Sex Remains Negative Prognostic Factor in Contemporary Cohort of High-Risk Non-Muscle-Invasive Bladder Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Quality of Primary TURBT and the Risk of Disease Understaging at Primary TURBT
3.2. BCG Response
3.3. Radical Cystectomy
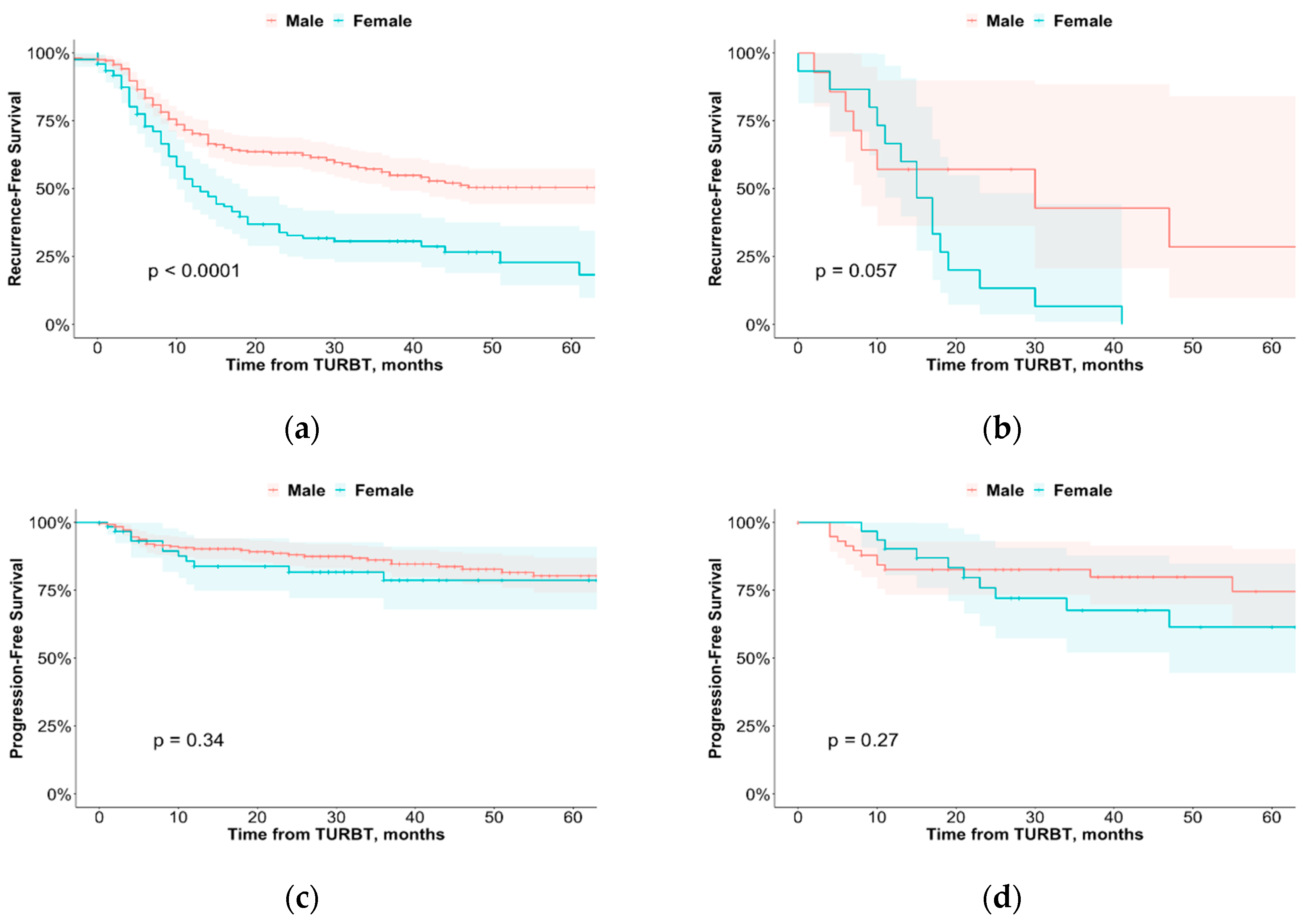
3.4. Disease Recurrence
3.5. Disease Progression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cumberbatch, M.G.K.; Jubber, I.; Black, P.C.; Esperto, F.; Figueroa, J.D.; Kamat, A.M.; Kiemeney, L.; Lotan, Y.; Pang, K.; Silverman, D.T.; et al. Epidemiology of Bladder Cancer: A Systematic Review and Contemporary Update of Risk Factors in 2018. Eur. Urol. 2018, 74, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Fajkovic, H.; Halpern, J.A.; Cha, E.K.; Bahadori, A.; Chromecki, T.F.; Karakiewicz, P.I.; Breinl, E.; Merseburger, A.S.; Shariat, S.F. Impact of gender on bladder cancer incidence, staging, and prognosis. World J. Urol. 2011, 29, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Dobruch, J.; Daneshmand, S.; Fisch, M.; Lotan, Y.; Noon, A.P.; Resnick, M.J.; Shariat, S.F.; Zlotta, A.R.; Boorjian, S.A. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur. Urol. 2016, 69, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Krimphove, M.J.; Szymaniak, J.; Marchese, M.; Tully, K.H.; D’Andrea, D.; Mossanen, M.; Lipsitz, S.R.; Kilbridge, K.; Kibel, A.S.; Kluth, L.A.; et al. Sex-specific Differences in the Quality of Treatment of Muscle-invasive Bladder Cancer Do Not Explain the Overall Survival Discrepancy. Eur. Urol. Focus 2021, 7, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.; Catto, J.W.F.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, R.J.; Rodríguez, O.; Hernández, V.; Turturica, D.; Bauerová, L.; Bruins, H.M.; Bründl, J.; van der Kwast, T.H.; Brisuda, A.; Rubio-Briones, J.; et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non-muscle-invasive Bladder Cancer (NMIBC) Incorporating the WHO 2004/2016 and WHO 1973 Classification Systems for Grade: An Update from the EAU NMIBC Guidelines Panel. Eur. Urol. 2021, 79, 480–488. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, S.; Alfred Witjes, J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: A systematic review. Eur. Urol. 2011, 60, 493–500. [Google Scholar] [CrossRef]
- D’Andrea, D.; Soria, F.; Grotenhuis, A.J.; Cha, E.K.; Malats, N.; di Stasi, S.; Joniau, S.; Cai, T.; van Rhijn, B.W.G.; Irani, J.; et al. Association of patients’ sex with treatment outcomes after intravesical bacillus Calmette-Guérin immunotherapy for T1G3/HG bladder cancer. World J. Urol. 2021, 39, 3337–3344. [Google Scholar] [CrossRef]
- Otto, W.; May, M.; Fritsche, H.-M.; Dragun, D.; Aziz, A.; Gierth, M.; Trojan, L.; Herrmann, E.; Moritz, R.; Ellinger, J.; et al. Analysis of sex differences in cancer-specific survival and perioperative mortality following radical cystectomy: Results of a large German multicenter study of nearly 2500 patients with urothelial carcinoma of the bladder. Gend. Med. 2012, 9, 481–489. [Google Scholar] [CrossRef]
