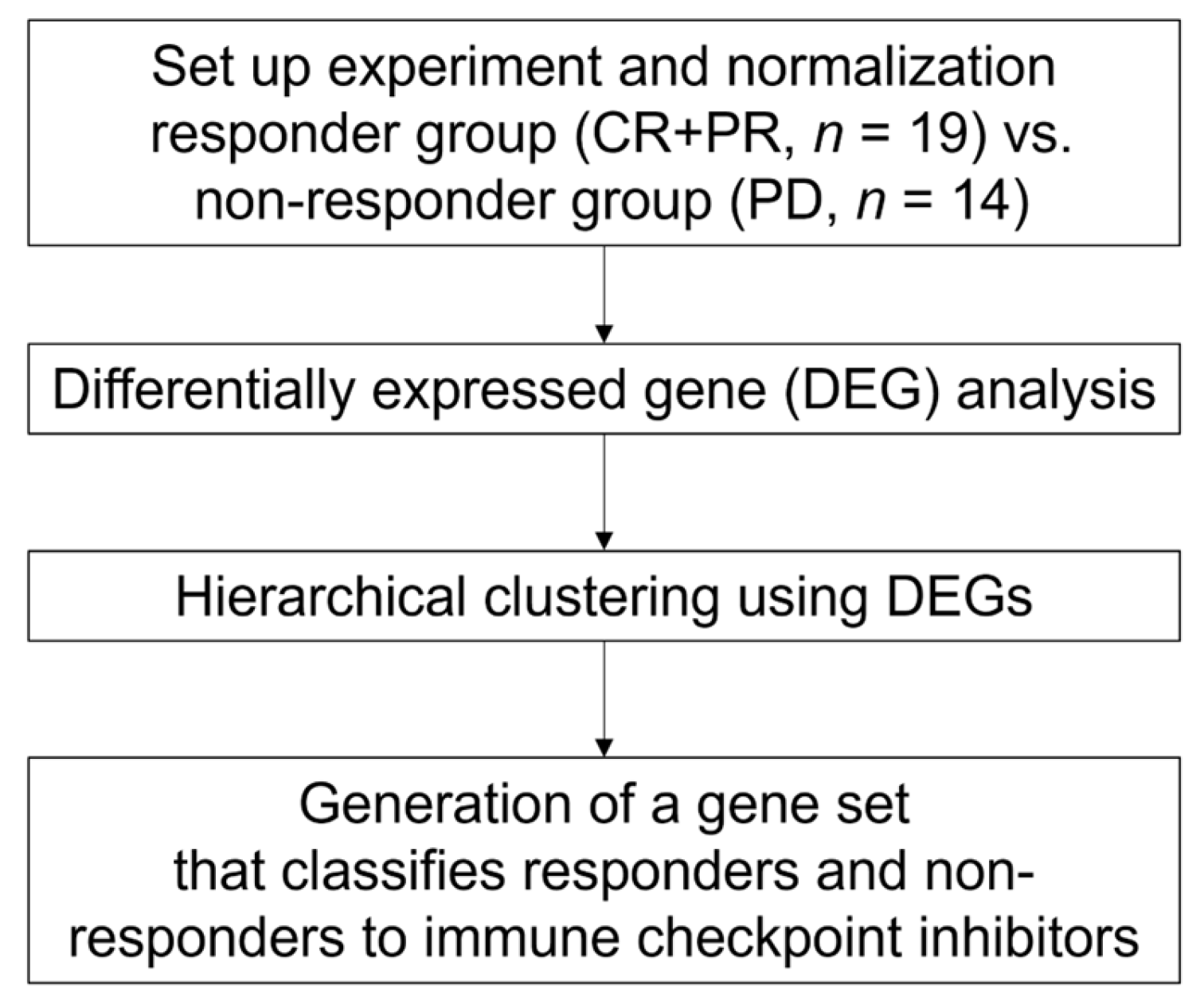
2. Materials and Methods
2.1. Patients and Sample Collection
2.2. RNA Isolation and RNA Sequencing (RNA-seq)
2.3. RNA-seq Analysis
2.4. Cluster Analysis
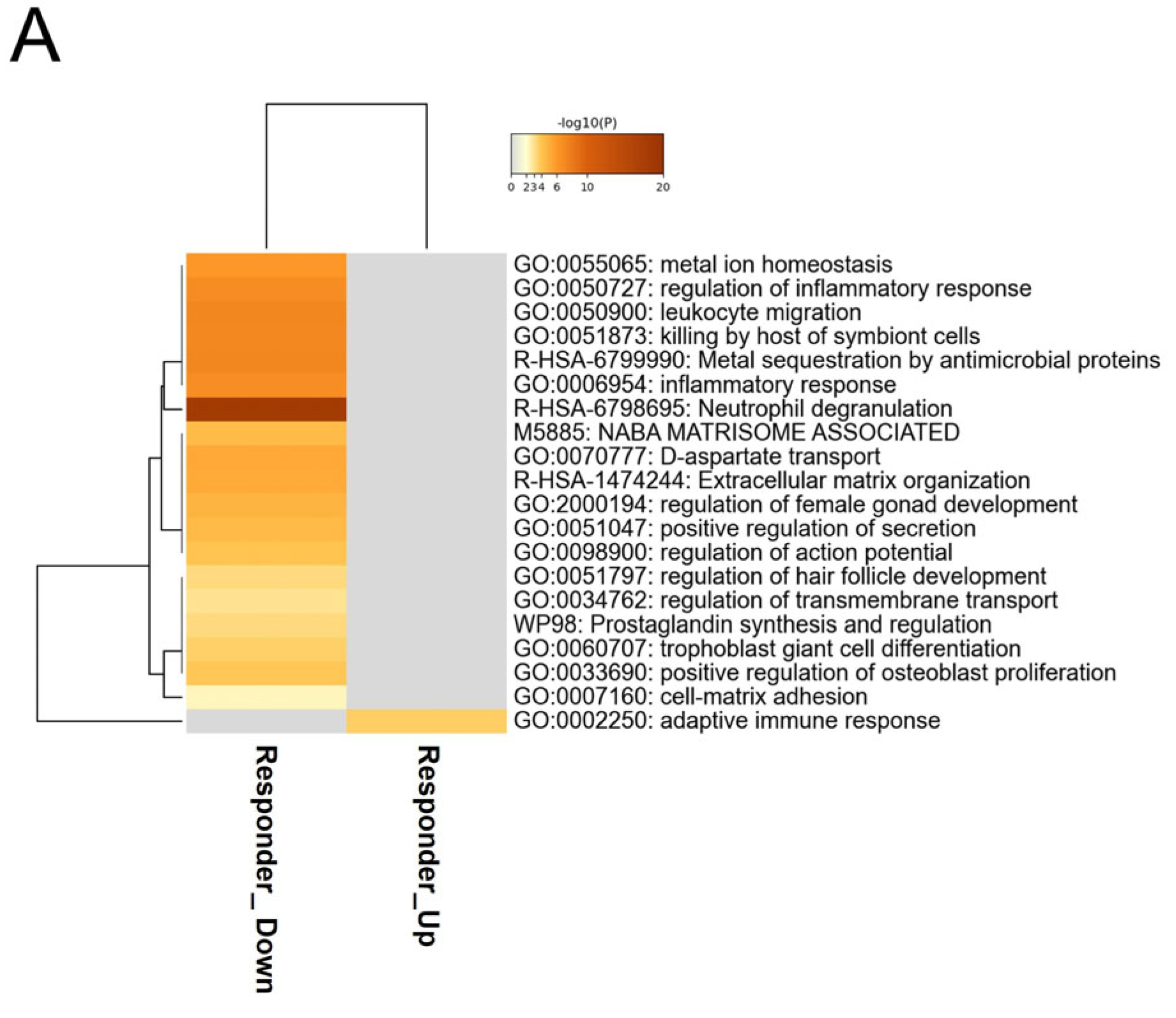
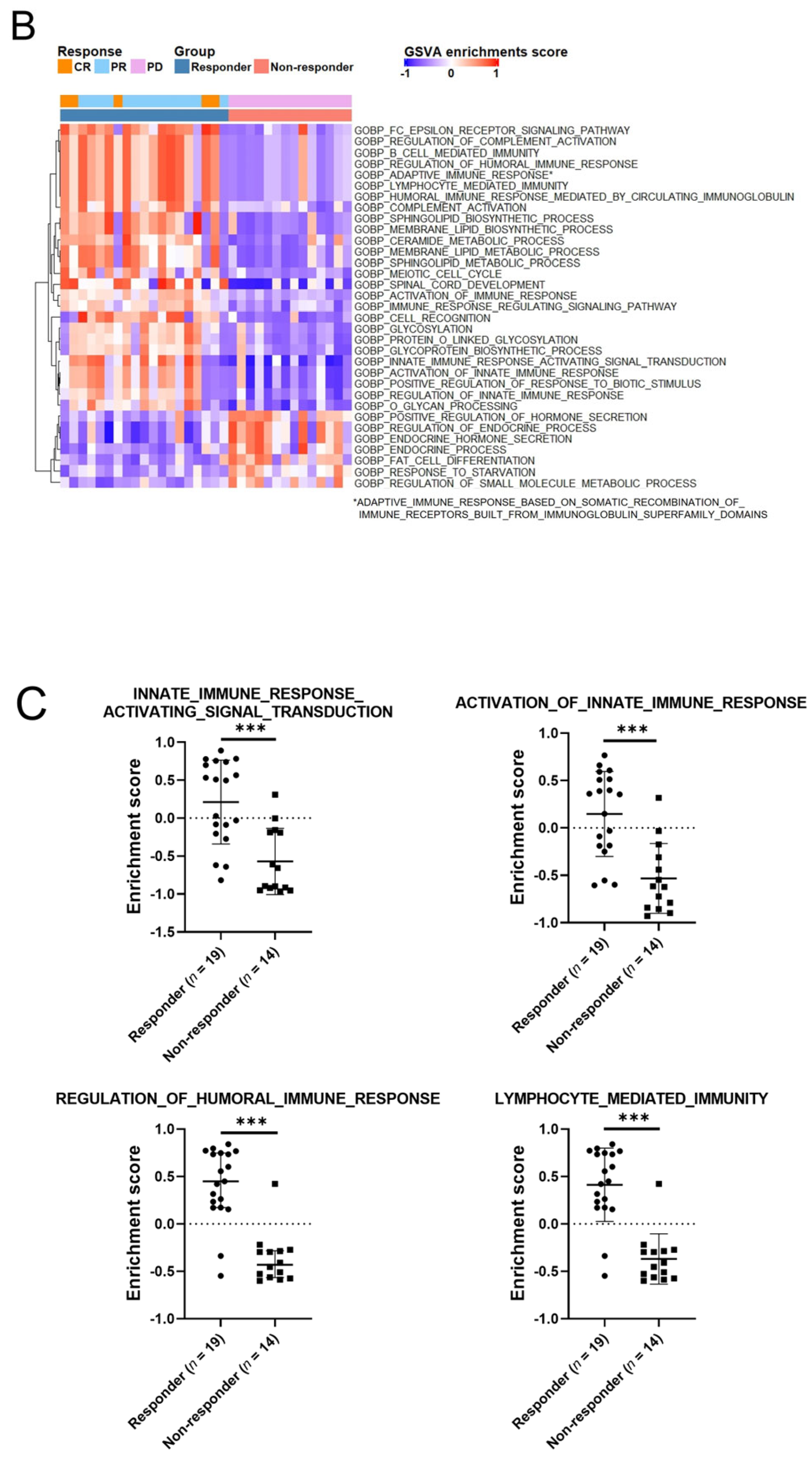
2.5. Enrichment Analysis
2.6. Statistical Analysis
2.7. Data Availability
3. Results
3.1. Patient Characteristics of the Discovery Dataset
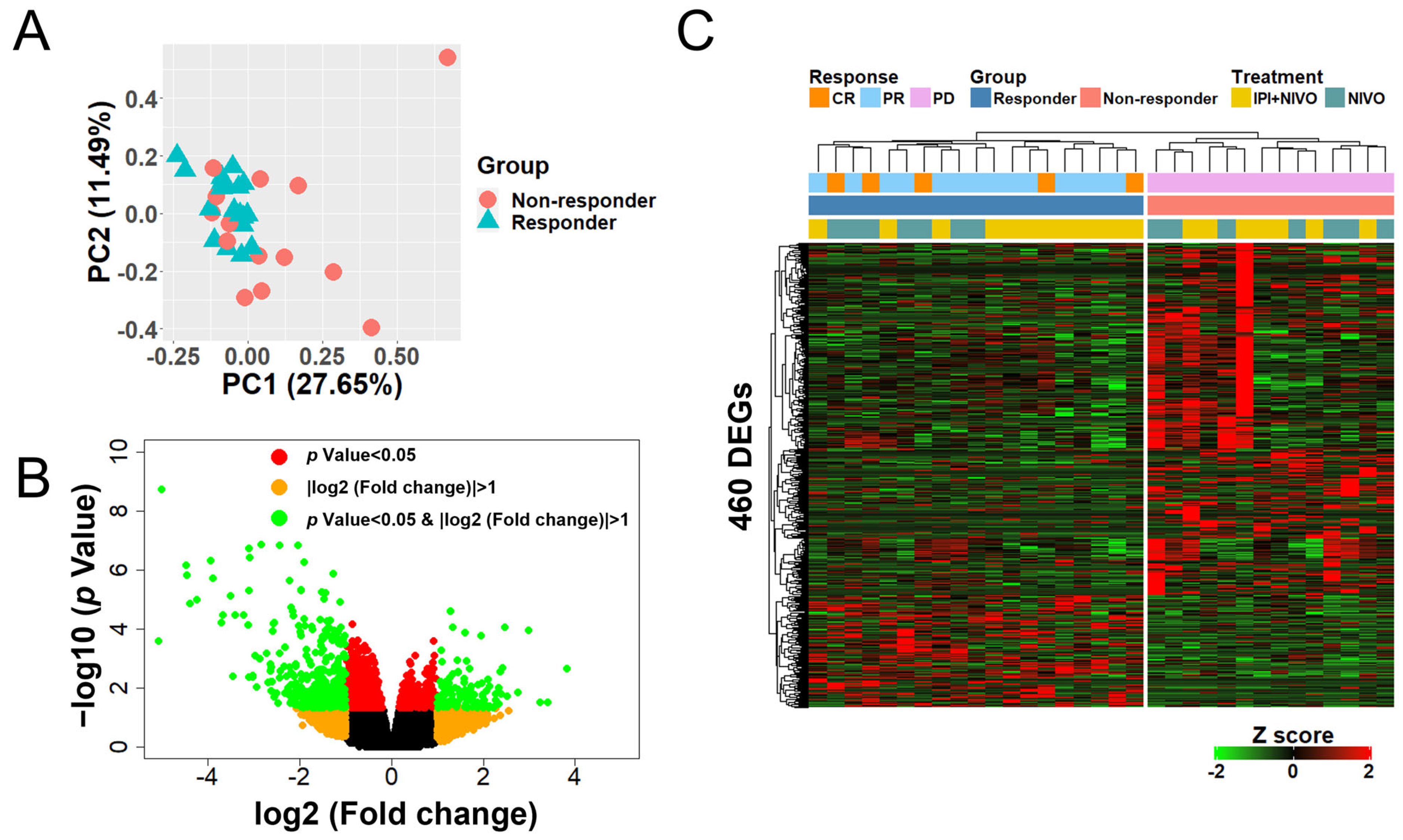
3.2. Whole-Blood Gene Expression Profiles in Patients with ICI-Treated mRCC Were Significantly Different between Responders and Non-Responders
3.3. Hierarchical Clustering Using 460 DEGs Clearly Defines Two Large Clusters with Differing Responses
3.4. Whole-Blood Gene Expression Profiles Stratified by ICI Type Were Also Significantly Different between Responders and Non-Responders
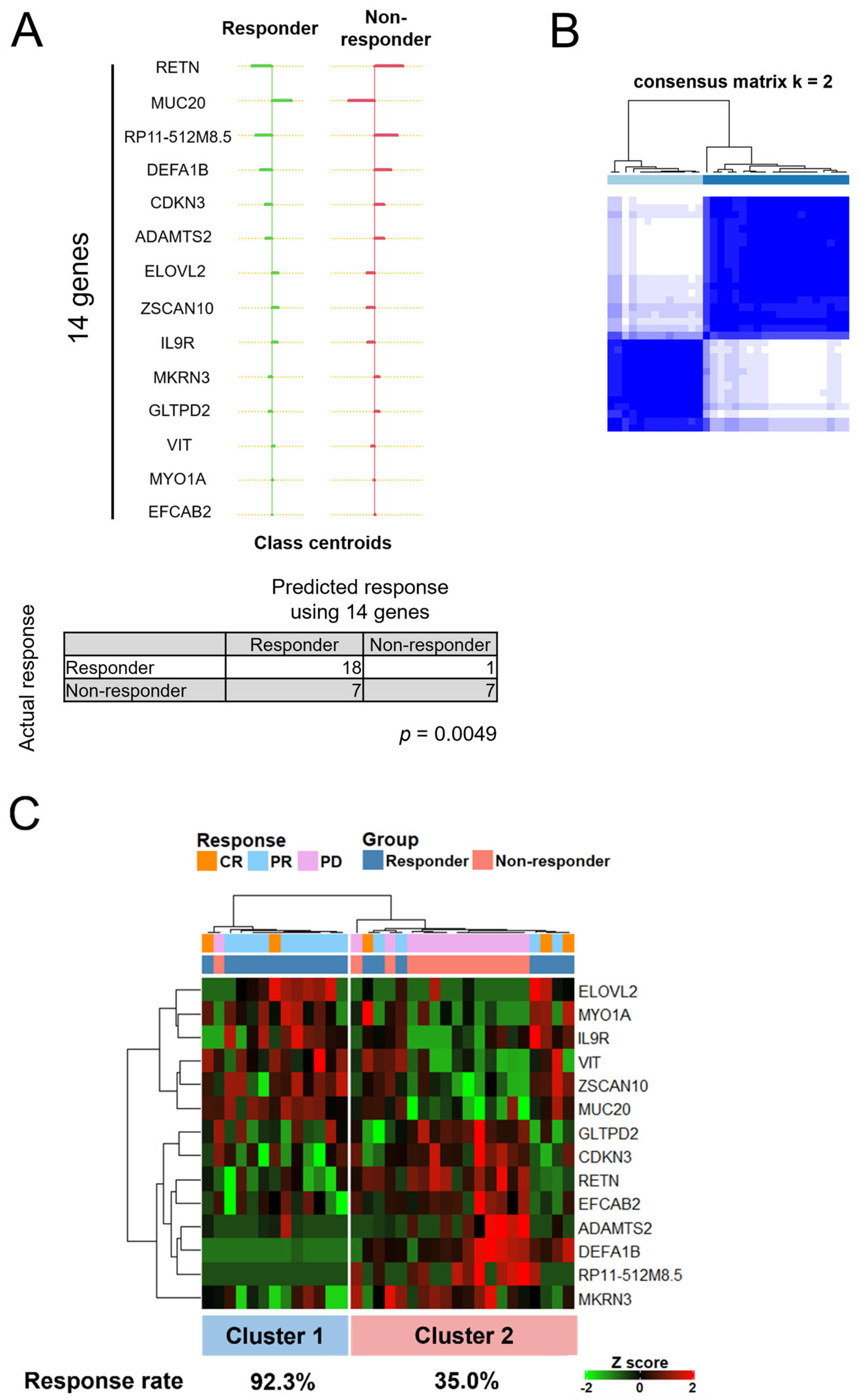
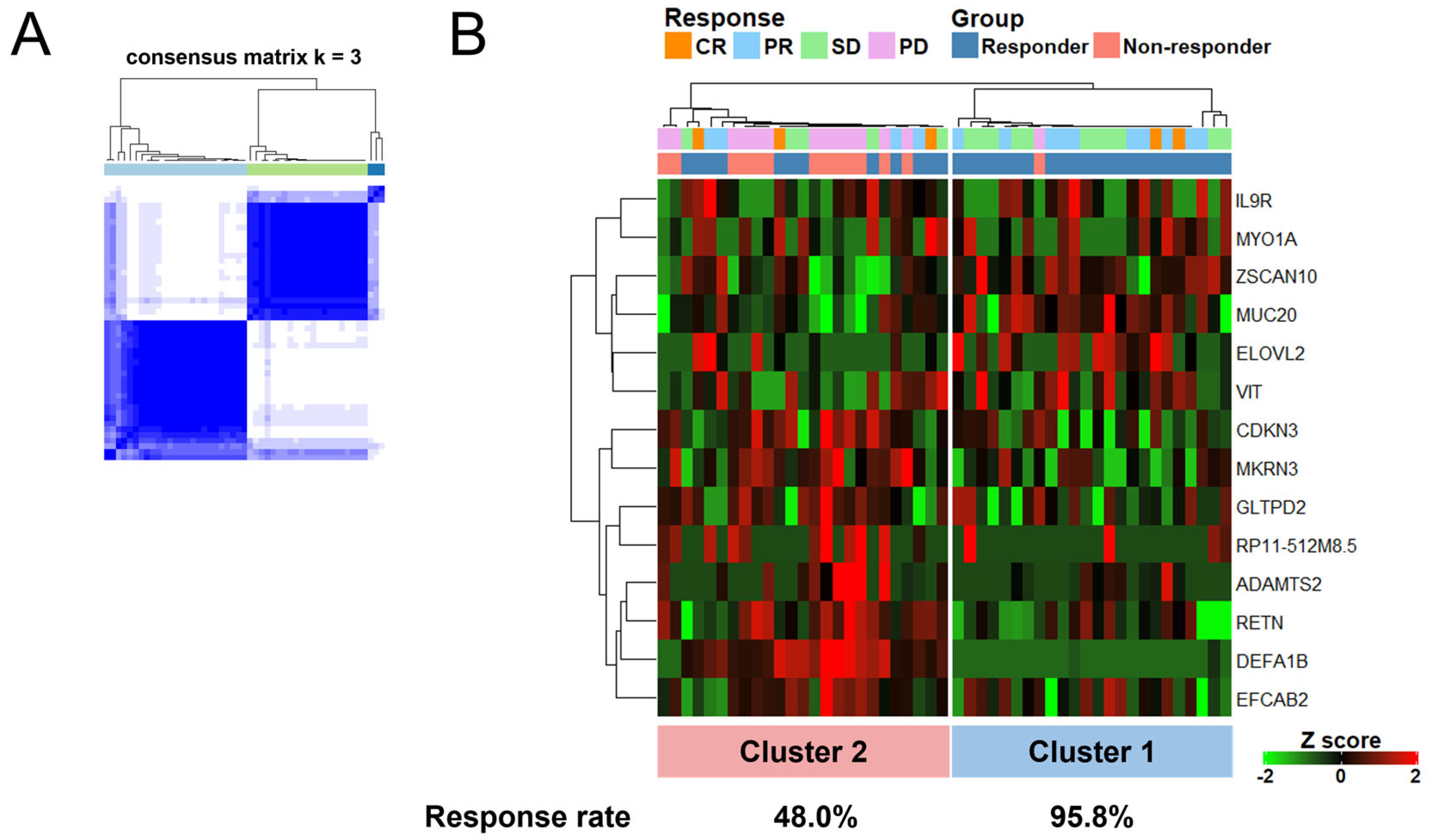