- Tilki, D.; Reich, O.; Svatek, R.S.; Karakiewicz, P.I.; Kassouf, W.; Novara, G.; Ficarra, V.; Chade, D.C.; Fritsche, H.-M.; Gerwens, N.; et al. Characteristics and outcomes of patients with clinical carcinoma in situ only treated with radical cystectomy: An international study of 243 patients. J. Urol. 2010, 183, 1757–1763. [Google Scholar] [CrossRef]
- Messer, J.C.; Shariat, S.F.; Dinney, C.P.; Novara, G.; Fradet, Y.; Kassouf, W.; Karakiewicz, P.I.; Fritsche, H.-M.; Izawa, J.I.; Lotan, Y.; et al. Female gender is associated with a worse survival after radical cystectomy for urothelial carcinoma of the bladder: A competing risk analysis. Urology 2014, 83, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Kluth, L.A.; Rieken, M.; Xylinas, E.; Kent, M.; Rink, M.; Rouprêt, M.; Sharifi, N.; Jamzadeh, A.; Kassouf, W.; Kaushik, D.; et al. Gender-specific differences in clinicopathologic outcomes following radical cystectomy: An international multi-institutional study of more than 8000 patients. Eur. Urol. 2014, 66, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Palou, J.; Sylvester, R.J.; Faba, O.R.; Parada, R.; Peña, J.A.; Algaba, F.; Villavicencio, H. Female gender and carcinoma in situ in the prostatic urethra are prognostic factors for recurrence, progression, and disease-specific mortality in T1G3 bladder cancer patients treated with bacillus Calmette-Guérin. Eur. Urol. 2012, 62, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gomez, J.; Solsona, E.; Unda, M.; Martinez-Piñeiro, L.; Gonzalez, M.; Hernandez, R.; Madero, R.; Ojea, A.; Pertusa, C.; Rodriguez-Molina, J.; et al. Prognostic factors in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guérin: Multivariate analysis of data from four randomized CUETO trials. Eur. Urol. 2008, 53, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.; Soave, A.; Shariat, S.F.; Fajkovic, H.; Fisch, M.; Rink, M. Female with bladder cancer: What and why is there a difference? Transl. Androl. Urol. 2016, 5, 668–682. [Google Scholar] [CrossRef]
- Boorjian, S.A.; Zhu, F.; Herr, H.W. The effect of gender on response to bacillus Calmette-Guérin therapy for patients with non-muscle-invasive urothelial carcinoma of the bladder. BJU Int. 2010, 106, 357–361. [Google Scholar] [CrossRef]
- Kluth, L.A.; Fajkovic, H.; Xylinas, E.; Crivelli, J.J.; Passoni, N.; Rouprêt, M.; Becker, A.; Comploj, E.; Pycha, A.; Holmang, S.; et al. Female gender is associated with higher risk of disease recurrence in patients with primary T1 high-grade urothelial carcinoma of the bladder. World J. Urol. 2013, 31, 1029–1036. [Google Scholar] [CrossRef]
- Gontero, P.; Sylvester, R.; Pisano, F.; Joniau, S.; Vander Eeckt, K.; Serretta, V.; Larré, S.; Di Stasi, S.; van Rhijn, B.; Witjes, A.J.; et al. Prognostic factors and risk groups in T1G3 non-muscle-invasive bladder cancer patients initially treated with Bacillus Calmette-Guérin: Results of a retrospective multicenter study of 2451 patients. Eur. Urol. 2015, 67, 74–82. [Google Scholar] [CrossRef]
- Soave, A.; Dahlem, R.; Hansen, J.; Weisbach, L.; Minner, S.; Engel, O.; Kluth, L.A.; Chun, F.K.; Shariat, S.F.; Fisch, M.; et al. Gender-specific outcomes of bladder cancer patients: A stage-specific analysis in a contemporary, homogenous radical cystectomy cohort. Eur. J. Surg. Oncol. 2015, 41, 368–377. [Google Scholar] [CrossRef]
- Mitra, A.P.; Skinner, E.C.; Schuckman, A.K.; Quinn, D.I.; Dorff, T.B.; Daneshmand, S. Effect of gender on outcomes following radical cystectomy for urothelial carcinoma of the bladder: A critical analysis of 1,994 patients. Urol. Oncol. 2014, 32, 52.e1–52.e9. [Google Scholar] [CrossRef]
- Herkommer, K.; Hofer, C.; Gschwend, J.E.; Kron, M.; Treiber, U. Gender and body mass index as risk factors for bladder perforation during primary transurethral resection of bladder tumors. J. Urol. 2012, 187, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, X.; Fan, J.; Li, L.; He, D.; Wu, K. Risk Stratification for the Rate and Location of Residual Bladder Tumor for the Decision of Re-Transurethral Resection of Bladder Tumor. Front. Oncol. 2022, 12, 788568. [Google Scholar] [CrossRef]
- Herr, H.W. Role of re-resection in non-muscle-invasive bladder cancer. Sci. World J. 2011, 11, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Holz, S.; Albisinni, S.; Gilsoul, J.; Pirson, M.; Duthie, V.; Quackels, T.; Vanden Bossche, M.; Roumeguère, T. Risk factor assessment in high-risk, bacillus Calmette–Guérin-treated, non-muscle-invasive bladder cancer. Res. Rep. Urol. 2017, 9, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.M.; Sylvester, R.J.; Böhle, A.; Palou, J.; Lamm, D.L.; Brausi, M.; Soloway, M.; Persad, R.; Buckley, R.; Colombel, M.; et al. Definitions, End Points, and Clinical Trial Designs for Non–Muscle-Invasive Bladder Cancer: Recommendations from the International Bladder Cancer Group. J. Clin. Oncol. 2016, 34, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Bree, K.K.; Hensley, P.J.; Brooks, N.; Matulay, J.; Nogueras-Gonzalez, G.M.; Navai, N.; Dinney, C.P.; Kamat, A.M. Impact of sex on response to BCG in non-muscle invasive bladder cancer patients: A contemporary review from a tertiary care center. World J. Urol. 2021, 39, 4143–4149. [Google Scholar] [CrossRef]
- Herr, H.W. Tumor progression and survival of patients with high grade, noninvasive papillary (TaG3) bladder tumors: 15-year outcome. J. Urol. 2000, 163, 60–61, discussion 61–62. [Google Scholar] [CrossRef]
- Bree, K.K.; Hensley, P.J.; Lobo, N.; Brooks, N.A.; Nogueras-Gonzalez, G.M.; Guo, C.C.; Navai, N.; Grossman, H.B.; Dinney, C.P.; Kamat, A.M. All High-Grade Ta Tumors Should Be Classified as High Risk: Bacillus Calmette-Guérin Response in High-Grade Ta Tumors. J. Urol. 2022, 208, 284–291. [Google Scholar] [CrossRef]