3.5. A Set of 14 Genes Could Accurately Classify Responders Treated with ICIs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atkins, M.B.; Tannir, N.M. Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer Treat. Rev. 2018, 70, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.W.; Klaassen, Z.; Agarwal, N.; Haaland, B.; Esther, J.; Ye, X.Y.; Wang, X.; Pal, S.K.; Wallis, C.J.D. First-line Treatment of Metastatic Renal Cell Carcinoma: A Systematic Review and Network Meta-analysis. Eur. Urol. Oncol. 2019, 2, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Plimack, E.R.; Procopio, G.; McDermott, D.F.; et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer 2020, 126, 4156–4167. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, M.; Ghidini, A.; Tomasello, G. Outcomes Following Immune Checkpoint Inhibitor Treatment of Patients With Microsatellite Instability-High Cancers: A Systematic Review and Meta-analysis. JAMA Oncol. 2020, 6, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, M.D.; Hayes, D.N. ConsensusClusterPlus: A class discovery tool with confidence assessments and item tracking. Bioinformatics 2010, 26, 1572–1573. [Google Scholar] [CrossRef]
- Tibshirani, R.; Hastie, T.; Narasimhan, B.; Chu, G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc. Natl. Acad. Sci. USA 2002, 99, 6567–6572. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Butterfield, L.H.; Disis, M.L.; Fox, B.A.; Kaufman, D.R.; Khleif, S.N.; Wang, E. SITC 2018 workshop report: Immuno-Oncology Biomarkers: State of the Art. J. Immunother. Cancer 2018, 6, 138. [Google Scholar] [CrossRef]
- Obermoser, G.; Presnell, S.; Domico, K.; Xu, H.; Wang, Y.; Anguiano, E.; Thompson-Snipes, L.; Ranganathan, R.; Zeitner, B.; Bjork, A.; et al. Systems Scale Interactive Exploration Reveals Quantitative and Qualitative Differences in Response to Influenza and Pneumococcal Vaccines. Immunity 2013, 38, 831–844. [Google Scholar] [CrossRef]
- Chaussabel, D.; Pascual, V.; Banchereau, J. Assessing the human immune system through blood transcriptomics. BMC Biol. 2010, 8, 84. [Google Scholar] [CrossRef]