- Amin, M.B.; Smith, S.C.; Reuter, V.E.; Epstein, J.I.; Grignon, D.J.; Hansel, D.E.; Lin, O.; McKenney, J.K.; Montironi, R.; Paner, G.P.; et al. Update for the practicing pathologist: The International Consultation on Urologic Disease-European association of urology consultation on bladder cancer. Mod. Pathol. 2015, 28, 612–630. [Google Scholar] [CrossRef]
- Cao, D.; Vollmer, R.T.; Luly, J.; Jain, S.; Roytman, T.M.; Ferris, C.W.; Hudson, M.A. Comparison of 2004 and 1973 World Health Organization grading systems and their relationship to pathologic staging for predicting long-term prognosis in patients with urothelial carcinoma. Urology 2010, 76, 593–599. [Google Scholar] [CrossRef]
- Otto, W.; Denzinger, S.; Fritsche, H.-M.; Burger, M.; Wieland, W.F.; Hofstädter, F.; Hartmann, A.; Bertz, S. The WHO classification of 1973 is more suitable than the WHO classification of 2004 for predicting survival in pT1 urothelial bladder cancer. BJU Int. 2011, 107, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Kardoust Parizi, M.; Enikeev, D.; Glybochko, P.V.; Seebacher, V.; Janisch, F.; Fajkovic, H.; Chłosta, P.L.; Shariat, S.F. Prognostic value of T1 substaging on oncological outcomes in patients with non-muscle-invasive bladder urothelial carcinoma: A systematic literature review and meta-analysis. World J. Urol. 2020, 38, 1437–1449. [Google Scholar] [CrossRef] [PubMed]
- de Jong, F.C.; Hoedemaeker, R.F.; Kvikstad, V.; Mensink, J.T.M.; de Jong, J.J.; Boevé, E.R.; van der Schoot, D.K.E.; Zwarthoff, E.C.; Boormans, J.L.; Zuiverloon, T.C.M. T1 Substaging of Nonmuscle Invasive Bladder Cancer is Associated with bacillus Calmette-Guérin Failure and Improves Patient Stratification at Diagnosis. J. Urol. 2021, 205, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.I.; Angulo, J.C. The prognostic significance of vascular invasion in stage T1 bladder cancer. Histopathology 1995, 27, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Kikuchi, E.; Mikami, S.; Miyajima, A.; Oya, M. Lymphovascular invasion status at transurethral resection of bladder tumors may predict subsequent poor response of T1 tumors to bacillus Calmette-Guérin. BMC Urol. 2016, 16, 5. [Google Scholar] [CrossRef]
- Tilki, D.; Shariat, S.F.; Lotan, Y.; Rink, M.; Karakiewicz, P.I.; Schoenberg, M.P.; Lerner, S.P.; Sonpavde, G.; Sagalowsky, A.I.; Gupta, A. Lymphovascular invasion is independently associated with bladder cancer recurrence and survival in patients with final stage T1 disease and negative lymph nodes after radical cystectomy. BJU Int. 2013, 111, 1215–1221. [Google Scholar] [CrossRef]
- Yoneda, K.; Kamiya, N.; Utsumi, T.; Wakai, K.; Oka, R.; Endo, T.; Yano, M.; Hiruta, N.; Ichikawa, T.; Suzuki, H. Impact of Lymphovascular Invasion on Prognosis in the Patients with Bladder Cancer—Comparison of Transurethral Resection and Radical Cystectomy. Diagnostics 2021, 11, 244. [Google Scholar] [CrossRef]
- Ukai, R.; Hashimoto, K.; Nakayama, H.; Iwamoto, T. Lymphovascular invasion predicts poor prognosis in high-grade pT1 bladder cancer patients who underwent transurethral resection in one piece. Jpn. J. Clin. Oncol. 2017, 47, 447–452. [Google Scholar] [CrossRef]
- Yuk, H.D.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. Lymphovascular invasion have a similar prognostic value as lymph node involvement in patients undergoing radical cystectomy with urothelial carcinoma. Sci. Rep. 2018, 8, 15928. [Google Scholar] [CrossRef]
| Variable | Men | Women | p-Value |
|---|---|---|---|
| Number of patients | 388 (75%) | 131 (25%) | NA |
| Age at primary TUR (mean) | 69 | 70 | 0.27 |
| Follow-up (months) | 44 | 43 | 0.65 |
| pTstage | 0.20 | ||
| Ta | 17 (4%) | 10 (8%) | |
| T1 | 363 (94%) | 118 (90%) | |
| Tx | 8 (2%) | 3 (2%) | |
| Muscle presence at TURBT | 0.78 | ||
| Present | 287 (74%) | 74 (56%) | |
| Absent | 101 (26%) | 57 (44%) | |
| Grade | 0.48 | ||
| High | 291 (75%) | 91 (69%) | |
| Low | 97 (25%) | 40 (31%) | |
| Tumor diameter (<3 cm/>3 cm) | 0.78 | ||
| <3 cm | 197 (51%) | 69 (53%) | |
| >3 cm | 191 (49%) | 62 (47%) | |
| Number of tumors | 0.57 | ||
| Single | 233 (60%) | 77 (59%) | |
| Multiple | 155 (40%) | 54 (41%) | |
| Concomitant Cis | 106 (27%) | 29 (22%) | 0.49 |
| LVI | 31 (8%) | 11 (8%) | 0.93 |
| ReTUR | 312 (80%) | 94 (72%) | 0.70 |
| Adequate BCG | 155 (40%) | 63 (48%) | 0.75 |
| Recurrence | 156 (40%) | 82 (63%) | 0.22 |
| Progression | 57 (15%) | 29 (22%) | 0.25 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Factor | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Age at TURBT | 0.99 (0.98–1.01) | 0.40 | 1.00 (0.98–1.01) | 0.20 |
| Grade at TURBT (high vs. low) | 1.00 (0.73 -136) | 0.98 | 1.05 (0.63–1.44) | 0.81 |
| Muscle presence (yes vs. now) | 0.90 (0.68–1.19) | 0.45 | 0.96 (0.76–1.43) | 0.79 |
| T stage from TURBT (T1 vs. Ta) | 1.06 (0.66–1.70) | 0.81 | 1.07 (0.57–1.53) | 0.78 |
| Tumor size (<3 cm vs. >3 cm) | 0.99 (0.78–1.31) | 0.94 | 0.97 (0.77–1.38) | 0.85 |
| Concomitant Cis (yes vs. no) | 1.08 (0.80–1.44) | 0.62 | 1.10 (0.79–1.54) | 0.56 |
| LVI (yes vs. no) | 1.45 (0.97–2.22) | 0.07 | 1.59 (1.04–2.43) | 0.03 |
| Sex (female vs. male) | 1.94 (1.48–2.55) | <0.001 | 1.91 (1.39–2.60) | <0.001 |
| (a) | ||||
| Univariate | Multivariate | |||
| Factor | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Age at TURBT | 1.02 (0.96–1.01) | 0.15 | 1.00 (0.96–1.05) | 0.93 |
| Grade at TURBT (high vs. low) | 1.20 (0.43–3.32) | 0.73 | 4.37 (0.54–3.99) | 0.99 |
| Muscle presence (yes vs. no) | 0.90 (0.52–1.54) | 0.69 | 0.92 (0.50–1.68) | 0.78 |
| T stage from TURBT (T1 vs. Ta) | 1.08 (0.40–2.16) | 0.86 | 1.68 (0.25–1.44) | 0.25 |
| Tumor size (<3 cm vs. >3 cm) | 0.73 (0.81–2.32) | 0.23 | 0.64 (0.86–2.88) | 0.14 |
| Concomitant Cis (yes vs. no) | 1.30 (0.77–2.19) | 0.32 | 1.45 (0.81–2.61) | 0.21 |