- Singhania, A.; Graham, C.M.; Gabryšová, L.; Moreira-Teixeira, L.; Stavropoulos, E.; Pitt, J.M.; Chakravarty, P.; Warnatsch, A.; Branchett, W.J.; Conejero, L.; et al. Transcriptional profiling unveils type I and II interferon networks in blood and tissues across diseases. Nat. Commun. 2019, 10, 2887. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, P.; Wassmann, K.; Christenfeld, A.M.; Fisher, D.; Kyi, C.; Kirkwood, J.M.; Bhardwaj, N.; Oh, W.K. Whole-blood RNA transcript-based models can predict clinical response in two large independent clinical studies of patients with advanced melanoma treated with the checkpoint inhibitor, tremelimumab. J. Immunother. Cancer 2017, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Patel, L.; Buckels, A.C.; Kinghorn, I.J.; Murdock, P.R.; Holbrook, J.D.; Plumpton, C.; Macphee, C.H.; Smith, S.A. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem. Biophys. Res. Commun. 2003, 300, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Silswal, N.; Singh, A.K.; Aruna, B.; Mukhopadhyay, S.; Ghosh, S.; Ehtesham, N.Z. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem. Biophys. Res. Commun. 2005, 334, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Karapanagiotou, E.M.; Tsochatzis, E.A.; Dilana, K.D.; Tourkantonis, I.; Gratsias, I.; Syrigos, K.N. The significance of leptin, adiponectin, and resistin serum levels in non-small cell lung cancer (NSCLC). Lung Cancer 2008, 61, 391–397. [Google Scholar] [CrossRef]
- Bonaventura, A.; Grossi, F.; Carbone, F.; Vecchié, A.; Minetti, S.; Bardi, N.; Elia, E.; Ansaldo, A.M.; Ferrara, D.; Rijavec, E.; et al. Resistin is associated with overall survival in non-small cell lung cancer patients during nivolumab treatment. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2020, 22, 1603–1610. [Google Scholar] [CrossRef]
- Wang, C.; Lu, Y.; Chen, L.; Gao, T.; Yang, Q.; Zhu, C.; Chen, Y. Th9 cells are subjected to PD-1/PD-L1-mediated inhibition and are capable of promoting CD8 T cell expansion through IL-9R in colorectal cancer. Int. Immunopharmacol. 2020, 78, 106019. [Google Scholar] [CrossRef]