| LVI (yes vs. no) | 1.38 (0.69–2.75) | 0.36 | 1.06 (0.49–2.30) | 0.88 |
| Sex (female vs. male) | 1.81 (1.07–3.06) | 0.03 | 1.99 (0.98–4.02) | 0.06 |
| (b) | ||||
| Univariate | Multivariate | |||
| Factor | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Age at TURBT | 1.02 (0.99–1.04) | 0.15 | 1.02 (0.99–1.04) | 0.22 |
| Grade at TURBT (high vs. low) | 1.42 (0.79–2.53) | 0.24 | 1.06 (0.49–2.27) | 0.89 |
| Muscle presence (yes vs. now) | 0.69 (0.44–1.09) | 0.11 | 0.75 (0.45–1.24) | 0.26 |
| T stage from TURBT (T1 vs. Ta) | 1.05 (0.48–2.29) | 0.91 | 1.05 (0.46–2.40) | 0.91 |
| Tumor size (<3 cm vs. >3 cm) | 1.53 (0.99–2.37) | 0.054 | 1.55 (0.95–2.52) | 0.08 |
| Concomitant Cis (yes vs. no) | 1.53 (0.97–2.42) | 0.07 | 1.75 (1.03–2.95) | 0.04 |
| LVI (yes vs. no) | 3.57 (2.15–5.94) | <0.001 | 3.36 (1.97–5.71) | <0.001 |
| Sex (female vs. male) | 0.73 (0.72–2.62) | 0.34 | 1.38 (0.82–2.32) | 0.23 |
| (c) | ||||
| Univariate | Multivariate | |||
| Factor | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Age at TURBT | 1.01 (0.96–1.03) | 0.63 | 1.00 (0.96–1.05) | 0.93 |
| Grade at TURBT (high vs. low) | 1.32 (0.83–14.13) | 0.99 | 1.04 (0.54–3.99) | 0.99 |
| Muscle presence (yes vs. now) | 1.84 (0.23–1.26) | 0.16 | 1.78 (0.22–1.38) | 0.20 |
| T stage from TURBT (T1 vs. Ta) | 0.63 (0.54–4.77) | 0.40 | 1.57 (0.47–5.30) | 0.46 |
| Tumor size (<3 cm vs. >3 cm) | 0.53 (0.82–4.23) | 0.14 | 2.03 (080–5.17) | 0.14 |
| Concomitant Cis (yes vs. no) | 1.45 (0.65–3.25) | 0.36 | 1.61 (0.65–4.00) | 0.30 |
| LVI (yes vs. no) | 2.99 (1.27–7.06) | 0.01 | 2.71 (1.03–7.13) | 0.04 |
| Sex (female vs. male) | 1.56 (0.70–3.48) | 0.28 | 1.09 (0.33–2.52) | 0.87 |
| (d) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilski, K.; Kozikowski, M.; Skrzypczyk, M.A.; Dobruch, A.; Hendricksen, K.; D’Andrea, D.; Czech, A.K.; Dobruch, J. Sex Remains Negative Prognostic Factor in Contemporary Cohort of High-Risk Non-Muscle-Invasive Bladder Cancer. Cancers 2022, 14, 6110. https://doi.org/10.3390/cancers14246110
Bilski K, Kozikowski M, Skrzypczyk MA, Dobruch A, Hendricksen K, D’Andrea D, Czech AK, Dobruch J. Sex Remains Negative Prognostic Factor in Contemporary Cohort of High-Risk Non-Muscle-Invasive Bladder Cancer. Cancers. 2022; 14(24):6110. https://doi.org/10.3390/cancers14246110
Chicago/Turabian StyleBilski, Konrad, Mieszko Kozikowski, Michał A. Skrzypczyk, Aleksandra Dobruch, Kees Hendricksen, David D’Andrea, Anna Katarzyna Czech, and Jakub Dobruch. 2022. "Sex Remains Negative Prognostic Factor in Contemporary Cohort of High-Risk Non-Muscle-Invasive Bladder Cancer" Cancers 14, no. 24: 6110. https://doi.org/10.3390/cancers14246110
APA StyleBilski, K., Kozikowski, M., Skrzypczyk, M. A., Dobruch, A., Hendricksen, K., D’Andrea, D., Czech, A. K., & Dobruch, J. (2022). Sex Remains Negative Prognostic Factor in Contemporary Cohort of High-Risk Non-Muscle-Invasive Bladder Cancer. Cancers, 14(24), 6110. https://doi.org/10.3390/cancers14246110