- Hashimoto, M.; Kamphorst, A.O.; Im, S.J.; Kissick, H.T.; Pillai, R.N.; Ramalingam, S.S.; Araki, K.; Ahmed, R. CD8 T Cell Exhaustion in Chronic Infection and Cancer: Opportunities for Interventions. Annu. Rev. Med. 2018, 69, 301–318. [Google Scholar] [CrossRef]
- Li, H.; van der Leun, A.M.; Yofe, I.; Lubling, Y.; Gelbard-Solodkin, D.; van Akkooi, A.C.J.; van den Braber, M.; Rozeman, E.A.; Haanen, J.; Blank, C.U.; et al. Dysfunctional CD8 T Cells Form a Proliferative, Dynamically Regulated Compartment within Human Melanoma. Cell 2019, 176, 775–789.e18. [Google Scholar] [CrossRef]


| Responders (CR/PR) | Non-Responders (PD) | p Value | ||
|---|---|---|---|---|
| Number of Patients | 19 | 14 | ||
| Sex | Male | 17 (89.5) | 9 (64.3) | 0.11 |
| Female | 2 (10.5) | 5 (35.7) | ||
| Median Age (Range) | 66.0 (46–82) | 71.5 (61–84) | 0.03 | |
| Histology | Clear Cell Carcinoma | 15 (78.9) | 11 (78.6) | 0.70 |
| Clear Cell Carcinoma + Sarcomatoid Variant | 2 (10.5) | 1 (7.1) | ||
| Sarcomatoid | 1 (5.3) | 1 (7.1) | ||
| Papillary | 1 (5.3) | 0 | ||
| Xp 11 Translocation | 0 | 1 (7.1) | ||
| IMDC Classification | Favorable | 4 (21.1) | 0 | 0.10 |
| Intermediate | 11 (57.9) | 9 (64.3) | ||
| Poor | 4 (21.1) | 5 (35.7) | ||
| Treatment | Nivolumab | 7 (36.8) | 7 (50.0) | 0.50 |
| Ipilimumab + Nivolumab | 12 (63.2) | 7 (50.0) | ||
| KPS | 100 | 14 (73.7) | 4 (28.6) | 0.0063 |
| 90 | 2 (10.5) | 4 (28.6) | ||
| 80 | 3 (15.8) | 1 (7.1) | ||
| 70 > = | 0 | 5 (35.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagumo, Y.; Kandori, S.; Kojima, T.; Hamada, K.; Nitta, S.; Chihara, I.; Shiga, M.; Negoro, H.; Mathis, B.J.; Nishiyama, H. Whole-Blood Gene Expression Profiles Correlate with Response to Immune Checkpoint Inhibitors in Patients with Metastatic Renal Cell Carcinoma. Cancers 2022, 14, 6207. https://doi.org/10.3390/cancers14246207
Nagumo Y, Kandori S, Kojima T, Hamada K, Nitta S, Chihara I, Shiga M, Negoro H, Mathis BJ, Nishiyama H. Whole-Blood Gene Expression Profiles Correlate with Response to Immune Checkpoint Inhibitors in Patients with Metastatic Renal Cell Carcinoma. Cancers. 2022; 14(24):6207. https://doi.org/10.3390/cancers14246207
Chicago/Turabian StyleNagumo, Yoshiyuki, Shuya Kandori, Takahiro Kojima, Kazuki Hamada, Satoshi Nitta, Ichiro Chihara, Masanobu Shiga, Hiromitsu Negoro, Bryan J. Mathis, and Hiroyuki Nishiyama. 2022. "Whole-Blood Gene Expression Profiles Correlate with Response to Immune Checkpoint Inhibitors in Patients with Metastatic Renal Cell Carcinoma" Cancers 14, no. 24: 6207. https://doi.org/10.3390/cancers14246207
APA StyleNagumo, Y., Kandori, S., Kojima, T., Hamada, K., Nitta, S., Chihara, I., Shiga, M., Negoro, H., Mathis, B. J., & Nishiyama, H. (2022). Whole-Blood Gene Expression Profiles Correlate with Response to Immune Checkpoint Inhibitors in Patients with Metastatic Renal Cell Carcinoma. Cancers, 14(24), 6207. https://doi.org/10.3390/cancers14246207







